In this issue of Blood, Wang et al1 report findings that significantly advance our understanding of the pathogenesis of reticular dysgenesis (RD), one of the most devastating severe immunodeficiency syndromes. The following critical question was rigorously answered: Why do inborn errors of metabolism, such as those seen in RD, lead to severe defects in specific blood cells but not in others?
Cellular metabolism is a complex process essential for the physiological functioning of hematopoiesis, which regulates the homeostasis, stemness, proliferation, and differentiation of hematopoietic stem cells (HSCs) into mature blood cells. Several metabolic processes have been described that fine-tune the functions of HSCs; therefore, failure of these metabolic processes can result in defective functioning of mature blood cells and abnormal differentiation of immature HSCs. Mutations in genes directly or indirectly involved in the regulation of cellular metabolism may cause severe neutropenia, as an isolated manifestation or as part of a multisystem disease. Thus, inherited mutations in the glucose-6-phosphatase catalytic subunit 3 gene cause severe congenital neutropenia,2 and mutations in glucose-6-phosphate transporter lead to glycogen storage disease Ib with neutropenia.3 Autosomal dominant mutations in the caseinolytic peptidase B homolog, CLPB, encoding an adenosine triphosphatase involved in protein folding and mitochondrial function,4 have also been identified in patients with neutropenia, thus expanding the genetic heterogeneity of metabolic dysregulation causes of neutropenia. The association of metabolic genetic defects with bone marrow failure syndromes can be explained by the fact that, during differentiation, metabolic processes are finely tuned to meet distinct needs of HSCs and their differentiated progeny. For example, HSCs primarily rely on glycolysis for energy production.5 As HSCs differentiate, mitochondrial oxidative phosphorylation becomes the preferred method of energy generation, with a metabolic shift toward oxidative phosphorylation, particularly in myeloid and erythroid lineages. The metabolism of neutrophils differs significantly from their less mature progenitor cells, due to its specialization to support rapid, energy-intensive immune functions. Surprisingly, little is known about the regulation of metabolism during stage-specific granulopoiesis.
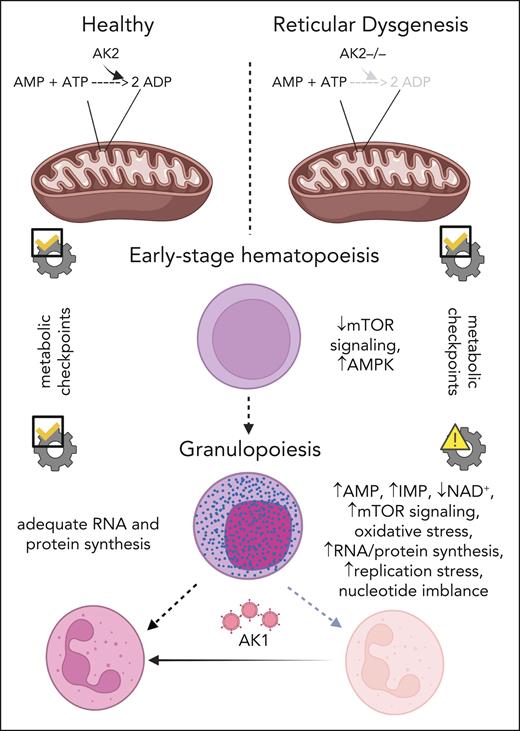
In their study, Wang et al tackled this complex problem by studying the metabolic processes involved in the neutrophilic differentiation of HSCs with mutations in adenylate kinase 2 (AK2) (see figure). AK2 controls cellular energy homeostasis and the metabolism of adenine nucleotides by regulating the reversible transfer of the terminal phosphate group between adenosine triphosphate and adenosine monophosphate (AMP) in the mitochondria. Autosomal recessive loss-of-function or missense mutations in AK2 were found in patients with RD. RD is characterized by severe neutropenia, profoundly reduced numbers of T lymphocytes and natural killer cells, hypoplasia of the thymus and secondary lymphoid organs, lack of innate and adaptive immune functions, and bilateral sensorineural deafness.6
Mechanism of diminished granulopoiesis in AK2-deficient hematopoietic stem and progenitor cells. ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMPK, AMP-activated kinase; mTOR, mammalian target of rapamycin; IMP, inosine monophosphate; NAD+, nicotinamide adenine dinucleotide.
Mechanism of diminished granulopoiesis in AK2-deficient hematopoietic stem and progenitor cells. ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMPK, AMP-activated kinase; mTOR, mammalian target of rapamycin; IMP, inosine monophosphate; NAD+, nicotinamide adenine dinucleotide.
The authors performed single-cell transcriptomics of bone marrow from 2 patients with RD and 9 healthy individuals. They identified expanded populations of immature cells, but significantly reduced populations of more mature granulocytic progenitors, and both mature lymphocytes and natural killer cells. Consistent with this, expression of ribonucleoprotein-related gene sets was decreased in immature progenitors, but was paradoxically increased in more mature granulocytic progenitors. To understand how AK2 deficiency affects mitochondrial function at distinct stages of granulocytic differentiation, the authors successfully created a model of AK2 deficiency in healthy donor CD34+ cells using gene editing. They found that AK2-deficient myelocytes and neutrophils paradoxically increased anabolic pathways and mitochondrial function, unlike their immature counterparts wherein these functions were significantly reduced. AK2 deficiency also altered purine metabolism, depleted mitochondrial substrates, and shifted the redox balance toward reductive stress in myelocytes and neutrophils, but not in the more immature myeloid cells, promyelocytes. These data suggest that metabolic checkpoints respond favorably to metabolic stress during early stages of granulopoiesis, but not in later stages. In addition, AK2-deficient cells exhibit increased activity of the de novo and salvage purine synthesis pathways during late stages of granulopoiesis, with concomitant elevated levels of inosine monophosphate and AMP. This nucleotide imbalance reduces proliferation and differentiation during granulopoiesis. To test whether AK2-deficient cells actively deaminate AMP to inosine monophosphate, the authors pharmacologically inhibited AMP deaminase in AK2-deficient cells and observed no improvement in granulocytic differentiation. In addition, the defective in vitro granulopoiesis of AK2-deficient cells was rescued by cytosolic AK1. They concluded that ectopic mammalian target-of-rapamycin activation, nucleotide imbalance, and proliferation arrest result from ineffective metabolic checkpoints in AK2-deficient granulocytic progenitors.
The work by Wang and colleagues contributes to the broader understanding of how inborn errors of metabolism can lead to selective cellular defects. The selective vulnerability of late-stage granulocyte precursors in RD, despite the ubiquitous expression of AK2, underscores the importance of metabolic context- and stage-specific requirements in determining the impact of metabolic disorders.
It would be of interest to investigate whether similar metabolic processes are deregulated in AK2-deficient lymphocytes and natural killer cells. It is also unclear how metabolic processes are linked to signaling pathways known to specifically activate granulopoiesis. It has been shown that granulocyte-macrophage colony-stimulating factor activates NAD+-dependent protein deacetylase SIRT1 through induction of a NAD+-generating enzyme, nicotinamide phosphoribosyltransferase (NAmPRTase or NAMPT). SIRT1-dependent deacetylation and activation of the transcription factors C/EBPα and C/EBPβ induces granulopoiesis.7 The authors observed reduced NAD+ levels in AK2-deficient myeloid cells, so it would be of interest to investigate whether NAMPT is still activated by granulocyte-macrophage colony-stimulating factor in the absence of AK2, and whether the remaining amounts of NAD+ can activate SIRT1 sufficiently to induce C/EBPs in patients with RD.
Another area of interest would be to identify the extent to which the findings in RD can be extrapolated to other forms of neutropenia or bone marrow failure syndromes. Finally, the study’s findings also raise the intriguing possibility of metabolic checkpoint manipulation as an attractive therapeutic strategy in other diseases characterized by dysregulated hematopoiesis. However, the challenges encountered in pharmacologically rescuing AK2-deficient cells underscore the need for a nuanced approach that considers the complex and context-dependent nature of metabolic regulation in hematopoietic stem and progenitor cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal