Issue Archive
Table of Contents
BLOOD COMMENTARIES
SPECIAL REPORT
Consensus recommendations on the management of toxicity associated with CD3×CD20 bispecific antibody therapy
CD3×CD20 bispecific antibody therapies instigate a T-cell mediated immune response to B-cell lymphoid cancers and are a major new class of treatment for patients with B-cell lymphomas. This new class of agents is associated with both rapid responses and distinct major toxicities, including cytokine release syndrome (CRS). Prevention and management of CRS, cytopenia, infections, and neurotoxicity are central to their successful use in routine care; this consensus statement from a multidisciplinary, international panel of clinicians, examined by Crombie and coauthors, outlines best practice, tailored for application in both the community and hospital settings.
CLINICAL TRIALS AND OBSERVATIONS
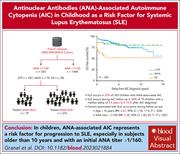
Antinuclear antibody–associated autoimmune cytopenia in childhood is a risk factor for systemic lupus erythematosus
Clinical Trials & Observations
A minority of patients presenting as children with chronic autoimmune cytopenia (AIC), manifesting as immune thrombocytopenic purpura or autoimmune hemolytic anemia, will subsequently develop systemic lupus erythematosus (SLE), but which ones? In a prospective cohort study, Granel and colleagues identified a 22% incidence rate of SLE after diagnosis of AIC if the antinuclear antibody (ANA) was ever positive, with the highest risk in children diagnosed after 10 years of age and where the ANA titer exceeded 160. The authors’ work identified a subgroup of patients needing long-term monitoring for later emergence of SLE.
HEMATOPOIESIS AND STEM CELLS
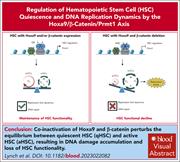
Hematopoietic stem cell quiescence and DNA replication dynamics maintained by the resilient β-catenin/Hoxa9/Prmt1 axis
Hematopoietic stem cells (HSCs) must retain both the ability to self-renew and to produce large numbers of mature progeny. Lynch et al reveal that Hoxa9 and β-catenin act in concert to maintain HSC integrity, collaborating to promote quiescence and safeguard DNA fidelity. Their actions converge to regulate the expression of overlapping target genes, including PRMT1, which is critical for HSC maintenance.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
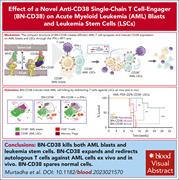
A CD38-directed, single-chain T-cell engager targets leukemia stem cells through IFN-γ–induced CD38 expression
LYMPHOID NEOPLASIA
Follow-up from the A041202 study shows continued efficacy of ibrutinib regimens for older adults with CLL
CME
Clinical Trials & Observations
In this month’s CME article, Woyach et al report on the longer follow-up of a randomized trial of ibrutinib vs ibrutinib plus rituximab (IR) vs bendamustine plus rituximab (BR) as first-line therapy for older patients with chronic lymphocytic leukemia (CLL). The 4-year data confirm the major progression-free survival advantage of using ibrutinib over BR and that the addition of rituximab to ibrutinib does not increase this benefit. The greatest benefits are observed in those patients with high-risk CLL, but, as yet, no overall survival advantage is evident. The cumulative incidence of atrial fibrillation of patients on ibrutinib is 18%, and other safety elements are updated. These maturing data provide updated information that informs treatment choices in clinical practice.
MYELOID NEOPLASIA
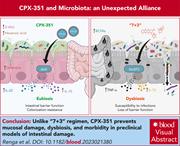
CPX-351 exploits the gut microbiota to promote mucosal barrier function, colonization resistance, and immune homeostasis
CPX-351 encapsulates cytosine arabinoside and daunorubicin in liposomes at a fixed molar ratio, and its use in patients with acute myeloid leukemia incurs less gastrointestinal toxicity than standard 7 + 3 regimens of these key antileukemia drugs. Renga and colleagues reveal through elegant experiments in mice that CPX-351 caused less disturbance of the gut microbiota and less fungal colonization than 7 + 3 and better maintained intestinal mucosa integrity. The liposomal membrane of CPX-351 activated an aryl hydrocarbon receptor–mediated pathway in the gut mucosa and induced production of immunomodulatory metabolites by anaerobic bacteria, resulting in the gut protective effect.
THROMBOSIS AND HEMOSTASIS
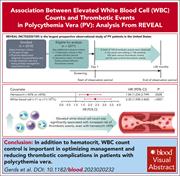
Association between elevated white blood cell counts and thrombotic events in polycythemia vera: analysis from REVEAL
Clinical Trials & Observations
Thrombosis risk stratification is the basis for treatment selection in patients with polycythemia vera (PV). In the prospective REVEAL observational study of 2510 patients, Gerds and colleagues identified persistent leukocytosis as a risk factor for both arterial and venous thrombosis in both high- and low-risk patients and in patients whose hematocrit is adequately controlled. These data support the inclusion of leukocytosis in the definition of high-risk PV and suggest that normalization of leukocytosis should be an objective of treatment in patients receiving cytoreduction.
TRANSPLANTATION
Regulatory T cells suppress myeloma-specific immunity during autologous stem cell mobilization and transplantation
Autograft remains a key component of management of fit patients diagnosed with multiple myeloma, in part because it establishes an antimyeloma immune response. Takahashi et al explored how this emerges, showing that during stem cell mobilization, both effector CD8 T cells necessary for the antimyeloma response and immunosuppressive regulatory T cells (Tregs), which dampen the response, are collected in high numbers. In murine models, manipulation of the graft to reduce Tregs and expand effector CD8 T cells improved the antitumor effect of autografts, setting the scene for an ongoing phase 1 study in patients.
LETTER TO BLOOD
BLOOD WORK
ERRATUM
CONTINUING MEDICAL EDUCATION (CME) QUESTIONS
-
Cover Image
Cover Image
![issue cover]()
Immunofluorescent staining shows excessive γH2Ax DNA damage foci in hematopoietic stem and progenitor cells from β-catenin- and Hoxa9-coinactivated mouse bone marrow, where these 2 proteins cooperate to sustain transcriptional programs for DNA replication and damage responses. See the article by Lynch et al on page 1586.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals