Issue Archive
Table of Contents
EDITORIAL
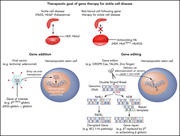
Introduction to a review series on gene therapy and gene editing for sickle cell disease and hemophilia
Introduced by Associate Editor Catherine Bollard, this review series focuses on the state of the art of gene therapy for sickle cell disease and hemophilia. These papers review the range of clinical trials for gene therapy of these two inherited diseases, with a focus on outcomes and safety. In addition, we include a Perspective article reflecting on the development of hematologic malignancy in 2 patients after gene therapy for sickle cell disease, which led to a brief suspension of 1 clinical trial that has now resumed enrollment.
BLOOD COMMENTARIES
REVIEW SERIES
PERSPECTIVE
LYMPHOID NEOPLASIA
Molecular classification improves risk assessment in adult BCR-ABL1–negative B-ALL
Paietta and colleagues performed extensive genomic analysis of a cohort of over 200 adults with BCR-ABL-negative B-cell acute lymphoblastic leukemia (B-ALL) treated in a large clinical trial. Adding gene expression profiling, immunophenotypic analysis, and fusion polymerase chain reaction predicted survival better than traditional stratification by age and white blood cell count, mainly by allowing reassignment of high risk patients to standard or immediate risk groups based on genomic analysis.
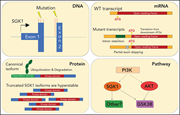
SGK1 mutations in DLBCL generate hyperstable protein neoisoforms that promote AKT independence
Brief Report
The serum and glucocorticoid-regulated kinase 1 gene (SGK1), one of the most commonly mutated genes in diffuse large B-cell lymphoma (DLBCL), has been widely assumed to be a tumor suppressor that is inactivated by loss-of-function mutations. Gao et al challenge this paradigm, demonstrating that N-terminal truncation mutations remove the degradation domain, leading to truncated hyperstable proteins that retain kinase activity, thereby enhancing proliferation and resistance to AKT inhibition. This suggests SGK1 inhibition as a potential target for therapy of DLBCL.
MYELOID NEOPLASIA
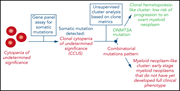
Relationship between clone metrics and clinical outcome in clonal cytopenia
CME
Clinical Trials & Observations
Clonal cytopenia of undetermined significance (CCUS) is associated with increased risk of myeloid neoplasm (MN); however, predicting risk of evolution to MN is difficult. In this month’s CME article, Gallì et al describe clonal dynamics in a large cohort of patients with (1) idiopathic cytopenia of undetermined significance (ICUS), (2) no hematologic abnormalities, (3) unexplained anemia, and (4) overt MN. Thirty percent of patients with ICUS could be reassigned as having CCUS. The authors further report that the nature of the clonal mutations, the combination of different mutations, and the variant allele fractions allow patients to be separated into groups with divergent likelihood of developing MN.
PHAGOCYTES, GRANULOCYTES, AND MYELOPOIESIS
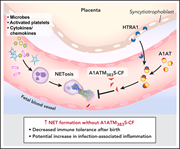
Placental HTRA1 cleaves α1-antitrypsin to generate a NET-inhibitory peptide
Neutrophil extracellular traps (NETs) are important in the response to infection but can also mediate excessive inflammation. Neonatal neutrophils do not form NETs because of circulating NET-inhibitory peptides (NIPs). Campbell and colleagues report that α1-antitrypsin (A1AT) is cleaved by high-temperature requirement serine protease A1 (HTRA1) to form NIPs. HTRA1-null mice become NET competent, and administration of the A1AT cleavage fragment improves survival in a neonatal sepsis model, confirming that HTRA1 cleaves A1AT to modulate NET formation.
LETTERS TO BLOOD
Validation of the international working group proposal for SF3B1 mutant myelodysplastic syndromes
Clinical Trials & Observations
Komrokji et al examined the proposed international working group proposal to designate SF3B1-mutant myelodysplastic syndrome (MDS) as a unique disease entity, using a single-institution validation cohort of 1779 MDS patients, of whom 320 harbored SF3B1 mutations. They confirmed the demographics of older age, low-risk disease, ring sideroblasts, and low blast percentage. They further confirmed better overall survival that is negatively impacted in those patients with concomitant mutations, high variant allele fraction, and increased blast percentage.
The use of IV immunoglobulin in the treatment of vaccine-induced immune thrombotic thrombocytopenia
Clinical Trials & Observations
BLOOD WORK
CONTINUING MEDICAL EDUCATION (CME) QUESTIONS
-
Cover Image
Cover Image
![issue cover]()
Live cell confocal microscopy with cell-permeable and impermeable DNA stains demonstrates neutrophil extracellular trap (NET) formation (magenta) by lipopolysaccharide-stimulated human neutrophils. Pretreatment with the scrambled peptide control for the A1ATM383S cleavage fragment, a placentally derived NET-inhibitory peptide, fails to inhibit NET formation. Non-NET-forming neutrophils retain intact nuclei (green). See the article by Campbell et al on page 977.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals





ALL is not the same in the era of genetics