Key Points
Zanubrutinib sustained PFS benefit over ibrutinib; sensitivity analyses suggest this was driven by both antileukemic effect and tolerability.
Zanubrutinib maintained a favorable safety/tolerability profile with prevalence of most adverse events remaining stable or decreasing year-over-year.
Visual Abstract
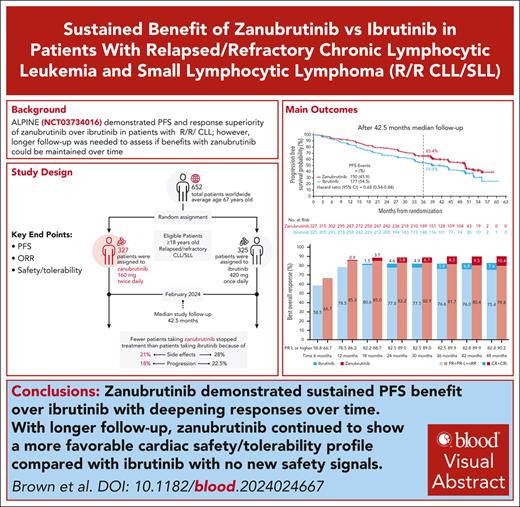
The ALPINE trial established the superiority of zanubrutinib over ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma; here, we present data from the final comparative analysis with extended follow-up. Overall, 652 patients received zanubrutinib (n = 327) or ibrutinib (n = 325). At an overall median follow-up of 42.5 months, progression-free survival benefit with zanubrutinib vs ibrutinib was sustained (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.54-0.84), including in patients with del(17p)/TP53 mutation (HR, 0.51; 95% CI, 0.33-0.78) and across multiple sensitivity analyses. Overall response rate remained higher with zanubrutinib compared with ibrutinib (85.6% vs 75.4%); responses deepened over time with complete response/complete response with incomplete bone marrow recovery rates of 11.6% (zanubrutinib) and 7.7% (ibrutinib). Although median overall survival has not been reached in either treatment group, fewer zanubrutinib patients have died than ibrutinib patients (HR, 0.77 [95% CI, 0.55-1.06]). With median exposure time of 41.2 and 37.8 months in zanubrutinib and ibrutinib arms, respectively, the most common nonhematologic adverse events included COVID-19–related infection (46.0% vs 33.3%), diarrhea (18.8% vs 25.6%), upper respiratory tract infection (29.3% vs 19.8%), and hypertension (27.2% vs 25.3%). Cardiac events were lower with zanubrutinib (25.9% vs 35.5%) despite similar rates of hypertension. Incidence of atrial fibrillation/flutter was lower with zanubrutinib vs ibrutinib (7.1% vs 17.0%); no cardiac deaths were reported with zanubrutinib vs 6 cardiac deaths with ibrutinib. This analysis, at 42.5 months median follow-up, demonstrates that zanubrutinib remains more efficacious than ibrutinib with an improved overall safety/tolerability profile. This trial was registered at www.ClinicalTrials.gov as #NCT03734016.
Introduction
Bruton tyrosine kinase (BTK) is a key component of the B-cell receptor signaling pathway in various B-cell malignancies. Ibrutinib was the first BTK inhibitor (BTKi) approved for the treatment of chronic lymphocytic leukemia (CLL).1 Acalabrutinib, a second-generation BTKi, was directly compared with ibrutinib in the phase 3 ELEVATE-RR study (ClinicalTrials.gov identifier: NCT02477696) in patients with previously treated CLL and small lymphocytic lymphoma (SLL) who had del(17p) and/or del(11q). After a median follow-up of 40.9 months, acalabrutinib showed noninferiority in progression-free survival (PFS) to ibrutinib (hazard ratio [HR], 1.0; 95% confidence interval [CI], 0.79-1.27) with a lower incidence of adverse events (AEs).2
Zanubrutinib is a uniquely designed BTKi specifically developed for greater BTK specificity and increased potency compared with ibrutinib and acalabrutinib.3 With exposure coverage above the half-maximal inhibitory concentration during the entire dosing interval,4 zanubrutinib can provide sustained BTK occupancy across disease-relevant tissues.5,6 In a global, phase 3 study (ALPINE; ClinicalTrials.gov identifier: NCT03734016), efficacy and safety/tolerability of zanubrutinib were compared head-to-head with ibrutinib in patients with relapsed/refractory (R/R) CLL/SLL. At the predefined, event-driven PFS analysis of this study, which occurred at 29.6 months median follow-up, zanubrutinib demonstrated statistically significant and clinically meaningful PFS superiority over ibrutinib (HR, 0.65; 95% CI, 0.49-0.86; P = .0024) when assessed by both the investigator and by an independent review committee. Additionally, zanubrutinib demonstrated superiority over ibrutinib in overall response rate (ORR) at 2 prespecified analyses7,8 and showed a favorable safety/tolerability profile.7,8 Because this study evaluated continuous therapy, longer-term follow-up can provide additional insights into the impact of zanubrutinib in this patient population. Here, we report the final comparative analysis between zanubrutinib and ibrutinib in ALPINE after 42.5 months of median follow-up.
Methods
The ALPINE study design and methodology have been previously described.7,8 Briefly, adults (aged ≥18 years) with a confirmed diagnosis of CLL or SLL, requiring treatment per International Workshop on CLL criteria, who relapsed or were refractory to at least 1 prior line of therapy, and had measurable disease by imaging were eligible. Enrolled patients were randomized (1:1) to receive open-label zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily until disease progression or unacceptable toxicity. Crossover was not allowed during the study. This study was conducted in accordance with the principles of the Declaration of Helsinki, good clinical practice, and International Council on Harmonisation guidelines, and all applicable regulatory requirements, and approved by institutional review board or independent ethics committee at each site. Participants gave written informed consent.
Disease response and progression were assessed per International Workshop on CLL 2008 criteria,9 with modification for treatment-related lymphocytosis for patients with CLL,10 and per Lugano Classification for patients with SLL.11 ORR, defined as the proportion of patients achieving complete response (CR) or complete response with incomplete bone marrow recovery (CRi), nodular partial response, or partial response (PR), was the primary end point and assessed by both investigator and independent review committee in prespecified analyses. PR with lymphocytosis was included in ORR. Key secondary end points included PFS and rate of atrial fibrillation or flutter; other secondary end points included overall survival (OS) and safety parameters.
The ad hoc analyses presented here evaluated investigator-assessed efficacy and safety/tolerability profiles of zanubrutinib and ibrutinib. For tolerability reporting, preferred terms were pooled for clinically relevant AEs, including COVID-19, hypertension, neutropenia, anemia, thrombocytopenia, and atrial fibrillation and/or flutter (see supplemental Data, available on the Blood website). To address the impact of subsequent CLL treatments in the absence of progressive disease (PD), PFS events occurring while receiving study treatment, and COVID-19–related deaths, multiple sensitivity analyses were performed to assess PFS under different assumptions. At the close of the ALPINE study, all patients were given an opportunity to roll over into a separate zanubrutinib long-term extension study (LTE-1; ClinicalTrials.gov identifier: NCT04170283).
Statistical testing of the primary end point of ORR was performed using a stratified Cochran-Mantel-Haenszel test. Statistical testing of time-to-event end points including PFS and OS was performed using a stratified log-rank test. Because the prespecified analyses per the study statistical analysis plan have previously been completed, no P values are presented. For time-to-event end points, including PFS and OS, a stratified HR was calculated along with its 95% CI from a stratified Cox proportional hazards model, and the distribution of these end points for each arm was summarized using the median and other quartiles as well as PFS rates at selected time points based on the Kaplan-Meier method. For time-to-AE cumulative incidence curves (eg, time to cardiac disorders), estimates were calculated as 1 minus Kaplan-Meier estimates, and treatment-emergent deaths were considered for censoring only and not as competing risks. Exposure-adjusted incidence rates (EAIRs) were calculated for COVID-19–related infections; EAIRs, in units of persons per 100 person-months, were calculated as: (number of patients having the AE/time from first dose to the first onset date or treatment-emergent end date if there was no such AE) × 100. Efficacy analyses were stratified by the 4 randomization stratification factors; safety and subgroup analyses were not stratified.
Results
Patient disposition and characteristics
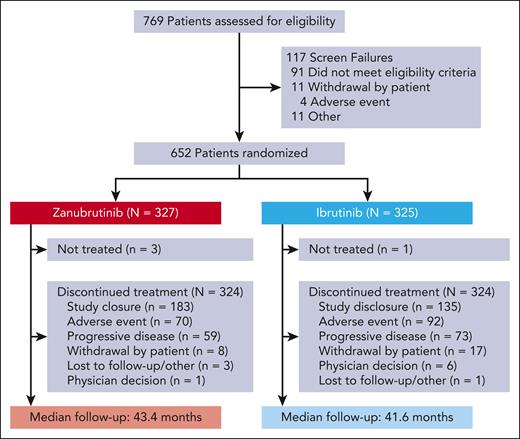
As previously detailed, 652 patients from North America, Europe, and Asia-Pacific were randomized to receive zanubrutinib (n = 327) or ibrutinib (n = 325) between 1 November 2018, and 14 December 2020 (Figure 1).7,8 At baseline, demographics and disease characteristics were generally balanced (Table 1). As of 28 February 2024, overall median study follow-up was 42.5 months (range, 0.1-60.5); median follow-up was 43.4 months (range, 0.1-59.6) with zanubrutinib and 41.6 months (range, 0.1-60.5) with ibrutinib.
Demographics and baseline characteristics
| . | Zanubrutinib (n = 327) . | Ibrutinib (n = 325) . |
|---|---|---|
| Age, median (range), y | 67 (35-90) | 68 (35-89) |
| Male | 213 (65.1) | 232 (71.4) |
| ECOG PS score of ≥1, n (%) | 198 (60.6) | 203 (62.5) |
| Race, n (%) | ||
| White | 261 (79.8) | 265 (81.5) |
| Asian | 47 (14.4) | 44 (13.5) |
| Black or African American | 4 (1.2) | 2 (0.6) |
| Native Hawaiian, Pacific Islander or other | 3 (0.9) | 0 |
| Multiple | 1 (0.3) | 0 |
| Other/not reported/unknown | 11 (3.4) | 14 (4.3) |
| Prior lines of systemic therapy, median (range) | 1 (1-6) | 1 (1-12) |
| >3 prior lines, n (%) | 24 (7.3) | 30 (9.2) |
| del(17p) and/or TP53mut, n (%) | 75 (22.9) | 75 (23.1) |
| del(17p) | 45 (13.8) | 50 (15.4) |
| TP53mut without del(17p) | 30 (9.2) | 25 (7.7) |
| IGHV mutational status, n (%) | ||
| Mutated | 80 (24.5) | 70 (21.5) |
| Unmutated | 240 (73.4) | 241 (74.2) |
| Missing | 7 (2.1) | 14 (4.3) |
| Complex karyotype∗(≥3 abnormalities) | ||
| Yes | 56 (17.1) | 70 (21.5) |
| No | 153 (46.8) | 130 (40.0) |
| Missing | 118 (36.1) | 125 (38.5) |
| Complex karyotype∗(≥5 abnormalities) | ||
| Yes | 32 (9.8) | 38 (11.7) |
| No | 177 (54.1) | 162 (49.8) |
| Missing | 118 (36.1) | 125 (38.5) |
| Bulky disease (≥5 cm), n (%) | 145 (44.3) | 149 (45.8) |
| . | Zanubrutinib (n = 327) . | Ibrutinib (n = 325) . |
|---|---|---|
| Age, median (range), y | 67 (35-90) | 68 (35-89) |
| Male | 213 (65.1) | 232 (71.4) |
| ECOG PS score of ≥1, n (%) | 198 (60.6) | 203 (62.5) |
| Race, n (%) | ||
| White | 261 (79.8) | 265 (81.5) |
| Asian | 47 (14.4) | 44 (13.5) |
| Black or African American | 4 (1.2) | 2 (0.6) |
| Native Hawaiian, Pacific Islander or other | 3 (0.9) | 0 |
| Multiple | 1 (0.3) | 0 |
| Other/not reported/unknown | 11 (3.4) | 14 (4.3) |
| Prior lines of systemic therapy, median (range) | 1 (1-6) | 1 (1-12) |
| >3 prior lines, n (%) | 24 (7.3) | 30 (9.2) |
| del(17p) and/or TP53mut, n (%) | 75 (22.9) | 75 (23.1) |
| del(17p) | 45 (13.8) | 50 (15.4) |
| TP53mut without del(17p) | 30 (9.2) | 25 (7.7) |
| IGHV mutational status, n (%) | ||
| Mutated | 80 (24.5) | 70 (21.5) |
| Unmutated | 240 (73.4) | 241 (74.2) |
| Missing | 7 (2.1) | 14 (4.3) |
| Complex karyotype∗(≥3 abnormalities) | ||
| Yes | 56 (17.1) | 70 (21.5) |
| No | 153 (46.8) | 130 (40.0) |
| Missing | 118 (36.1) | 125 (38.5) |
| Complex karyotype∗(≥5 abnormalities) | ||
| Yes | 32 (9.8) | 38 (11.7) |
| No | 177 (54.1) | 162 (49.8) |
| Missing | 118 (36.1) | 125 (38.5) |
| Bulky disease (≥5 cm), n (%) | 145 (44.3) | 149 (45.8) |
ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy chain variable.
Centrally assessed.
Fewer patients treated with zanubrutinib than those treated with ibrutinib had discontinued treatment because of AEs (zanubrutinib, n = 70 [21.4%]; ibrutinib, n = 92 [28.3%]) or disease progression (zanubrutinib, n = 59 [18.0%]; ibrutinib; n = 73 [22.5%]). Dose interruption and dose reduction because of AEs occurred in 59.3% vs 62.0% and 14.8% vs 18.2% of patients with zanubrutinib vs ibrutinib, respectively. Furthermore, fewer zanubrutinib patients than ibrutinib patients reported AEs leading to hospitalization (48.5% vs 57.1%).
Efficacy
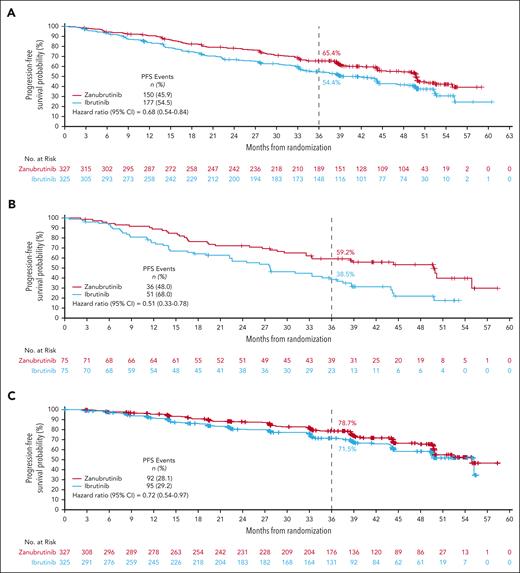
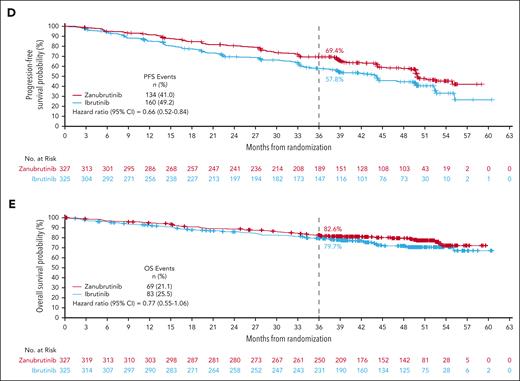
With 42.5 months of median follow-up, zanubrutinib PFS benefit was sustained over ibrutinib (HR, 0.68; 95% CI, 0.54-0.84; Figure 2A); the 36-month PFS rate was 65.4% in the zanubrutinib treatment arm and 54.4% in the ibrutinib treatment arm (supplemental Table 1). Improvement in PFS of zanubrutinib over ibrutinib was sustained in high-risk patients with del(17p)/TP53mut (HR, 0.51; 95% CI, 0.33-0.78; Figure 2B) as well as in patients without del(17p)/TP53mut (HR, 0.79; 95% CI, 0.61-1.02; supplemental Figure 1). Across most other major subgroups, PFS improvement with zanubrutinib was also maintained (supplemental Figure 2), including by prior lines of therapy (supplemental Figure 3). Zanubrutinib’s PFS benefit over ibrutinib remained consistent across multiple sensitivity analyses (supplemental Table 2), including assessment of progression and death events that occurred only while patients remained on active treatment (HR, 0.72; 95% CI, 0.54-0.97; Figure 2C), and when censoring for deaths attributed to COVID-19 (HR, 0.66; 95% CI, 0.52-0.84; Figure 2D). The 36-month PFS rates for zanubrutinib and ibrutinib in these sensitivity analyses were 78.7% and 71.5% (active treatment) and 69.4% and 57.8% (COVID-19), respectively (supplemental Table 1).
Investigator-assessed PFS and OS. (A) PFS in the overall population. (B) PFS in patients with del(17p)/TP53mut. (C) PFS when assessing only for progression or death events occurring on active treatment. (D) PFS when adjusted for COVID-19 deaths. (E) OS.
Investigator-assessed PFS and OS. (A) PFS in the overall population. (B) PFS in patients with del(17p)/TP53mut. (C) PFS when assessing only for progression or death events occurring on active treatment. (D) PFS when adjusted for COVID-19 deaths. (E) OS.
At 42.5 months follow-up, ORR remained higher with zanubrutinib compared with ibrutinib (85.6% vs 75.4%; response ratio, 1.13; 95% CI, 1.05-1.22); the rate of PR with lymphocytosis or better was 90.2% vs 82.8%, respectively. Although clinical responses deepened in both arms over time (supplemental Figure 3), zanubrutinib-treated patients reached CR/CRi earlier and more of them achieved CR/CRi than did ibrutinib-treated patients. Median OS had not been reached in either treatment group (Figure 2E). Overall, 69 and 83 zanubrutinib- and ibrutinib-treated patients, respectively, have died (OS: HR, 0.77; 95% CI, 0.55-1.06).
Safety/tolerability profile
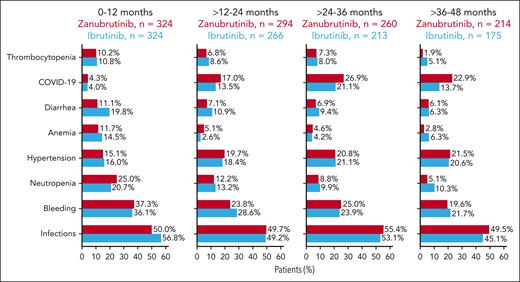
The prevalence of most AEs remained stable year-over-year (Figure 3). Although decreases in neutropenia and anemia were reported over time, the highest proportion of COVID-19–related infections were reported between 24 and 36 months. EAIRs for COVID-19–related infections were 1.51 per 100 person-months for zanubrutinib and 1.21 per 100 person-months for ibrutinib. Overall, 46.9% of zanubrutinib patients reported COVID-19 vaccination compared with 38.6% of ibrutinib patients. Overall, 43 COVID-19 deaths were reported; 21 (6.4%) in the zanubrutinib arm and 22 (6.8%) in the ibrutinib arm.
Yearly prevalence of clinically relevant AEs from 0 to 48 months. Pooled MedDRA preferred terms for all reported AEs.
Yearly prevalence of clinically relevant AEs from 0 to 48 months. Pooled MedDRA preferred terms for all reported AEs.
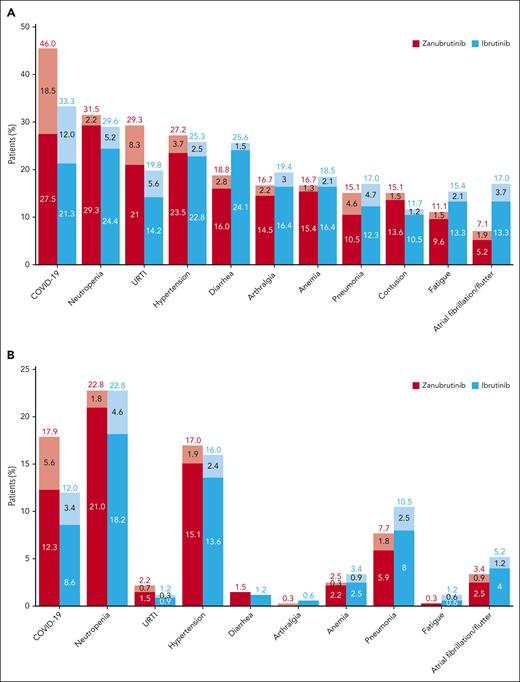
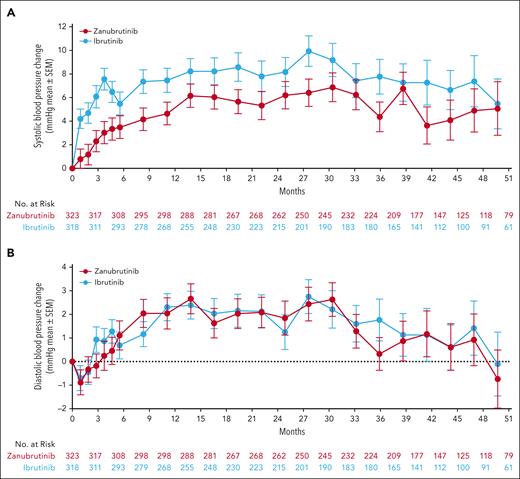
At this data cutoff, reflecting 42.5 months median follow-up, the most common nonhematologic treatment-emergent AEs of any grade with zanubrutinib vs ibrutinib were COVID-19–related infections (46.0% vs 33.3%), upper respiratory tract infection (29.3% vs 19.8%), diarrhea (18.8% vs 25.6%), and hypertension (27.2% vs 25.3%). The most commonly reported nonhematologic grade ≥3 AEs were hypertension (17.0% vs 16.0%), COVID-19–related infections (17.9% vs 12.0%), and pneumonia (7.7% vs 10.5%) with zanubrutinib vs ibrutinib, respectively. Neutropenia was the most common hematological AE of any grade (31.5% vs 29.6%) and grade ≥3 (22.8% vs 22.8%) with zanubrutinib vs ibrutinib, respectively; febrile neutropenia was low in both arms (n = 4, 1.2% each). Occurrence of hemolytic anemia (HA), including autoimmune HA, was rare. Two patients receiving ibrutinib experienced HA; 1 patient treated with zanubrutinib experienced autoimmune HA. The percentage of patients with all-grade and grade ≥3 AEs is presented in Figure 4A-B. Although hypertension rates were similar between treatments when evaluated as AEs using Medical Dictionary for Regulatory Activities (MedDRA) coded terms, mean changes from baseline in systolic blood pressure (Figure 5A) over time were generally lower in patients treated with zanubrutinib vs ibrutinib; changes in diastolic blood pressure (Figure 5B) were similar between treatment arms.
Most common treatment-emergent AEs. (A) All-grade treatment-emergent AEs occurring in ≥15% of patients. (B) Most common grade ≥3 treatment-emergent AEs. Value reported in cumulative prevalence rate at 42.5-month follow-up. Dark fill indicates percent of patients with an AE at 29.6-month median follow-up; lighter fill indicates change in percent of patients with AE from 29.6 months to 42.5-month median follow-up. URTI, upper respiratory tract infection.
Most common treatment-emergent AEs. (A) All-grade treatment-emergent AEs occurring in ≥15% of patients. (B) Most common grade ≥3 treatment-emergent AEs. Value reported in cumulative prevalence rate at 42.5-month follow-up. Dark fill indicates percent of patients with an AE at 29.6-month median follow-up; lighter fill indicates change in percent of patients with AE from 29.6 months to 42.5-month median follow-up. URTI, upper respiratory tract infection.
Mean change from baseline in systolic and diastolic blood pressure. (A) Mean change from baseline in systolic blood pressure. (B) Mean change from baseline in diastolic blood pressure. SEM, standard error of the mean.
Mean change from baseline in systolic and diastolic blood pressure. (A) Mean change from baseline in systolic blood pressure. (B) Mean change from baseline in diastolic blood pressure. SEM, standard error of the mean.
Of the AEs of special interest, defined in Brown et al,8 infections were the most prevalent (supplemental Table 3). Opportunistic infection rates (any grade; grade ≥3) were 2.8% vs 4.3%; 1.9% vs 1.9% between zanubrutinib and ibrutinib, respectively. Grade ≥3 neutropenia, concurrent with grade ≥3 infections, was 8.3% with zanubrutinib and 12.6% with ibrutinib
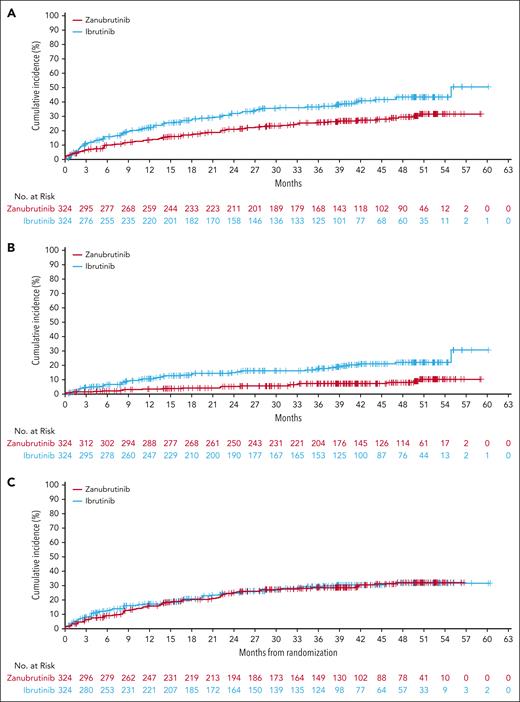
Overall cardiac events remained considerably lower with zanubrutinib compared with ibrutinib (Figure 6A) and the rate of atrial fibrillation/flutter was lower with zanubrutinib vs ibrutinib (7.1% vs 17.0%; Figure 6B) despite similar hypertension rates (Figure 6C). Overall incidence of cardiac events (25.9% vs 35.5%) and discontinuations because of cardiac events (0.9% vs 4.9%) were also lower with zanubrutinib compared with ibrutinib (supplemental Table 4). Overall, 6 patients treated with ibrutinib died because of cardiac AEs; in the zanubrutinib arm, no deaths due to cardiac AEs occurred (supplemental Table 5). Rate of fatal AEs was comparable between treatment arms (zanubrutinib, n = 43; ibrutinib; n = 41); more than half of the fatal AEs were due to infections, such as COVID-19 and/or pneumonia, in both treatment arms (zanubrutinib, n = 27/43; ibrutinib, n = 24/41).
Time to cardiac disorders and cardiovascular AEs of interest. (A) Time to occurrence of cardiac disorders; based on MedDRA version 26.0 cardiac disorder system organ class. (B) Time to occurrence of atrial fibrillation or flutter. (C) Time to occurrence of hypertension.
Time to cardiac disorders and cardiovascular AEs of interest. (A) Time to occurrence of cardiac disorders; based on MedDRA version 26.0 cardiac disorder system organ class. (B) Time to occurrence of atrial fibrillation or flutter. (C) Time to occurrence of hypertension.
Discussion
The ALPINE study is, to our knowledge, the only head-to-head study of covalent or noncovalent BTKis to show superior efficacy. Now, with median study follow-up of 42.5 months, zanubrutinib has been shown to offer a sustained PFS benefit vs ibrutinib, with a 32% reduction in risk of progression or death. The PFS benefits with zanubrutinib continue to extend to the predefined high-risk del(17p)/TP53mut population, for which risk of progression or death was 49% lower with zanubrutinib than with ibrutinib. This differs from an analysis after a similar median follow-up from the ELEVATE-RR study comparing acalabrutinib with ibrutinib, in which patients with previously treated CLL with del(17p) or del(11) showed no difference in the risk of progression or death, suggesting a clear differentiation of zanubrutinib among BTKi in this disease.
The improvement in PFS of zanubrutinib over ibrutinib was consistent across various sensitivity analyses that examined the impact of different variables (eg, discontinuation of treatment; COVID deaths) on PFS. Given that study treatment discontinuation because of AEs was more frequent in patients treated with ibrutinib than with zanubrutinib, we sought to investigate how much of the difference in PFS favoring zanubrutinib was because of subsequent progression after drug discontinuation for AEs. As such, a PFS sensitivity analysis was conducted that was restricted to the patients who remained on study treatment, to evaluate the differential impact of each drug among patients still receiving the intended study treatment. In this sensitivity analysis, which counted only progression and death events that occurred during active treatment, PFS with zanubrutinib remained better than with ibrutinib, demonstrating that the PFS advantage of zanubrutinib was substantially driven by treatment effect, not tolerability. Additionally, because ALPINE was conducted during the peak of COVID-19 incidence (2020-2023) and patients with CLL have tended to have more severe consequences from COVID-19 infection, a sensitivity analysis adjusting for death due to COVID-19 was conducted. Results of this PFS sensitivity analysis further reinforced the PFS advantage with zanubrutinib although the pandemic affected patients in both arms.
Although direct cross-trial comparisons are discouraged, landmark analyses provide insightful information to assure the performance of the ibrutinib arm in ALPINE was as observed in earlier trials. In the RESONATE study, a phase 3 study that compared the efficacy and safety of ibrutinib with ofatumumab, 18-month PFS for ibrutinib-treated patients was 76%; the 18-month PFS for ibrutinib-treated patients in ALPINE was consistent without (75%)8 and with (77%) COVID-19 adjustment. The patients on RESONATE were likely a higher-risk population, with more prior therapies and a higher rate of TP53 aberration. Yet, they were also enrolled from 67 sites in the United States, Australia, and 7 European countries compared with the wider enrollment of ALPINE from >150 sites in North America, Eastern and Western Europe, and Asia-Pacific, including China. Importantly, more than half of the total ALPINE enrollment came from Eastern Europe (43%; Poland, Turkey, and the Czech Republic) and China (14%), neither of which were represented in the RESONATE study. Furthermore, the lack of alternative treatment options for patients coming off ibrutinib on RESONATE, in comparison with those coming off ibrutinib on ALPINE, may have contributed to a greater effort to keep patients on RESONATE as long as possible. Multiple real-world studies of ibrutinib since have shown results comparable with the control arm of ALPINE, consistent with the global reach of this study.12-14
Indirect comparison of ibrutinib arms across separate trials can be made using matching-adjusted indirect comparison (MAIC) methodology, in which individual patient-level data from 1 trial are combined with published aggregate data from another trial, followed by propensity score weighting.15 Initial results of an indirect comparison of the ibrutinib arms across the ALPINE, ELEVATE-RR, and RESONATE studies suggested ibrutinib underperformance in ALPINE16; however, this MAIC analysis did not match on key characteristics critical when comparing data across studies (eg, TP53, immunoglobulin heavy chain variable gene mutation status, complex karyotype, and Binet stage). The results of a subsequent MAIC analysis, using a comprehensive list of matching variables, were recently published and are in contrast with the earlier results.17 Given the variability of results seen depending on the methodology of a given MAIC, these results should be interpreted with great caution, and underscore that our clinical decision-making must ultimately rely on randomized trials.15,18 Even with the generalization limitations inherent with open-label studies and follow-up limited to 42.5 months, ALPINE was a well-conducted randomized trial with balanced enrollment to each arm; the results reflect what can be expected of each drug in this patient population, and zanubrutinib showed superior efficacy to ibrutinib.
The primary analysis for ALPINE demonstrated superior ORR of zanubrutinib compared with ibrutinib. With longer follow-up, the higher response rates, both ORR and CR/CRi, persisted among patients treated with zanubrutinib compared with those treated with ibrutinib. Similar to other phase 3 zanubrutinib studies,19,20 ALPINE demonstrated a deepening of response over time, with consistently higher CR/CRi rates in the zanubrutinib arm over the course of the study. The improvements in CR/CRi rates over time were also seen in patients treated with zanubrutinib in the SEQUOIA study (NCT03336333) in patients with CLL with/without del(17p)/TP53mut, in which CR rates improved from 9.1% (both with/without del(17p)/TP53mut) at 26.2 months to 14.5% (with del(17p)/TP53mut) and 17.4% (without del(17p)/TP53mut) at 43.7 months.20
With median exposure time of 41.2 and 37.8 months with zanubrutinib and ibrutinib, respectively, zanubrutinib was generally more tolerable than ibrutinib. Fewer zanubrutinib-treated patients discontinued treatment because of AEs, and fewer patients exhibited cardiac side effects, such as atrial fibrillation. Although hypertension rates between zanubrutinib and ibrutinib were similar in ALPINE, the degree of increase in systolic blood pressure was less with zanubrutinib and the overall incidence of, and discontinuations because of, cardiac disorders were lower with zanubrutinib. Despite similar hypertension rates, significantly fewer patients treated with zanubrutinib than ibrutinib developed atrial fibrillation/flutter (7.1% vs 17.0%) and none of the zanubrutinib-treated patients died due to a cardiac disorder, whereas 6 deaths due to cardiac disorders were reported in patients treated with ibrutinib, confirming the cardiac safety advantage. It is also important to note the rate of hypertension reported with zanubrutinib in ALPINE is higher than in other zanubrutinib monotherapy studies.3,20,21 In a retrospective analysis of safety across 9 different clinical studies in the zanubrutinib clinical study database (N = 1550), the rate of hypertension in ALPINE was clearly higher than in other zanubrutinib studies; however, this could be because of differences in trial populations or greater investigator awareness of hypertension as a class effect of BTKis.
Although higher rates of any-grade COVID-19 were reported in the zanubrutinib arm, fewer COVID-19–related deaths and fewer treatment discontinuations because of COVID-19 occurred on the zanubrutinib arm compared with ibrutinib. As the incidence of COVID-19 infection increased during the trial, the longer duration of drug exposure for zanubrutinib likely contributed to COVID-19 AE rates with EAIR of 1.5 and 1.2 per 100 person-months for zanubrutinib and ibrutinib, respectively.
This updated ALPINE analysis demonstrates a durable and sustained efficacy benefit of zanubrutinib over ibrutinib in patients with R/R CLL/SLL. Furthermore, with longer follow-up, zanubrutinib continues to show a more favorable cardiac safety/tolerability profile compared with ibrutinib, and no new safety signals have been observed. Given the demonstrated superior efficacy and safety profile of zanubrutinib vs ibrutinib longer than 3 years, we felt it was not in the best interest of patients to continue ibrutinib. As such, a decision was made to close the study and allow eligible patients, including those originally assigned to ibrutinib, to enroll in LTE-1. Although long-term effects of zanubrutinib treatment in the ALPINE patients will be available from the LTE-1 study, there will be no additional comparative analyses. Thus, this ad hoc analysis represents the final comparative analysis of the ALPINE study and confirms the improved safety and efficacy of zanubrutinib compared with ibrutinib in patients with R/R CLL/SLL.
Acknowledgments
The authors thank the study patients, their supporters, the investigators, and clinical research staff at the study centers. Assistance with medical writing and editorial support, under the direction of the authors, was provided by Regina Switzer and Elizabeth Hermans and was funded by BeiGene.
Authorship
Contribution: The trial protocol was developed by BeiGene, in collaboration with J.R.B., B.E., S.M.O., L.Q., and C.S.T.; all authors contributed to data interpretation, analysis, and data collection; J.R.B., M. Shadman, A.C., T.S., L.W., and K.W. developed the first draft of the manuscript; and all authors were involved in reviewing/revision of the manuscript, provided approval for submission, and are accountable for all aspects of the work.
Conflicts-of-interest disclosure: J.R.B. reports consultancy with AbbVie, Acerta/AstraZeneca, Alloplex Biotherapeutics, BeiGene, Galapagos NV, Genentech/Roche, Grifols Worldwide Operations, InnoCare Pharma Inc, iOnctura, Kite, Loxo/Lilly, Merck, Numab Therapeutics, Pfizer, and Pharmacyclics; and research funding from BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, TG Therapeutics. B.E. reports research funding from Janssen, Gilead, Roche, AbbVie, BeiGene, and AstraZeneca; received honoraria from Janssen, BeiGene, Roche, AbbVie, Novartis, Celgene, and AstraZeneca; serves on speakers bureau for Roche, AbbVie, Merck Sharp & Dohme, BeiGene, and AstraZeneca; and received travel, accommodations, and expenses funding from BeiGene. N.L. reports consultancy with AbbVie, AstraZeneca, BeiGene, Lilly/Loxo, Genentech, Janssen, and Pharmacyclics; and research funding from AbbVie, AstraZeneca, BeiGene, Lilly/Loxo, Genentech, Octapharma, Oncternal, MingSight, and TG Therapeutics. S.M.O. reports consultancy with AbbVie, AstraZeneca, BeiGene, Lilly, Janssen, Johnson & Johnson, Pfizer, and Pharmacyclics; research funding from BeiGene, Lilly, Pfizer, Pharmacyclics, Regeneron; and membership with the CLL Society (unpaid). C.S.T. reports research funding from Janssen, AbbVie, and BeiGene; received honoraria from Janssen, AbbVie, BeiGene, Loxo, and AstraZeneca. L.Q. reports consultancy with Janssen, AstraZeneca, Takeda, Roche, AbbVie, and BeiGene. W.J. reports consultancy with Janssen, AstraZeneca, MEI Pharma, Lilly, Takeda, Roche, AbbVie, and BeiGene; reports research funding: from AbbVie, Bayer, BeiGene, Celgene, Janssen, Roche, Takeda, TG Therapeutics, AstraZeneca, MEI Pharma, and Lilly. M. Simkovic received consulting fees from AbbVie, AstraZeneca, and Janssen-Cilag; reports individual stocks in AbbVie, AstraZeneca, Johnson & Johnson, BeiGene, Gilead, Baxter, Novartis, Abbot, and Sanofi; reports honoraria from AbbVie, Janssen-Cilag, and AstraZeneca; reports membership on an entity’s board of directors or advisory committees of AbbVie, Janssen-Cilag, and AstraZeneca; and received travel, accommodations, and expenses funding from AbbVie, Janssen-Cilag, and AstraZeneca. J.M. received research funding from BeiGene. A.F. received honoraria from AbbVie, AstraZeneca, and Genentech; and reports research funding from AbbVie, AstraZeneca, BeiGene, and Genmab. R.W. reports honoraria from Janssen, AbbVie, and BeiGene; received research funding from BioOra; and is a current equity holder in the publicly traded company Fisher & Paykel Healthcare. T.R. reports honoraria from AbbVie, Janssen, BeiGene, AstraZeneca, Octopharma, Regeneron, and GlaxoSmithKline; reports consultancy with Janssen, BeiGene, and AstraZeneca; and received research funding from Janssen, BeiGene, AstraZeneca, OctoPharma, Regeneron, and GlaxoSmithKline. H.A.Y. serves on the speakers bureau for AbbVie, Amgen, AstraZeneca, BeiGene, GlaxoSmithKline, Jenssen, Karyopharm, and Takeda. M.W., T.S., L.W., J.L., and K.W. are employed by BeiGene; and are equity holders in BeiGene. A.C. was an employee of BeiGene at the time of the study, which included equity holder, and funds for travel, accommodations, and expenses. M. Shadman reports consultancy with, and roles on advisory boards, steering committees, or data safety monitoring committees of, AbbVie, Genentech, AstraZeneca, Genmab, Janssen, BeiGene, Bristol Myers Squibb, Morphosys/Incyte, Kite Pharma, Eli Lilly, Mustang Bio, Fate therapeutics, Nurix, and Merck; reports institutional research funding from Mustang Bio, Genentech, AbbVie, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; reports stock options in Koi Biotherapeutics; and reports employment by Bristol Myers Squibb (spouse). The remaining authors declare no competing financial interests.
Correspondence: Jennifer R. Brown, Harvard Medical School, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: jennifer_brown@dfci.harvard.edu.
References
Author notes
BeiGene voluntarily shares anonymous data on completed studies responsibly and provides qualified scientific and medical researchers access to anonymous data and supporting clinical trial documentation for clinical trials in dossiers for medicines and indications after submission and approval in the United States, China, and Europe. Clinical trials supporting subsequent local approvals, new indications, or combination products are eligible for sharing once corresponding regulatory approvals are achieved. BeiGene shares data only when permitted by applicable data privacy and security laws and regulations. In addition, data can only be shared when it is feasible to do so without compromising the privacy of study participants. Qualified researchers may submit data requests/research proposals for BeiGene review and consideration through BeiGene’s clinical trial webpage at https://www.beigene.com/our-science-and-medicines/our-clinical-trials/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal