Key Points
Leukemic cells rapidly adapt to targeted therapy by elevating prosurvival protein expression.
Cell attrition and enhanced bioavailability of BAFF drive increased survival, highlighting avenues for more potent combination therapies.
Visual Abstract
Venetoclax, a first-in-class BH3 mimetic drug that targets B-cell lymphoma-2 (BCL-2), has improved the outcomes of patients with chronic lymphocytic leukemia (CLL). Early measurements of the depth of the venetoclax treatment response, assessed by minimal residual disease, are strong predictors of long-term clinical outcomes. However, there are limited data on the early changes induced by venetoclax treatment that might inform strategies to improve responses. To address this gap, we conducted longitudinal mass cytometric profiling of blood cells from patients with CLL during the first 5 weeks of venetoclax monotherapy. At baseline, we resolved CLL heterogeneity at the single-cell level to define multiple subpopulations in all patients based on proliferative, metabolic, and cell survival proteins. Venetoclax induced a significant reduction in all CLL subpopulations and caused rapid upregulation of the prosurvival BCL-2, BCL-extra large, and mantle cell lymphoma-1 proteins in surviving cells, which had reduced sensitivity to the drug. In mouse models, the venetoclax-induced elevation of survival proteins in B cells and CLL-like cells that persisted was recapitulated, and genetic models demonstrated that extensive apoptosis and access to the B-cell cytokine, B-cell activating factor (BAFF), were essential. Accordingly, in patients with CLL who were treated with venetoclax or the anti-CD20 antibody obinutuzumab there was marked elevation in BAFF and an increase in prosurvival proteins in leukemic cells that persisted. Overall, these data highlight the rapid adaptation of CLL cells to targeted therapies through homeostatic factors and support cotargeting of cytokine signals to achieve deeper and more durable long-term responses.
Introduction
Chronic lymphocytic leukemia (CLL), the most prevalent adult leukemia in many developed countries, is characterized by the accumulation of long-lived CD5+CD19+ B cells in the blood, bone marrow (BM), and secondary lymphoid organs. Most CLL cells are quiescent,1 but there is a small population of proliferating cells detectable in lymph nodes and blood that respond to growth factor signals.2 Defective apoptosis is a hallmark of CLL and is mediated by elevated expression of the prosurvival protein BCL-2.3 Venetoclax (ABT-199), a small molecule inhibitor of B-cell lymphoma-2 (BCL-2), is a highly efficacious treatment for CLL, either as monotherapy4,5 or in combination with other agents.6-9 Venetoclax induces rapid apoptosis, and the majority of susceptible cells die within 4 to 8 hours of exposure in vitro and in vivo.10 Administration of high concentrations of venetoclax in patients with bulky disease can cause tumor lysis syndrome,4 and therefore a weekly dose ramp-up regimen is typically used.
Despite the high efficacy of venetoclax in CLL (and acute myeloid leukemia [AML]), incomplete responses and emergent therapeutic resistance remain important challenges. Studies of patients who relapse after long-term venetoclax therapy have revealed multiple, nonmutually exclusive drug resistance mechanisms, such as (1) mutations in the binding groove of BCL-2,11-13 (2) overexpression of other members of the BCL-2 family prosurvival proteins not targeted by venetoclax (eg, B-cell lymphoma extra large [BCL-XL] and mantle cell lymphoma-1 [MCL-1]),11,14 or (3) loss of BCL-2-associated protein X (BAX)15,16 and/or TP53 dysfunction.17 However, how leukemic cells initially evade venetoclax-induced apoptosis and the setting in which resistance emerges remain unclear.
In this study, we sought to address these questions by longitudinal deep profiling of blood cells from patients during the ramp-up period. We found striking elevation of prosurvival BCL-2 protein levels in residual CLL cells after treatment that was recapitulated in murine models. We found evidence that heightened BCL-2 in surviving CLL cells was caused by both the preferential loss of cells with relatively lower BCL-2 expression and B-cell activating factor (BAFF)-driven upregulation of BCL-2. Abrogation of BAFF receptor (BAFF-R) signaling blocked venetoclax-induced BCL-2 upregulation in vivo in normal B cells. Moreover, obinutuzumab, a targeted therapy that kills CLL cells in a BCL-2–independent manner, also led to BCL-2 upregulation in persisting cells, accompanied by marked elevation of serum BAFF. These findings highlight a mechanism by which targeted therapies alleviate cell competition for cytokine-mediated survival signals in CLL cells, suggesting new avenues to achieve deeper responses.
Methods
Patient samples
Patients treated with venetoclax monotherapy (VENICE study; NCT0298073118), venetoclax-ibrutinib (CAPTIVATE; NCT0291058319), or combination time-limited venetoclax-obinutuzumab7 were recruited from the Department of Clinical Haematology. The Royal Melbourne Hospital and Peter MacCallum Cancer Centre (Victoria, Australia; supplemental Tables 1 and 3, available on the Blood website); healthy donors were from the Victorian Blood Donor Registry. All donors provided written informed consent, and the research was approved by human research ethics committees/institutional review boards. Peripheral blood (PB) mononuclear cells were isolated by density gradient centrifugation and resuspended in Iscove modified Dulbecco medium with 10% heat-inactivated fetal calf serum. Cells from patients with breast cancer (ACTRN1261500070251620; supplemental Table 2) were analyzed using cellular indexing of transcriptomes and epitopes sequencing as previously described.21
Mass cytometry
Cells were prepared as previously described21,22 and frozen at −80°C. Thawed cisplatin-labeled cells underwent 20-plex palladium barcoding and were batched (supplemental Figure 1A) with common anchor samples. After methanol permeabilization and staining with antibody conjugates (supplemental Table 4) and 125 nM 191Ir/193Ir DNA intercalator (Fluidigm, San Francisco, CA), cells were washed, filtered, and resuspended with EQ normalization beads and acquired on a Helios CyTOF (Fluidigm). Data were processed using the CATALYST package.23 Replicated anchor samples were used to identify and correct for batch effects using CytofRUV.24 FlowSOM with 22 lineage proteins was used to identify clusters, and all clusters were used to define pseudoreplicates with k = 3. Data were visualized using uniform manifold approximation and projection (UMAP).25
Mice
C57BL/6, Bak–/–BaxΔCd23, vav-huBcl2, Bak–/–BaxΔCd23Tnfrsf13c–/–, and Eμ-TCL-1 mice were backcrossed onto the C57BL/6 background from foundation strains (supplemental Table 6). All mice were housed at Walter and Eliza Hall Institute of Medical Research or The University of Melbourne in specific pathogen-free conditions, and experiments were performed according to the relevant animal ethics committee requirements. Wild-type mice received 5 × 105 splenocytes from leukemic Eμ-TCL1 donors (percentage of CD19+CD5+ cells >95%) by IV injection. Recipients were treated with venetoclax or vehicle after reaching a threshold of 80% CD19+CD5+ blood lymphocytes. Venetoclax (100 mg/kg body weight) or vehicle was administered by daily oral gavage for 1 week.
Hematopoietic reconstitution
CD45.1/CD45.2 C57BL/6 F1 mice (6- to 8 weeks old) were lethally irradiated with 2 × 5.5 Gy 3 hours apart and reconstituted by IV injection of 2 × 106 BM cells. Irradiated recipients received anti-thymocyte antigen-1 (clone T24; 100 μg) intraperitoneally 24 hours later and were then reconstituted for >6 weeks.
Flow cytometry
Single-cell suspensions were stained with antibody conjugates to detect cell surface and intracellular proteins (supplemental Table 7). Samples were acquired on an LSRII or a Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Cytokine measurements
Serum cytokines from human samples were measured using the Luminex xMAP technology on the Bio-Plex 200 platform (Bio-Plex Manager 5.0) with the Bio-Plex Pro Human Cytokine Screening Test Kit (48-Plex, Bio-Rad) and the Bio-Plex Pro Human Inflammation Panel-1 Kit (37-Plex, Bio-Rad). Mouse BAFF was measured using the Mouse BAFF Quantikine enzyme-linked immunosorbent assay Kit (R&D Systems).
Quantification and statistical analysis
Data were analyzed using GraphPad Prism software. Student 2-tailed t tests (paired or unpaired) were used for comparisons and differences were considered significant when P < .05.
Results
Mass cytometry resolves heterogeneity among CLL cells
To assess the impact of venetoclax on CLL heterogeneity and survival, we assayed PB from 18 patients with relapse/refractory CLL who were receiving venetoclax monotherapy,18 healthy donors, and 2 treatment-naive patients with CLL who received venetoclax-ibrutinib after ibrutinib run-in7 (supplemental Table 1). PB samples were drawn before each weekly venetoclax dose escalation until 400 mg/day was reached (Figure 1A). As expected, venetoclax induced substantial dose-related reductions in PB lymphocytes in all patients (Figure 1B). Samples were barcoded with palladium and pooled into batches that included shared anchor samples and then stained with a panel of 43 conjugates to discriminate between CLL and normal immune subpopulations based on BCL-2 family proteins, cell cycle regulators, and key signaling pathways, including NF-κB, extracellular signal-regulated kinase/p38, mammalian target of rapamycin signaling, DNA damage, cyclic-AMP response element binding protein (CREB), and cancer-related proteins (TP53 and c-MYC)22 (supplemental Figure 1A; supplemental Table 4) using CyTOF analysis.
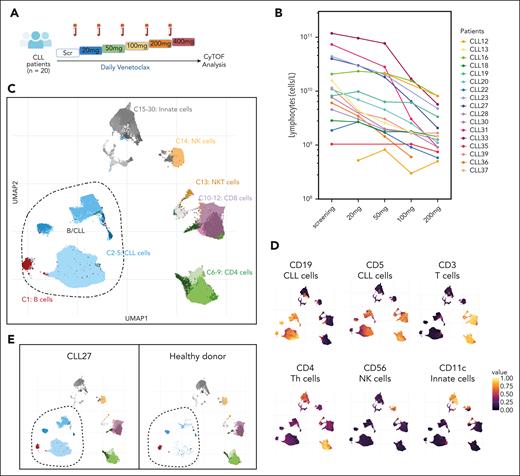
Mass cytometric analysis resolves CLL heterogeneity. (A) Schematic representation of the experimental strategy with PB samples from 20 patients with CLL collected at screening and during weekly venetoclax dose escalation. (B) Lymphocyte counts (cells per liter) during venetoclax dose escalation in each patient. (C) UMAP projection of PB cells (subsampling of 2000 cells per each sample) from patients with CLL (n = 18 VEN patients plus n = 2 VEN+IBR patients, 6 timepoints) and healthy donors colored by the following clusters: B cells (C1), CLL cells (C2-5), T cells (C6-C12), NK cells (C14), and myeloid cells (C15:C30). (D) UMAP plots colored by expression levels of the indicated markers used to identify the major immune cell populations. (E) UMAP plots of a patient with CLL (CLL27) and healthy donor with B/CLL clusters circled. IBR, ibrutinib; NKT, natural killer T; Scr, screening.
Mass cytometric analysis resolves CLL heterogeneity. (A) Schematic representation of the experimental strategy with PB samples from 20 patients with CLL collected at screening and during weekly venetoclax dose escalation. (B) Lymphocyte counts (cells per liter) during venetoclax dose escalation in each patient. (C) UMAP projection of PB cells (subsampling of 2000 cells per each sample) from patients with CLL (n = 18 VEN patients plus n = 2 VEN+IBR patients, 6 timepoints) and healthy donors colored by the following clusters: B cells (C1), CLL cells (C2-5), T cells (C6-C12), NK cells (C14), and myeloid cells (C15:C30). (D) UMAP plots colored by expression levels of the indicated markers used to identify the major immune cell populations. (E) UMAP plots of a patient with CLL (CLL27) and healthy donor with B/CLL clusters circled. IBR, ibrutinib; NKT, natural killer T; Scr, screening.
FlowSOM was used to identify 30 clusters, and the data were visualized using 2-dimensional UMAP (Figure 1C). These clusters were annotated based on the expression of lineage markers to distinguishing among healthy B (C1: CD19highCD5low), CLL (C2-5: CD19highCD5high), T (C6-12: CD3high), natural killer T (C13), natural killer (C14: CD56high), and myeloid (C15-30: CD11chigh) cell populations (Figure 1C-D). Cluster distinctions within lineages were driven by the expression of BCL-2 family proteins, cell cycle regulators, core signaling pathway constituents, including NF-kB, extracellular signal-regulated kinase/p38, mammalian target of rapamycin signaling, and JAK/STAT, and the transcription factors phosphorylated CREB (pCREB), TP53, and c-MYC (supplemental Figure 1B-C). Overall, the initial analysis identified 4 putative CLL clusters (C2-5: CD19highCD5high) that were prominent in PB from patients with CLL but not in PB from a healthy donor (Figure 1E).
Next, we performed finer mapping of normal B/CLL clusters into 10 subclusters using FlowSOM (Figure 2A). Overlaying the expression profile of various markers (supplemental Figure 1D) on UMAPs distinguished CD5lowCD20highpS6high normal B cells (C2.1-2.3)26 from CLL cells (Figure 2B). We also observed a small population of CD5+ B cells in healthy donors that phenotypically resembled CLL (C2.3) (Figures 1E and 2B,E), as previously reported.26,27 CLL clusters could be broadly divided into immunoglobulin M (IgM)low (C2.4-2.5) and IgMhigh cells (C2.6-2.10) (Figure 2C). In addition, we detected minor CLL subpopulations, including the pRBhigh TACIhigh BAXhigh (C2.9) and pH2AXhigh (C2.10) subsets (Figure 2A,D).
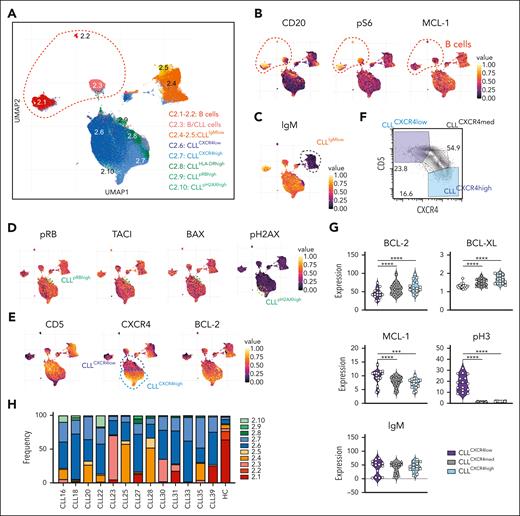
Intrapatient and interpatient heterogeneity of CLL cell subpopulations. (A) UMAP of reclustered healthy B/CLL cells (from Figure 1C) from patients and healthy controls. (B) UMAP plots colored by median protein expression of CD20, pS6, and MCL-1, distinguishing between healthy B cells (CD20high pS6high MCL-1low) and CLL cells (CD20lowpS6lowMCL-1high). (C) UMAP plots colored by protein expression level of IgM, distinguishing IgMhigh and IgMlow B cells. (D) UMAP plots colored by amounts of pRB, TACI, BAX, and pH2AX, thereby distinguishing between 2 minor subpopulations of CLL cells, namely pRBhighTACIhighBAXhigh (cluster 2.9) and pH2AXhigh (cluster 2.10). (E) UMAP plots colored by protein expression of CD5, CXCR4, and BCL-2, thereby distinguishing 2 main subpopulations of CLL cells, namely CD5highCXCR4lowBCL-2high (cluster 2.6) and CD5lowCXCR4highBCL-2v.high (cluster 2.7). (F) Representative 2-dimensional plot of CXCR4 vs CD5 expression showing CD5highCXCR4low and CD5lowCXCR4high CLL cell populations from patient CLL20. (G) Violin plots showing the mean intensities of BCL-2, BCL-XL, MCL-1, pH3, and IgM of old and new CLL cells that have low, medium, and high levels of CXCR4 protein. (H) Frequency of each cluster in the PB of patients before venetoclax treatment. Student paired t test was used. ∗∗∗P < .005; ∗∗∗∗P < .001. BAX, BCL-2 associated protein X; HC, healthy control; pH2AX, phosporylated histone H2AX; pRB, retinoblastoma protein; TACI, transmembrane activator and CAML interactor.
Intrapatient and interpatient heterogeneity of CLL cell subpopulations. (A) UMAP of reclustered healthy B/CLL cells (from Figure 1C) from patients and healthy controls. (B) UMAP plots colored by median protein expression of CD20, pS6, and MCL-1, distinguishing between healthy B cells (CD20high pS6high MCL-1low) and CLL cells (CD20lowpS6lowMCL-1high). (C) UMAP plots colored by protein expression level of IgM, distinguishing IgMhigh and IgMlow B cells. (D) UMAP plots colored by amounts of pRB, TACI, BAX, and pH2AX, thereby distinguishing between 2 minor subpopulations of CLL cells, namely pRBhighTACIhighBAXhigh (cluster 2.9) and pH2AXhigh (cluster 2.10). (E) UMAP plots colored by protein expression of CD5, CXCR4, and BCL-2, thereby distinguishing 2 main subpopulations of CLL cells, namely CD5highCXCR4lowBCL-2high (cluster 2.6) and CD5lowCXCR4highBCL-2v.high (cluster 2.7). (F) Representative 2-dimensional plot of CXCR4 vs CD5 expression showing CD5highCXCR4low and CD5lowCXCR4high CLL cell populations from patient CLL20. (G) Violin plots showing the mean intensities of BCL-2, BCL-XL, MCL-1, pH3, and IgM of old and new CLL cells that have low, medium, and high levels of CXCR4 protein. (H) Frequency of each cluster in the PB of patients before venetoclax treatment. Student paired t test was used. ∗∗∗P < .005; ∗∗∗∗P < .001. BAX, BCL-2 associated protein X; HC, healthy control; pH2AX, phosporylated histone H2AX; pRB, retinoblastoma protein; TACI, transmembrane activator and CAML interactor.
Interestingly, the major CLL clusters had graded expression of CD5, CXCR4, and BCL-2 (Figure 2E). Traditional 2-dimensional gating identified CXCR4lowCD5high and CXCR4highCD5low CLL populations (Figure 2F), previously identified as PB CLL cells that have recently migrated from or are en route to lymphoid tissues, respectively.28 Consistent with previous observations,29 CXCR4lowCD5high CLL cells had relatively lower levels of BCL-2 and BCL-XL than CXCR4highCD5low CLL cells but higher amounts of MCL-1 and mitosis-associated phosphorylated histone 3 (pH3) (Figure 2G). The amount of surface IgM was comparable (Figure 2G). With a few exceptions, all CLL clusters were found in all patients, albeit at varying frequencies (Figure 2H), suggesting relatively modest interpatient variability. Taken together, the CyTOF profiling of CLL cells revealed heterogeneity that was distinguished by the cell surface phenotype, signaling states (eg, pRB, pH2AX), and expression of BCL-2 prosurvival proteins (eg, BCL-2highCXCR4highCD5low vs BCL-2lowCXCR4lowCD5high CLL cells).
Venetoclax induced a dose-related increase in BCL-2 in surviving CLL cells
Having defined the baseline characteristics and heterogeneity of CLL cell populations among patients, we sought to resolve the impact of venetoclax monotherapy (n = 18). Venetoclax reduced the CLL burden in all patients with no dose-dependent changes in the CLL cluster proportions observed (Figure 3A; supplemental Figure 2A). Gating on CXCR4lowCD5high and CXCR4highCD5low populations identified some instances of CXCR4high CLL cell enrichment (with comparatively higher BCL-2), but this trend was not statistically significant (supplemental Figure 2B-C). To identify dose-related changes in the CLL cells independent of the subpopulations, we performed linear discriminant analysis (LDA). The LDA ranked proteins according to the extent of the differences observed in the CLL cells across venetoclax doses in individual patients (eg, patient CLL19; Figure 3B; supplemental Figure 3A-B) and compared it with the distribution of randomly oriented planes (green shaded area). A summary of the LDA from all patients showed that the top ranked dose-related change in CLL cells over time was BCL-2 (Figure 3C). Accordingly, we observed a significant increase in the mean level of BCL-2 in CLL cells after the 50 mg and 100 mg doses of venetoclax treatment when compared with baseline (supplemental Figure 3C). These CyTOF data showed that short-term venetoclax treatment enriched for or drove higher BCL-2 in surviving cells (hereafter termed VENsurv).
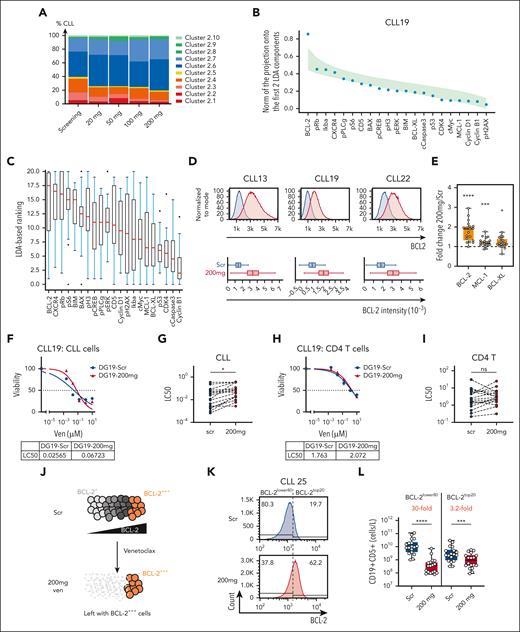
Venetoclax dose-dependent increase in BCL-2 protein detected in CLL cells. (A) Mean proportions of different CLL clusters from all patients in the cohort at the indicated timepoints during treatment. (B) Graph of the results of the LDA of data from the CLL clusters from patient CLL19 across increasing doses of venetoclax. The magnitude of the projection of each marker direction onto the plane of the first 2 LDA components is represented by blue dots. This has a maximum value of 1 if the marker direction lies in the planes of the first 2 LDA components. To reveal the markers that drive the changes, the markers are ordered on the x-axis by the magnitude of this projection. The green shaded area represents the distribution of ranking curves of randomly oriented planes (see “Methods”). (C) Summary of markers ranked by contributions to the first 2 LDA components in all patients for venetoclax dose escalation (seen in panel B for patient CLL19). The higher the ranking of a marker, the more it is affected by venetoclax dose changes with 20 being the highest ranked marker and 0 the lowest. (D) Representative histograms showing BCL-2 protein expression of curated pairs of patients with CLL at screening (blue) and after 200 mg VEN treatment (red) in patient CLL13, CLL19, and CLL22 as measured by flow cytometry. The distribution of BCL-2 levels in each sample is represented by the box and whisker plots in the lower panel. The box represents the 25th, 50th, and 75th percentiles of the population with x marking the mean and the whiskers representing the minimum and maximum values (excluding outliers). (E) Fold change in BCL-2, MCL-1, and BCL-XL protein expression in samples after 200 mg treatment normalized to screening in individual patients. P values were calculated using raw data and Student 2-tailed paired t test. (F) Representative results of venetoclax sensitivity in an in vitro assay of CLL cells from patient CLL19. (G) Summary of the LC50 values of CLL cells at screening and after 200 mg venetoclax treatment. (H) Representative results of venetoclax sensitivity in an in vitro assay in nontransformed CD4+ T cells from patient CLL19. (I) Summary of the LC50 values of CD4+ T cells at screening and after 200 mg venetoclax treatment. (J) Schematic representation of the hypothesis. CLL cells express a range of BCL-2 amounts, from BCL-2+ to BCL-2+++. CLL cells with relatively lower levels of BCL-2 (BCL-2+) are more sensitive to venetoclax treatment, thereby enriching for CLL cells with higher BCL-2 levels (BCL-2+++) after venetoclax monotherapy. (K) Distribution of BCL-2 in CLL cells before (Scr) and 1 week after 200 mg dose of venetoclax with a dashed line indicating the threshold distinguishing the top 20% of BCL-2 expressors (BCL-2top20) and 80% lower BCL-2 expressors (BCL-2lower80) at screening. This threshold was then applied to each paired profile after 200 mg treatment. (L) Estimated concentration of BCL-2top20 and BCL-2lower80 in CLL cells in the blood at screening and after 200 mg venetoclax treatment based on the BCL-2top20/BCL-2lower80 threshold assigned in the screening sample. P values calculated using ratio paired t test. For box and whisker plots in panel D, outliers were omitted from the plot with an outlier factor of 1.5. Outliers represented less than 2.5% of the total population in all samples. For panels G and I, Student 2-tailed paired t test was used (patients with missing values were excluded from analysis). For panels E and L, the mean ± standard error of the mean (SEM) is shown. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. BIM, BCL-2 interacting mediator of cell death; cMyc, myelocytomatosis oncogene protein; Ikba, NK-κ-B inhibitor α; LC50, lethal concentration 50; ns, not significant; pERK, phosphorylated extracellular signal-regulated kinase; pPLCg, phosphorylated phospholipase C γ; pRb, retinoblastoma protein.
Venetoclax dose-dependent increase in BCL-2 protein detected in CLL cells. (A) Mean proportions of different CLL clusters from all patients in the cohort at the indicated timepoints during treatment. (B) Graph of the results of the LDA of data from the CLL clusters from patient CLL19 across increasing doses of venetoclax. The magnitude of the projection of each marker direction onto the plane of the first 2 LDA components is represented by blue dots. This has a maximum value of 1 if the marker direction lies in the planes of the first 2 LDA components. To reveal the markers that drive the changes, the markers are ordered on the x-axis by the magnitude of this projection. The green shaded area represents the distribution of ranking curves of randomly oriented planes (see “Methods”). (C) Summary of markers ranked by contributions to the first 2 LDA components in all patients for venetoclax dose escalation (seen in panel B for patient CLL19). The higher the ranking of a marker, the more it is affected by venetoclax dose changes with 20 being the highest ranked marker and 0 the lowest. (D) Representative histograms showing BCL-2 protein expression of curated pairs of patients with CLL at screening (blue) and after 200 mg VEN treatment (red) in patient CLL13, CLL19, and CLL22 as measured by flow cytometry. The distribution of BCL-2 levels in each sample is represented by the box and whisker plots in the lower panel. The box represents the 25th, 50th, and 75th percentiles of the population with x marking the mean and the whiskers representing the minimum and maximum values (excluding outliers). (E) Fold change in BCL-2, MCL-1, and BCL-XL protein expression in samples after 200 mg treatment normalized to screening in individual patients. P values were calculated using raw data and Student 2-tailed paired t test. (F) Representative results of venetoclax sensitivity in an in vitro assay of CLL cells from patient CLL19. (G) Summary of the LC50 values of CLL cells at screening and after 200 mg venetoclax treatment. (H) Representative results of venetoclax sensitivity in an in vitro assay in nontransformed CD4+ T cells from patient CLL19. (I) Summary of the LC50 values of CD4+ T cells at screening and after 200 mg venetoclax treatment. (J) Schematic representation of the hypothesis. CLL cells express a range of BCL-2 amounts, from BCL-2+ to BCL-2+++. CLL cells with relatively lower levels of BCL-2 (BCL-2+) are more sensitive to venetoclax treatment, thereby enriching for CLL cells with higher BCL-2 levels (BCL-2+++) after venetoclax monotherapy. (K) Distribution of BCL-2 in CLL cells before (Scr) and 1 week after 200 mg dose of venetoclax with a dashed line indicating the threshold distinguishing the top 20% of BCL-2 expressors (BCL-2top20) and 80% lower BCL-2 expressors (BCL-2lower80) at screening. This threshold was then applied to each paired profile after 200 mg treatment. (L) Estimated concentration of BCL-2top20 and BCL-2lower80 in CLL cells in the blood at screening and after 200 mg venetoclax treatment based on the BCL-2top20/BCL-2lower80 threshold assigned in the screening sample. P values calculated using ratio paired t test. For box and whisker plots in panel D, outliers were omitted from the plot with an outlier factor of 1.5. Outliers represented less than 2.5% of the total population in all samples. For panels G and I, Student 2-tailed paired t test was used (patients with missing values were excluded from analysis). For panels E and L, the mean ± standard error of the mean (SEM) is shown. ∗P < .05; ∗∗∗P < .005; ∗∗∗∗P < .001. BIM, BCL-2 interacting mediator of cell death; cMyc, myelocytomatosis oncogene protein; Ikba, NK-κ-B inhibitor α; LC50, lethal concentration 50; ns, not significant; pERK, phosphorylated extracellular signal-regulated kinase; pPLCg, phosphorylated phospholipase C γ; pRb, retinoblastoma protein.
To verify these findings, we used conventional flow cytometry with fluorochrome conjugates that offer a greater dynamic range than CyTOF. Analysis of paired samples at screening and after 200 mg of venetoclax validated the significant increase in BCL-2 protein expression in VENsurv cells (Figure 3D-E). Smaller, statistically significant increases in MCL-1 and BCL-XL were also apparent (Figure 3E). We then compared the in vitro venetoclax sensitivity of cells at screening and after 200 mg treatment and found reduced sensitivity in CLL cells (Figure 3F-G) but not in CD4+ T cells (Figure 3H-I). Those CLL cells that exhibited a greater reduction in sensitivity to venetoclax also had the greatest increase in BCL-2 levels (supplemental Figure 3D-E). These data align with a previous study of a smaller patient group30 and demonstrate that venetoclax selects for or induces cells with higher expression of prosurvival BCL-2 proteins and reduced sensitivity to venetoclax. To extend these findings to a setting of combination therapy, we analyzed the 2 patients with CLL who received first-line ibrutinib lead-in, followed by ibrutinib plus venetoclax and compared the cells just before and after venetoclax treatment. The addition of venetoclax substantially reduced the white blood cell counts (supplemental Figure 3F), and the VENsurv CLL cells in both patients had increased BCL-2 expression and reduced in vitro sensitivity to venetoclax (supplemental Figure 3G-H). These data suggest that the rapid adaptation processes acting on VENsurv CLL cells are pertinent to multiple settings.
Preferential loss of CLL cells with relatively lower BCL-2 protein partially accounts for VENsurv cell phenotype
To explore how venetoclax causes an apparent rapid increase in prosurvival proteins in VENsurv cells, we hypothesized that, in vivo, dose escalation may preferentially eliminate those cells with relatively lower amounts of BCL-2 (Figure 3J). To test this notion, we first set a baseline threshold to identify the 20% of cells with the highest amount of BCL-2 (BCL-2top20) vs the remaining 80% (BCL-2lower80) cells in each patient at screening. We then applied this threshold to the paired data obtained after 200 mg treatment (Figure 3K). Calculation of the ex vivo concentration of the 2 populations revealed a mean 30-fold decrease in BCL-2lower80 cells, whereas the BCL-2top20 cells decreased by only 3.2-fold (Figure 3L). These data support the notion that, during the venetoclax ramp-up, CLL cells with lower amounts of BCL-2 are preferentially killed. However, we also observed that VENsurv cells often manifest levels of BCL-2 that exceeded the range measured before venetoclax administration (Figure 3D,K), suggesting another (nonmutually exclusive) scenario in which cell extrinsic factors drive the upregulation of BCL-2.
In vivo elevation of BCL-2 in VENsurv cells in mice
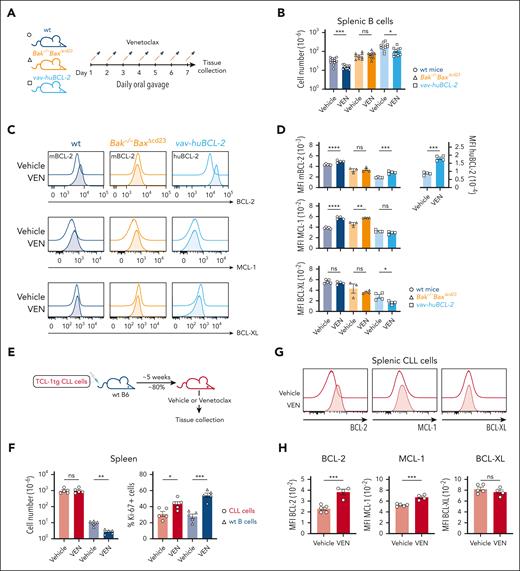
To further dissect the mechanisms that drive the increased prosurvival protein expression in VENsurv cells, we assayed normal and leukemic B cells in mice shortly after venetoclax treatment (Figure 4A). Consistent with previous studies,31 venetoclax swiftly reduced the total B-cell numbers by >2 fold in wildtype (wt) mice (Figure 4B), predominantly in peripheral B cells. As observed among patients with CLL (Figure 3E), VENsurv B cells had elevated levels of BCL-2 and MCL-1; the BCL-XL levels remained unchanged (Figure 4C-D). In Bak–/–BaxΔCd23 mice, all B cells lack the downstream effectors of apoptosis, BAX and BCL-2 homologs antagonist/killer, therefore cannot undergo apoptosis. Venetoclax did not affect the B-cell numbers or BCL-2 expression in these mice (Figure 4B-D), demonstrating that apoptotic death was essential for the elevation of BCL-2 in VENsurv B cells. Intriguingly, MCL-1 levels still increased in VENsurv B cells from Bak–/–BaxΔCd23 mice (Figure 5B-D), suggesting that a nonapoptotic or cell extrinsic mechanism influences MCL-1 protein expression in this scenario.
In vivo modeling demonstrates the elevation of BCL-2 in VENsurv cells in mice. (A) Schematic representation of the experimental strategy. wt, Bak–/–BaxΔcd23, and vav-huBcl-2 mice were treated daily with 100 mg/kg body weight venetoclax or vehicle (a mixture of 60% Phosal 50 propylene glycol, 30% polyethylene glycol 400, and 10% ethanol) for 1 week. (B) Absolute numbers of splenic B cells before and after venetoclax treatment in wt, Bak–/–BaxΔcd23, and vav-huBcl-2 mice. (C) Histograms of BCL-2, MCL-1, and BCL-XL protein in CD19+CD21+IgM+ B cells from wt, Bak–/–BaxΔcd23, or vav-huBcl-2 mice measured by flow cytometry after vehicle or venetoclax treatment. (D) Geometric mean (± SEM) of mBCL-2, huBCL-2, MCL-1, and BCL-XL protein levels in vehicle- and venetoclax-treated mice of the indicated genotypes. (E) Schematic representation of the Eμ-TCL-1 transgenic mice modeling of CLL cell response to venetoclax. Cohorts of C57BL/6 mice received 5 × 105Eμ-TCL-1 transgenic CLL cells from the same donor, and once they reached 80% leukemic burden in blood, they were treated with vehicle or venetoclax daily for 7 days. (F) Total splenic cell numbers and the proportions of Ki67+Eμ-TCL-1 transgenic CLL cells and wt B cells before and after treatment. (G) Histograms of BCL-2, MCL-1, and BCL-XL protein in CLL cells recovered from the spleen of mice treated with vehicle or venetoclax. (H) Quantification of BCL-2, MCL-1, and BCL-XL protein expression in CLL cells recovered from the spleen of mice treated with vehicle or venetoclax. Data from panels B-D are representative of 3 independent experiments with n = 2 to 5 mice per group. Data from panels F-H are representative of 3 independent experiments with n = 5 to 6 mice per group. For all bar graphs, the mean ± SEM are shown, and each symbol represents an individual mouse; Student 2-tailed t test was used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. hBCL-2, human BCL-2; mBCL-2, murine BCL-2.
In vivo modeling demonstrates the elevation of BCL-2 in VENsurv cells in mice. (A) Schematic representation of the experimental strategy. wt, Bak–/–BaxΔcd23, and vav-huBcl-2 mice were treated daily with 100 mg/kg body weight venetoclax or vehicle (a mixture of 60% Phosal 50 propylene glycol, 30% polyethylene glycol 400, and 10% ethanol) for 1 week. (B) Absolute numbers of splenic B cells before and after venetoclax treatment in wt, Bak–/–BaxΔcd23, and vav-huBcl-2 mice. (C) Histograms of BCL-2, MCL-1, and BCL-XL protein in CD19+CD21+IgM+ B cells from wt, Bak–/–BaxΔcd23, or vav-huBcl-2 mice measured by flow cytometry after vehicle or venetoclax treatment. (D) Geometric mean (± SEM) of mBCL-2, huBCL-2, MCL-1, and BCL-XL protein levels in vehicle- and venetoclax-treated mice of the indicated genotypes. (E) Schematic representation of the Eμ-TCL-1 transgenic mice modeling of CLL cell response to venetoclax. Cohorts of C57BL/6 mice received 5 × 105Eμ-TCL-1 transgenic CLL cells from the same donor, and once they reached 80% leukemic burden in blood, they were treated with vehicle or venetoclax daily for 7 days. (F) Total splenic cell numbers and the proportions of Ki67+Eμ-TCL-1 transgenic CLL cells and wt B cells before and after treatment. (G) Histograms of BCL-2, MCL-1, and BCL-XL protein in CLL cells recovered from the spleen of mice treated with vehicle or venetoclax. (H) Quantification of BCL-2, MCL-1, and BCL-XL protein expression in CLL cells recovered from the spleen of mice treated with vehicle or venetoclax. Data from panels B-D are representative of 3 independent experiments with n = 2 to 5 mice per group. Data from panels F-H are representative of 3 independent experiments with n = 5 to 6 mice per group. For all bar graphs, the mean ± SEM are shown, and each symbol represents an individual mouse; Student 2-tailed t test was used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. hBCL-2, human BCL-2; mBCL-2, murine BCL-2.
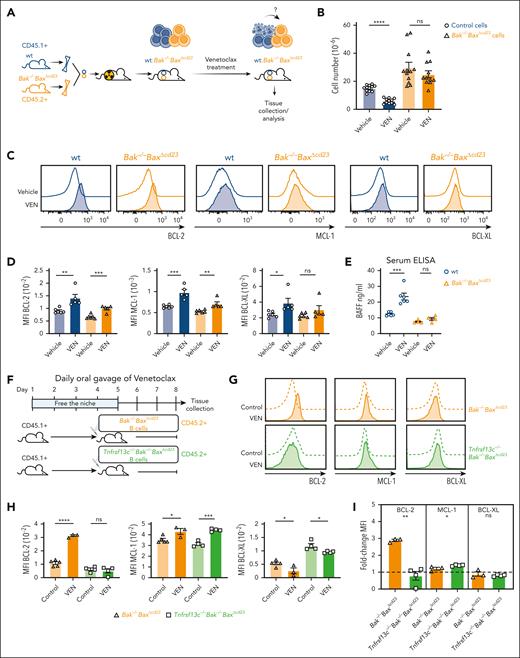
BAFF controls the homeostatic survival response to venetoclax treatment in B cells. (A) Schematic representation of hematopoietic chimeras reconstituted with a mixture of CD45.2+, Bak–/–BaxΔcd23, and CD45.1+ wt BM progenitors, followed by venetoclax treatment. (B) Total numbers of splenic B cells of Bak–/–BaxΔcd23 or wt origin recovered from vehicle- and venetoclax-treated chimeras. (C) Histograms of BCL-2, MCL-1, and BCL-XL staining gated on splenic B cells of wt or Bak–/–BaxΔcd23 origin recovered from the chimeric recipient mice treated with vehicle or venetoclax. (D) Geometric mean of BCL-2, MCL-1, and BCL-XL protein expression levels in splenic B cells of Bak–/–BaxΔcd23 or wt origin recovered from the vehicle- or venetoclax-treated chimeric recipient mice. (E) Serum BAFF from wt and Bak–/–BaxΔcd23 mice after 7-day treatment of venetoclax. Data are from 2 experiments with n = 2 to 3. (F) Schematic representation of the experimental design. Purified CD45.2+ C57BL/6 B cells from Bak–/–BaxΔcd23 or Tnfrsf13c–/–Bak–/–BaxΔcd23 mice were transferred into venetoclax-treated CD45.1+ C57BL/6 wt recipient mice, which were then maintained on daily venetoclax treatment before analysis. (G) Histograms of BCL-2, MCL-1, and BCL-XL protein expression in donor CD45.2+ B cells from unmanipulated control mice with the same genotype or from venetoclax treated recipients. (H) Geometric mean of BCL-2, MCL-1, and BCL-XL protein expression in Bak–/–BaxΔcd23 and Tnfrsf13c–/–Bak–/–BaxΔcd23 B cells from control (untreated) recipient mice or from venetoclax-treated recipient mice as indicated in (F), measured by flow cytometry. (I) Fold change in BCL-2, MCL-1, and BCL-XL protein levels in Bak–/–BaxΔcd23 and Tnfrsf13c–/–Bak–/–BaxΔcd23 B cells in venetoclax-treated mice. The data are expressed relative to the MFI in B cells recovered from unmanipulated control mice of the same genotype. Data from panels B-H are representative of 3 independent experiments with n = 3 to 6 mice per group. For all bar graphs, the mean ± SEM are shown and each symbol represents an individual mouse; Student 2-tailed t test was used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity.
BAFF controls the homeostatic survival response to venetoclax treatment in B cells. (A) Schematic representation of hematopoietic chimeras reconstituted with a mixture of CD45.2+, Bak–/–BaxΔcd23, and CD45.1+ wt BM progenitors, followed by venetoclax treatment. (B) Total numbers of splenic B cells of Bak–/–BaxΔcd23 or wt origin recovered from vehicle- and venetoclax-treated chimeras. (C) Histograms of BCL-2, MCL-1, and BCL-XL staining gated on splenic B cells of wt or Bak–/–BaxΔcd23 origin recovered from the chimeric recipient mice treated with vehicle or venetoclax. (D) Geometric mean of BCL-2, MCL-1, and BCL-XL protein expression levels in splenic B cells of Bak–/–BaxΔcd23 or wt origin recovered from the vehicle- or venetoclax-treated chimeric recipient mice. (E) Serum BAFF from wt and Bak–/–BaxΔcd23 mice after 7-day treatment of venetoclax. Data are from 2 experiments with n = 2 to 3. (F) Schematic representation of the experimental design. Purified CD45.2+ C57BL/6 B cells from Bak–/–BaxΔcd23 or Tnfrsf13c–/–Bak–/–BaxΔcd23 mice were transferred into venetoclax-treated CD45.1+ C57BL/6 wt recipient mice, which were then maintained on daily venetoclax treatment before analysis. (G) Histograms of BCL-2, MCL-1, and BCL-XL protein expression in donor CD45.2+ B cells from unmanipulated control mice with the same genotype or from venetoclax treated recipients. (H) Geometric mean of BCL-2, MCL-1, and BCL-XL protein expression in Bak–/–BaxΔcd23 and Tnfrsf13c–/–Bak–/–BaxΔcd23 B cells from control (untreated) recipient mice or from venetoclax-treated recipient mice as indicated in (F), measured by flow cytometry. (I) Fold change in BCL-2, MCL-1, and BCL-XL protein levels in Bak–/–BaxΔcd23 and Tnfrsf13c–/–Bak–/–BaxΔcd23 B cells in venetoclax-treated mice. The data are expressed relative to the MFI in B cells recovered from unmanipulated control mice of the same genotype. Data from panels B-H are representative of 3 independent experiments with n = 3 to 6 mice per group. For all bar graphs, the mean ± SEM are shown and each symbol represents an individual mouse; Student 2-tailed t test was used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. ELISA, enzyme-linked immunosorbent assay; MFI, mean fluorescence intensity.
We also assessed the impact of venetoclax on vav-huBCL-2 transgenic mice that overexpress human BCL-2 in all hematopoietic cells.32 B cells from vav-huBCL-2 mice remained sensitive to venetoclax-induced cell death in vivo (Figure 4B) and VENsurv cells exhibited substantially increased amounts of both human (ie, transgene encoded) and mouse BCL-2 (ie, endogenous) (Figure 4C-D). Together, these data indicate that the increase in cellular BCL-2 protein following short-term venetoclax treatment in vivo is (1) conserved between mouse and human BCL-2, (2) dependent on cells that undergo apoptosis, (3) independent of the BCL-2 antibodies used for detection, and (4) observed in B cells with varying baseline amounts of BCL-2.
Next, we tested whether the elevated BCL-2 observed in VENsurv B cells also occurs in a mouse model of CLL. We adoptively transferred CLL cells from Eμ-TCL-1 transgenic mice into wt mice and treated recipients with either vehicle or venetoclax (Figure 4E). Venetoclax caused a significant decline in splenic healthy B cells, but not in leukemic CD19+CD5+ cells (Figure 4F). The apparently lower sensitivity of CD19+CD5+Eμ-TCL-1 transgenic CLL cells, consistent with previous studies,33,34 is likely because of their high proliferation when compared with human CLL cells. Indeed, the proportions of Ki-67+ proliferating Eμ-TCL-1 transgenic B cells increased with venetoclax treatment (Figure 4F), presumably maintaining their numbers despite substantial apoptosis. Nevertheless, we observed increased expression levels of BCL-2 and MCL-1 in VENsurv leukemic cells (Figure 4G-H). Collectively, these data reveal that venetoclax-induced apoptosis, in vivo, rapidly drives elevated BCL-2 and MCL-1 protein levels in VENsurv cells, recapitulating the responses observed in CLL cells from patients.
Competition for BAFF controls the homeostatic response to venetoclax in murine B cells
We next sought to determine whether cell extrinsic factors drive the increase in BCL-2 proteins in VENsurv cells. To establish an in vivo model in which the impact of venetoclax on the microenvironment could be read-out in apoptosis-resistant B cells, we generated hematopoietic chimeras with a mixture of congenically labeled wt (CD45.1+) cells and Bak–/–BaxΔCd23 (CD45.2+) cells into CD45.1/2 irradiated recipients (Figure 5A). These chimeras were treated with venetoclax, which ablated wt B cells and elevated BCL-2, MCL-1, and BCL-XL in the surviving wt cells (Figure 5B-D). The numbers of B cells derived from Bak–/–BaxΔCd23 BM was unchanged by venetoclax treatment; however, their levels of BCL-2 increased (Figure 5B-D). This finding is in contrast with the observations in intact Bak–/–BaxΔCd23 mice (Figure 4C-D), indicating that BCL-2 upregulation is driven by cell extrinsic factors and dependent on the removal of wt cells in the chimeras. MCL-1 protein increased modestly in both wt and Bak–/–BaxΔCd23 B cells from the venetoclax-treated chimeras (Figure 5C-D), similar to findings in complete Bak–/–BaxΔCd23 mice (Figure 4C-D), indicating that the elevation in MCL-1 was independent of B-cell apoptosis. Collectively, these data demonstrate that cell extrinsic cues rapidly upregulate prosurvival proteins in VENsurv B cells.
A prominent extrinsic cue is BAFF, which binds to the BAFF-R on peripheral B cells to promote their maturation and survival.35 Indeed, we observed increased BAFF in the serum of wt mice treated with venetoclax but not in that of complete Bak–/–BaxΔCd23 mice in which there was no B-cell apoptosis (Figure 5E). To test this further, we used Bak–/–BaxΔCd23 mice that lacked the BAFF-R (Bak–/–BaxΔCd23Tnfrsf13c–/–),36 thereby generating B cells that were both resistant to apoptosis and refractory to BAFF signaling. We adoptively transferred B cells purified from Bak–/–BaxΔCd23 or Bak–/–BaxΔCd23Tnfrsf13c–/– mice into wt recipients that had been treated with venetoclax for 4 days to deplete their B cells (Figure 5F). After a further 3-day treatment with venetoclax, we detected increased BCL-2 in Bak–/–BaxΔCd23 but not in Bak–/–BaxΔCd23Tnfrsf13c–/– VENsurv total B cells (Figure 5G-I) or IgM+CD21+ B cells (supplemental Figure 4A-C). In contrast, both populations showed modestly increased MCL-1 (Figure 5G-I). These data demonstrate that venetoclax treatment rapidly induces BAFF-R signals in vivo that upregulate BCL-2 in VENsurv B cells, whereas a BAFF-independent pathway increases MCL-1 protein levels.
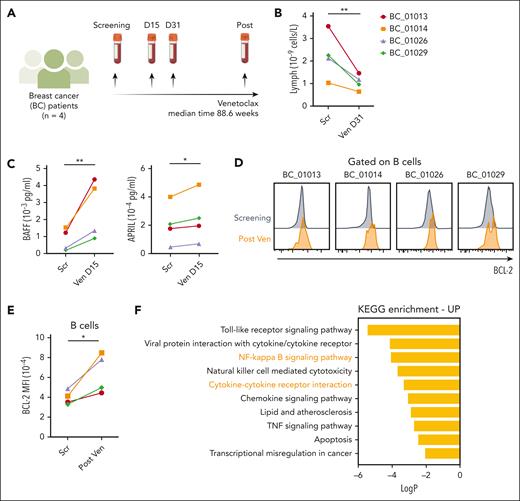
To assess whether a similar mechanism impacts human B cells, we investigated patients who received venetoclax for metastatic breast cancer (ie, without a hematologic malignancy) (Figure 6A) (patient characteristics in supplemental Table 2).20 Venetoclax treatment induced a striking reduction in circulating lymphocytes within 31 days (Figure 6B), consistent with the loss of circulating naive B cells.20 This decrease was accompanied by increased plasma concentrations of BAFF and APRIL (another cytokine that mediates differentiation and survival of B cells) (Figure 6C) and elevated BCL-2 expression in B cells (Figure 6D-E). The impact of venetoclax treatment on the B-cell transcriptome was analyzed using single-cell cellular indexing of transcriptomes and epitopes sequencing data from 5 paired samples.21 Kyoto Encyclopedia of Genes and Genomes pathway analysis of differentially expressed genes (48 down, 67 up; false discovery rate, <0.05; log fold change, >0.5) showed 17 pathways that were enriched, including BAFF-related NF-κB signaling, cytokine-cytokine receptor interactions, tumor necrosis factor signaling pathways, and apoptosis (Figure 6F). These findings support the concept that increased BAFF signaling drives BCL-2 upregulation in VENsurv B cells.
Healthy human B cells undergoing venetoclax treatment have increased BCL-2 expression. (A) Schematic representation of the study design. PB samples from 4 breast cancer patients were collected at screening and during venetoclax treatment (all patients received 800 mg venetoclax). (B) Change in lymphocyte concentration (cells per μL) during venetoclax treatment in each patient after 31 days of treatment. (C) Concentrations of BAFF and APRIL (pg/mL) detected in the serum samples of the breast cancer patients using a Luminex assay at screening and 15 days after venetoclax treatment. (D) Histograms of BCL-2 protein expression by flow cytometry in B cells in patients before and after venetoclax treatment (BC_01013 after 201.7 weeks, BC_01014 after 61.1 weeks, BC_01026 after 67.4 weeks, BC_01029 after 112.1 weeks). (E) Geometric mean of the BCL-2 levels of B cells in 4 patients. (F) KEGG enrichments analysis of differentially expressed genes in single-cell cellular indexing of transcriptomes and epitopes sequencing data from 5 paired samples under long-term venetoclax treatment. Student 2-tailed paired t test was used to calculate P values. ∗P < .05; ∗∗P < .01. APRIL, a proliferation-inducing ligand (also known as TNFSF13); KEGG, Kyoto Encyclopedia of Genes and Genomes; TNF, tumor necrosis factor.
Healthy human B cells undergoing venetoclax treatment have increased BCL-2 expression. (A) Schematic representation of the study design. PB samples from 4 breast cancer patients were collected at screening and during venetoclax treatment (all patients received 800 mg venetoclax). (B) Change in lymphocyte concentration (cells per μL) during venetoclax treatment in each patient after 31 days of treatment. (C) Concentrations of BAFF and APRIL (pg/mL) detected in the serum samples of the breast cancer patients using a Luminex assay at screening and 15 days after venetoclax treatment. (D) Histograms of BCL-2 protein expression by flow cytometry in B cells in patients before and after venetoclax treatment (BC_01013 after 201.7 weeks, BC_01014 after 61.1 weeks, BC_01026 after 67.4 weeks, BC_01029 after 112.1 weeks). (E) Geometric mean of the BCL-2 levels of B cells in 4 patients. (F) KEGG enrichments analysis of differentially expressed genes in single-cell cellular indexing of transcriptomes and epitopes sequencing data from 5 paired samples under long-term venetoclax treatment. Student 2-tailed paired t test was used to calculate P values. ∗P < .05; ∗∗P < .01. APRIL, a proliferation-inducing ligand (also known as TNFSF13); KEGG, Kyoto Encyclopedia of Genes and Genomes; TNF, tumor necrosis factor.
Elevated BAFF in patients with CLL treated with venetoclax or other targeted therapies
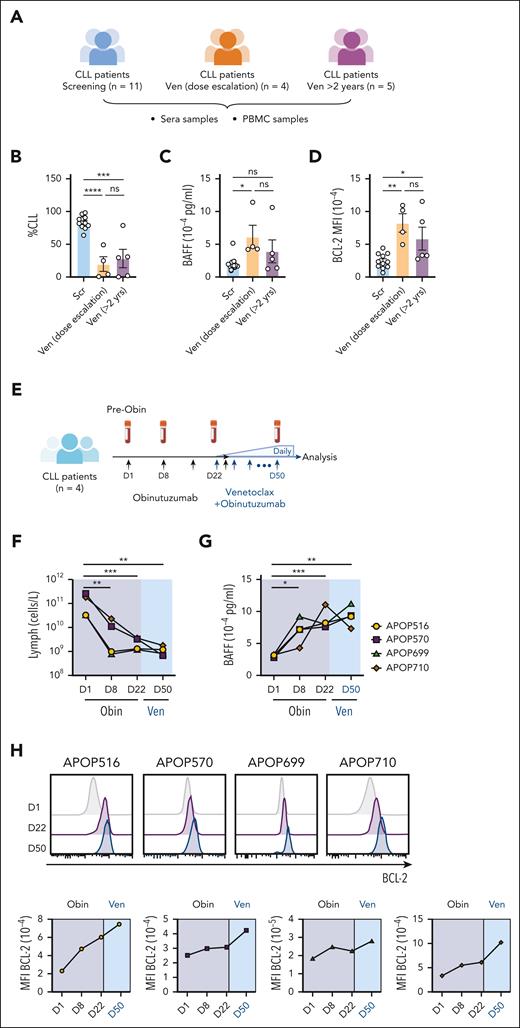
The data thus far support the hypothesis that venetoclax-induced killing of leukemic cells reduces competition among VENsurv cells for cytokines, such as BAFF, which leads to elevated BCL-2 protein expression and reduced sensitivity to venetoclax (supplemental Figure 5A). To test this hypothesis in the CLL setting, we measured serum BAFF in patients at screening, during venetoclax dose escalation, or more than 2 years after venetoclax monotherapy (Figure 7A; patient characteristics in supplemental Table 3). With reduced CLL burden (Figure 7B), patients who underwent venetoclax dose escalation had significantly higher amounts of BAFF than the screening group (Figure 7C). In addition, CLLsurv cells had increased BCL-2 protein (Figure 7D; supplemental Figure 5B). We modelled this process in vitro with CLL cells from treatment-naive patients cultured with or without recombinant BAFF and/or venetoclax. BAFF increased BCL-2 expression and promoted CLL cell survival upon venetoclax treatment (supplemental Figure 5C), supporting that a link between BAFF and BCL-2 impacts sensitivity to venetoclax.
Increase in serum BAFF levels correlates with BCL-2 upregulation in CLL cells in patients undergoing targeted therapies. (A) Schematic representation of the patient groups sampled before or after venetoclax treatment. (B) CLL burden (proportion of lymphocytes) for each patient at collection. (C) Concentration (pg/mL) of serum BAFF. (D) MFI of BCL-2 in CLL cells from each patient. (E) Schematic representation of the study design. PB samples from 3 patients with CLL were collected at screening and during obinutuzumab and venetoclax dose escalation. Patients received IV infusion of obinutuzumab on days 1/2 (first 1000 mg dose divided over 2 days 100/900 mg), 8 (1000 mg), 15 (1000 mg), and 29 (1000 mg). Venetoclax was introduced at 20 mg daily on day 22 and increased weekly (20, 50, 100, 200, 400 mg). (F) Change in lymphocyte concentrations (cells per liter) during obinutuzumab and venetoclax treatment in each patient. (G) Concentration (pg/mL) of BAFF detected in the serum samples of the patients with CLL using a Luminex assay. (H) Histograms (top panel) and MFI (lower panel) of BCL-2 protein expression in CLL cells in each patient at the indicated time points. For panels B-D, the mean ± SEM are shown and each symbol represents a measurement from an individual sample; Student 2-tailed t test was used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. Obin, obinutuzumab; PBMC, peripheral blood mononuclear cell.
Increase in serum BAFF levels correlates with BCL-2 upregulation in CLL cells in patients undergoing targeted therapies. (A) Schematic representation of the patient groups sampled before or after venetoclax treatment. (B) CLL burden (proportion of lymphocytes) for each patient at collection. (C) Concentration (pg/mL) of serum BAFF. (D) MFI of BCL-2 in CLL cells from each patient. (E) Schematic representation of the study design. PB samples from 3 patients with CLL were collected at screening and during obinutuzumab and venetoclax dose escalation. Patients received IV infusion of obinutuzumab on days 1/2 (first 1000 mg dose divided over 2 days 100/900 mg), 8 (1000 mg), 15 (1000 mg), and 29 (1000 mg). Venetoclax was introduced at 20 mg daily on day 22 and increased weekly (20, 50, 100, 200, 400 mg). (F) Change in lymphocyte concentrations (cells per liter) during obinutuzumab and venetoclax treatment in each patient. (G) Concentration (pg/mL) of BAFF detected in the serum samples of the patients with CLL using a Luminex assay. (H) Histograms (top panel) and MFI (lower panel) of BCL-2 protein expression in CLL cells in each patient at the indicated time points. For panels B-D, the mean ± SEM are shown and each symbol represents a measurement from an individual sample; Student 2-tailed t test was used. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005; ∗∗∗∗P < .001. Obin, obinutuzumab; PBMC, peripheral blood mononuclear cell.
Another prediction of this model is that any targeted therapy that reduce the leukemic burden would induce a similar effect in surviving CLL cells. We assayed samples from 4 patients who were treated with anti-CD20 antibody (obinutuzumab) before venetoclax ramp-up during standard-of-care venetoclax-obinutuzumab therapy (Figure 7E; patient characteristics in supplemental Table 3). Obinutuzumab monotherapy markedly reduced PB lymphocytes, and further reductions were observed in 3 patients with subsequent venetoclax treatment (Figure 7F). Within 8 days of obinutuzumab treatment, BAFF levels increased approximately 2-fold in all patients and remained high up to 50 days after treatment with no major changes observed in other cytokines (Figure 7G; supplemental Figure 5D). BCL-2 expression also increased in CLL cells that survived obinutuzumab monotherapy and increased further following venetoclax treatment (Figure 7H). Together, these findings show that the elevation in BCL-2 in cells that survive targeted therapies coincides with increased BAFF levels and is not unique to targeting BCL-2. Instead, it seems to be related to the reduction in leukemic cells, potentially reflecting reduced cell competition and enhanced access to BAFF.
Discussion
Venetoclax and other targeted therapies have improved the treatment outcomes for patients with CLL and AML in certain settings; however, therapeutic resistance remains an important problem. It is clear that long-term venetoclax treatment in patients can induce a variety of changes in the interplay among the BCL-2 family proteins that engender resistance.11-14,17 However, a detailed understanding of how targeting BCL-2 over the short term impacts the apoptosis pathway within the context of CLL heterogeneity is lacking. This timeframe is important because (1) patients with incomplete responses are more prone to relapse4 and (2) the prevailing environment that supports leukemic cell survival likely influences the manifestation of resistance. Our findings highlight the rapid elevation of BCL-2 in circulating CLL cells as a key feature in patients during venetoclax dose escalation. We found evidence that, in vivo, BAFF–BAFF-R signaling contributes to BCL-2 upregulation, which culminates in a reduction in sensitivity of CLL cells to treatment. Collectively, these data support the notion that alleviation of competition for BAFF-R–mediated survival signals among CLL cells during treatment may limit therapeutic responses and support resistance.
Our deep profiling of circulating CLL cells at the single-cell level revealed that they could be further distinguished from normal B cells by lower expression of MCL-1 and pS6, consistent with their low metabolic activity and turnover. Among CLL cells, a key distinction was the expression of prosurvival proteins, demarcated by CXCR4 expression.37,38 CXCR4low cells that had presumably recently emigrated from lymph nodes were MCL-1highBCL-2lowBCL-XLlow and proliferative (pH3high). In contrast, PB CXCR4high CLL cells that were capable of migrating into lymph nodes were MCL-1lowBCL-2highBCL-XLhigh and quiescent (pH3low). These data reveal dynamic regulation of the expression of prosurvival BCL-2 family proteins in CLL cells associated with division.
All PB CLL clusters were diminished by venetoclax dose escalation, concomitant with enrichment for cells with higher expression of BCL-2 and, to a lesser extent, BCL-XL and MCL-1. It is important to note that venetoclax is unlikely to be in excess during the dose escalation phase, perhaps explaining why cells with relatively higher BCL-2 were not more sensitive. These data are consistent with a previous study of 5 CLL patients that found heightened expression of BCL-2 in surviving cells after 2 weeks of venetoclax treatment.30 We found that VENsurv CLL cells exhibited reduced in vitro sensitivity to venetoclax and that the increased BCL-2 expression could only partially be explained by depletion of CLL cells with relatively lower BCL-2. In vivo mouse models provided clear evidence for a cell extrinsic mediator, transduced by BAFF-R, that drove up prosurvival BCL-2 in VENsurv B cells. Consistent with a mechanism in which increased BAFF availability drives BCL-2 upregulation upon extensive CLL cell apoptosis, obinutuzumab treatment also led to elevated BCL-2 in a small patient cohort, coincident with markedly increased circulating BAFF. This phenomenon was also observed in 2 patients who received venetoclax-ibrutinib after ibrutinib run-in, suggesting that cytokine-mediated BCL-2 upregulation may also be relevant to dual BCL-2–Bruton tyrosine kinase inhibition regimens.39,40 These data support the notion that competition for BAFF normally constrains expression of prosurvival BCL-2 in CLL cells.
We also detected modest increases in MCL-1 in surviving CLL cells, apparently driven by cell extrinsic signals that were independent of B-cell apoptosis. One potential explanation is that MCL-1, which has a high rate of protein turnover, is stabilized in the presence of venetoclax because of the displacement of proapoptotic BIM from BCL-2 to MCL-1.41,42 Another possibility is the existence of an alternative pathway that upregulates MCL-1 in presence of venetoclax perhaps via the alteration of stromal cells.43 It is important to note that MCL-1 and other unprofiled prosurvival proteins may also contribute to the reduced sensitivity of CLLsurv cells to venetoclax.
An implication of these data is that combining targeted therapies with agents that neutralize the relevant cytokines could be beneficial by restraining BCL-2 expression. More specifically, our work builds the rationale to explore the combination of venetoclax with BAFF blockade. Indeed, cotargeting of BAFF with ibrutinib provided a survival benefit in a murine CLL model44 and the in vitro killing of primary patient CLL cells treated with venetoclax, idelalisib, or ibrutinib.44-46 A clinical trial that combines venetoclax with a BAFF neutralizing antibody for treating CLL patients (NCT05069051) is currently in progress. In this study, we provide evidence for a potential in vivo mechanism of action that involves the prevention of BCL-2 protein upregulation and enhanced sensitivity to apoptosis.
More broadly, this cytokine/prosurvival protein axis may be relevant to other malignancies for which venetoclax has been tested, such as AML and myeloma, in which case malignant cells use distinct homeostatic cytokines to support their survival. Together, our discoveries underscore the potential of mitigating the bioavailability of prosurvival cytokines when designing more potent treatment strategies in diverse types of hematologic cancers, which can induce deeper response and minimize the risk for future relapse.
Acknowledgments
The authors thank the patients who enrolled in the venetoclax clinical trials and acknowledge the assistance from Naomi Sprigg and Kelli Gray who helped with the collection and curation of patient samples. In addition, the authors thank Andrew Mitchell and Tian Zheng at the Materials Characterisation and Fabrication Platform (MCFP) at The University of Melbourne for mass cytometry support.
This work was supported by grants and fellowships from the Australian National Health and Medical Research Council (NHMRC) through fellowships to C.E.T. (1089072), D.H.D.G. (1090236 and 1158024), G.J.L. (1078730 and 1175960), and D.C.S.H. (1043149 and 1156024) and investigator grants to M.A.A. (1177718), D.T.U. (1194779), and A.W.R. (1174902); a Fulbright Australia-America Postdoctoral Fellowship to C.E.T.; a Victorian Cancer Agency Fellowship to C.E.T. (MCRF20026); a Postdoctoral Fellowship from the German Cancer Aid to A.P. (57584078); a Cancer Council of Victoria Grant-in-Aid to D.H.D.G. and D.T.U. (1146518 and 1102104); Perpetual Impact Philanthropy funding to C.E.T. (grant IPAP2019/1437); Australian NHRMC grants to C.E.T. (2002618), A.W.R. and D.C.S.H. (1016647, 1113577, 1016701, 1113133, 1079560, 2013478, and 2011139), G.J.L. (1113133 and 1153049), and D.C.S.H. and R.T. (2013478); The Medical Advances Without Animals Trust, the Leukemia and Lymphoma Society, US, Specialized Center of Research grant to A.W.R., A.S., and D.C.S.H. (7015-18); a Tour de Cure Mid-Career Research grant to D.T.U. (RSP-070-FY2023); the National Breast Cancer Foundation Australia (grant IIRS-19-004); the Breast Cancer Research Foundation to G.J.L. (grant BCRF-20-182); the Rae Foundation to S.C.; and Investigator Initiated Study support from AbbVie and Genentech (Roche) for the breast cancer study to G.J.L. (ACTRN12615000702516). This work was performed, in part, at the MCFP at The University of Melbourne and the Victorian Node of the Australian National Fabrication Facility with support from the Victorian Comprehensive Cancer Centre. This work was also supported by the Australian Cancer Research Foundation and made possible through Victorian State Government Operational Infrastructure Support and the Australian Government NHMRC Independent Research Institutes Infrastructure Support Scheme.
Authorship
Contribution: M.-X.L., C.E.T., M.A.A., D.C.S.H., A.W.R., T.E.L., and D.H.D.G. devised the research strategy; C.E.T. and D.H.D.G. cosupervised the research; M.A.A., A.W.R., T.E.L., C.S.T., R.M.K., and D.R. were responsible for patient care and recruited patients; M.-X.L., C.E.T., T.T., E.B.-S., A.P., and T.M.H.N. performed the experiments; M.-X.L., C.E.T., T.T., M.T., H.P., M.E.R., and D.G.H. analyzed data; A.W.R., D.C.S.H., and T.E.L. helped with experimental design and interpreting of results; S.C. and D.G.H. developed the tools; M.-X.L. and C.E.T. wrote the first version of the manuscript with major input from D.H.D.G.; A.S., R.T., M.E.R., D.T.U., C.S., D.C.S.H., G.J.L., T.P.S., and S.C. advised on various aspects of the study; and all authors reviewed the data and contributed to critical revision of the manuscript.
Conflict-of-interest disclosure: M.A.A. and T.E.L. report receiving honoraria from AbbVie. D.H.D.G. reports receiving research funding from Servier. G.J.L. reports receiving honoraria from AbbVie and Pfizer and research funding (to the institution) from AbbVie, Amgen, Pfizer, and Servier. A.W.R. reports receiving research funding from AbbVie and is an inventor on a patent related to venetoclax dose ramp-up. C.S.T. reports receiving honoraria from Janssen, AbbVie, BeiGene, and AstraZeneca. The Walter and Eliza Hall Institute receives milestone and royalty payments related to venetoclax and employees are entitled to receive benefits related to these payments. The remaining authors declare no competing financial interests.
Correspondence: Charis E. Teh, Immunology, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Melbourne 3052, VIC, Australia; email: teh.c@wehi.edu.au; and Daniel H. D. Gray, Immunology, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Melbourne 3052, VIC, Australia; email: dgray@wehi.edu.au.
References
Author notes
M.-X.L. and T.T. are joint first authors.
C.E.T. and D.H.D.G. are joint senior authors.
Data are available on detailed email requests from the corresponding authors, Charis E. Teh (teh.c@wehi.edu.au) or Daniel H. D. Gray (dgray@wehi.edu.au).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal