In this issue of Blood, Galli and colleagues provide a sophisticated description of the genotypic and phenotypic features of patients with idiopathic cytopenia of undetermined significance (ICUS) and clonal cytopenia of undetermined significance (CCUS). CCUS is associated with an increased risk of progression to myeloid neoplasm (MN) associated with myelodysplasia (MDS), but an individual's rate of progression is highly variable. In their large, closely followed cohort, the authors demonstrate 3 major variables that predict outcome: the number of mutations, the types of mutations, and variant allele frequencies (VAFs) of those mutations.1 They drew clinical comparisons to patients with overt MN as well as a control group of older control patients, with and without anemia, to better characterize the clonal metrics. This study addresses a common problem in clinical practice: how to best advise cytopenic patients of their risk of transformation to overt MN based on their molecular mutational profile.
At the bedside, patients with peripheral blood cytopenia can present diagnostic challenges. Next-generation sequencing (NGS) is now widely available and no longer cost-limiting in most settings. Thus, it is often performed as part of a standard evaluation in the work up of patient with abnormally low blood counts.2,3 In some cases, NGS provides additional useful diagnostic information, which may allow earlier identification of a predisposition to MN or MDS. In other cases, NGS yields indeterminant or unactionable information. The ability to accurately prognosticate who will evolve to frank disease is an essential, and as yet, unmet clinical need.4,5 Although several novel molecular findings were incorporated into the 2016 revision of the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues,6 a closer integration of morphology and molecular genetics is still required to improve patient-centric endpoints. Better correlation between NGS data and disease prognosis is required to more fully use the NGS data.
In this paper, the authors clarified several clinical issues related to CCUS. Varied patterns of mutations and the VAFs of those mutations are associated with different degrees of dysplasia and clinical outcomes. The study provides greater information on the actual clonal burden than previously reported. In addition, the data may help identify the cause of anemia in community-dwelling older adults. In the prospective cohort of 311 patients with ICUS at baseline, nearly one-third of patients carried at least 1 genetic lesion that revised the diagnosis to CCUS. Mutated status is associated with a nearly fivefold increased risk of progression to MN overall and a nearly eightfold increase when 2 or more mutations are present. A significantly higher prevalence of clonal mutations was found in anemic community-dwelling older adults compared with similarly-aged persons without anemia .
Thresholds for VAF suggested by the Galli et al should improve the risk assessment and monitoring of patients, especially those predicted to be highest risk for earlier progression and evolution. Clonal size was the most significant predictor of risk for progression (hazard ratio of 11.6). However, VAF values <10% did not appear to carry an increased risk of progression. This suggests a level of mutational “tolerance” and perhaps may ultimately be a relevant threshold for diagnosis in MN or MDS. MN can evolve from CCUS from increased VAFs or acquisition of new mutations. We have previously known the value of serial monitoring,7 but this improved understanding of clonal metrics stresses its importance.
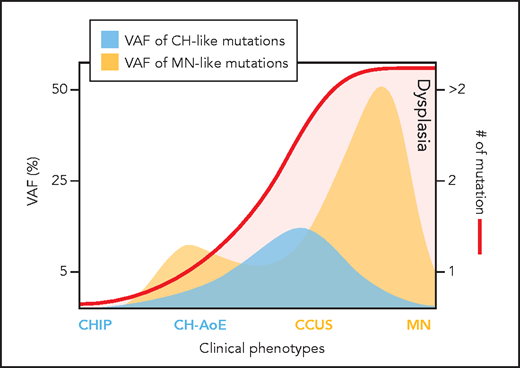
Clone metrics in clonal cytopenia. AoE, anemia of elderly; CHIP, clonal hematopoiesis indeterminate potential. Professional illustration by Patrick Lane, ScEYEnce Studios.
Clone metrics in clonal cytopenia. AoE, anemia of elderly; CHIP, clonal hematopoiesis indeterminate potential. Professional illustration by Patrick Lane, ScEYEnce Studios.
Significant associations were also found between mutated driver gene and risk of progression to MN. Patients harboring mutant splicing factors, ASXL1 or TET2, had significantly increased risks; conversely, no significant risk was found in patients carrying a DNMT3A mutation. There was prior recognition that not all mutations are created equal, but this data adds granularity. Furthermore, recurrent mutation patterns exhibited different VAF values associated with marrow dysplasia, indicating variable clinical expressivity of mutant clones as well as the subjectivity of the required morphology for MDS or MN diagnosis. This is additional confirmation of the antiquated nature of our current subjective criteria of dysplasia in 10% or more of myeloid cells, according to WHO criteria for MDS diagnosis. Having molecular quantification is valuable, as previous reports have found discordance (based on morphology alone) in MDS diagnoses among centers.8,9
The authors performed a cluster analysis and identified 2 major clusters, characterized by isolated DNMT3A mutations called CH-like (suggestive of “favorable clonal hematopoiesis”) and combinatorial mutation patterns labeled MN-like (more akin to MN disease genotype). The latter, less favorable group encompassed splicing factors, TP53, or DNMT3A, TET2, or ASXL1 genes in combination with additional mutated genes. The 2 clusters showed a significantly different overall survival in multivariable models, adjusted for salient clinical variables.
The examination of anemia in the elderly has clinical utility. SF3B1 and DNMT3a, TET2, and ASLX1 were associated with this anemia, which suggests causality in older patients with lower hemoglobin but no overt MN. These could be patients in whom a diagnostic marrow may ultimately have less value if the NGS is performed on blood and the clinical phenotype fits.
This manuscript provides answers to relevant questions for patients with CH. A simplified illustration of the author's results can be seen in the figure. Future directions include incorporation of molecular diagnostics, including VAFs, into the WHO classification of MNs. SF3B1 is an exemplary start.10 Given the high probability of CCUS progression to MN, perhaps “undetermined significance” is also no longer an ideal moniker, and this condition can be reclassified as neoplastic. Finally, the improvements in diagnosis and prognostication in CCUS applied from this study may ultimately pave the way for therapeutic interventions at the level of CCUS to alter the natural history of MN.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal