Activated protein C (APC) was shown to release extracellular vesicles (EVs). APC bound to the EVs was thought to be responsible for cytoprotection. Our study demonstrates that the cytoprotective effects of APC-released EVs are independent of APC. APC-released EVs carry anti-inflammatory microRNAs in their cargo.
TO THE EDITOR:
Activated protein C (APC), upon binding to endothelial protein C receptor (EPCR), exerts cytoprotective responses in endothelial and other cell types via activating protease-activated receptor-1 (PAR1).1 Previous studies showed that APC triggers the release of extracellular vesicles (EVs) from endothelial cells via EPCR-mediated PAR1 activation.2 APC-released EVs were found to contain significant amounts of APC,2 and the action of APC bound to EVs was thought to be responsible for cytoprotective effects associated with APC-released EVs in the recipient cells.3 Recent studies from our laboratory showed that factor VIIa (FVIIa) also promotes the release of EVs from endothelial cells via EPCR–dependent PAR1 activation.4 Interestingly, unlike significant amounts of APC association with APC-released EVs, very little FVIIa was associated with FVIIa-released EVs.4 FVIIa-released EVs were shown to confer anti-inflammatory and vascular protective effects through the transfer of microRNA 10a (miR10a) from its cargo to recipient cells.5,6 Although both APC and FVIIa induce cytoprotective responses and release endothelial EVs via PAR1-mediated cell signaling, there are distinctive differences between these proteases in how they activate PAR1 (eg, cleavage of PAR1 at R41 vs R46) and in the recruitment of intracellular signaling proteins (eg, β-arrestin-1 vs β-arrestin-2).7 These differences could lead to differential expression of various genes, miRs, and lipids, and determines EV’s cargo. The composition of EVs’ cargo would define EVs’ function and their therapeutic potential. In this study, we aimed to investigate whether APC-released EVs could exert cytoprotective effects independent of APC bound to EVs, and whether they carry anti-inflammatory miRs in their cargo.
Human umbilical vein endothelial cells were treated with a control vehicle or APC, EVs released into the supernatant serum-free medium were isolated by differential centrifugation, quantified by nanoparticle tracking analysis, and EVs’ function was evaluated in cytoprotecting assays, as previously described 4,5 and considering MISEV2018 guidelines.8 APC treatment markedly increased the release of EVs from endothelial cells (Figure 1A). Nanoparticle tracking analysis showed that the size of APC-released EVs was ⁓200 nm (215 ± 5 nm), like that of EVs isolated from cells treated with a control vehicle (control EVs, 203 ± 15 nm). Immunoblot analysis showed presence of transmembrane proteins associated with plasma membrane/endosomes (CD63, EPCR, and CD31) and cytosolic protein recovered in EVs (HPS70 and glyceraldehyde-3-phosphate dehydrogenase) but not protein associated with intracellular compartments (calnexin) in both control- and APC-released EVs but enriched in APC-released EVs (Figure 1B). As reported previously,2 significant amounts of APC were associated with APC-released EVs (Figure 1C). It is unclear at present the reason for the striking difference between APC and FVIIa for their ability to associate endothelial EVs because both APC and FVIIa bind EPCR with a similar affinity (⁓40 nM) on endothelial cells.9
APC-released EVs promote anti-inflammation and endothelial barrier protection. Primary cultures of human umbilical vein endothelial cells (HUVEC, passages between 3 to 6) were grown to confluency in endothelial basal growth medium (EBM-2; Lonza) in 6-well culture plates. When cells reached confluency, they were washed once with a serum-free EBM-2 medium (lacks growth factors and serum) before they were subjected to experimental treatments. (A) APC increases EV release from endothelial cells. HUVECs grown in 6-well culture plates were treated with a control vehicle (Control) or APC (100 nM) in 1 ml of serum-free EBM-2 medium for 8 hours. EVs were isolated from the culture supernatant media, as described previously.4 Briefly, the supernatant media was centrifugated at 2500g for 10 minutes at 4°C to sediment cells and cell debris. The pellet was suspended in 1 mL Hanks' balanced salt solution (HBSS) and centrifugated at 21 000g for 60 minutes at 4°C. The supernatant was discarded, and the pellet was resuspended in 1 mL of HBSS and the EV suspension was recentrifugated for 60 minutes at 4°C. The aforementioned step was repeated once more, and the final EV pellet was resuspended in 1 mL HBSS. EV count was quantified by nanoparticle tracking analysis (NTA) by Nano Sight NS300 (Malvern Panalytical). (B) Protein content–based EV characterization. Based on MISEV 2018 guidelines,8 EV nature and the degree of purity of EV preparation was analyzed by the presence of transmembrane proteins associated to plasma membrane (CD63, CD31, and EPCR), cytosolic proteins recovered in EVs (HSP70 and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), potential components of non-EV coisolated proteins (albumin), or soluble proteins associated with intracellular compartments other than plasma membrane/endosomes (calnexin) by immunoblot analysis. EVs isolated from an equal volume of conditioned media of endothelial cells treated with a control vehicle (control) or APC were used for the immunoblot analysis. Cell extracts of the same treatments were used as controls. (C) Levels of APC associated with the EVs. APC levels associated with control vehicle- or APC-released EVs were measured by chromogenic assay. (D-E) APC-released EVs confer anti-inflammatory phenotype ro recipient cells. THP-1 cells were incubated with a control vehicle (Control) or EVs derived from HUVECs treated with a control vehicle (Con EVs) or APC (APC EVs) for 4 hours in equal numbers (2 × 108; cells-to-EV ratio, 1:100). After the cells were washed to remove the free EVs, they were challenged with LPS (200 ng/mL). After 12 hours, the levels of proinflammatory cytokines, tumor necrosis factor α (TNF-α) (D) and interleukin-6 (IL-6) (E), in the supernatant medium were determined by enzyme-linked immunosorbent assay. (F) APC-released EVs protect aganist endothelial barrier disruption. HUVECs grown to confluence in transwells were incubated with a control vehicle (Control) or EVs (2 × 108) released from HUVECs treated with control vehicle (Con EVs) or APC (APC EVs) for 4 hours. After 4 hours, the monolayer was washed twice and challenged with LPS (200 ng/mL). Barrier permeability was measured 12 hours after the addition of LPS by endothelial barrier permeability in vitro assay using Evans blue–labeled bovine serum albumin. The barrier permeability (optical denisty readings) observed in cells treated with LPS that were not exposed to EVs were taken as 100%. (G-I) Cytoprotective effects of APC-EVs are independent of EVs’ bound APC. THP-1 cells or naïve HUVECs were preincubated with EPCR-blocking antibody (EPCR B-Ab; JRK1494; 100 μg/mL) or nonblocking control antibody (EPCR NB-Ab; JRK1500; 100 μg/mL) for 1 hour before the addition of control EVs or APC-EVs. In a subset, EVs were preincubated with anti-APC polyclonal antibodies (APC B-Ab; 100 μg/mL) or control immunoglobulin G (IgG; 100 μg/mL) for 1 hour followed by the fusion with recipient THP-1 cells and naïve HUVECs. EVs-fused recipient cells were challenged with LPS, and the release of TNF-α (G) and IL-6 (H) from THP-1 was measured by enzyme-linked immunosorbent assay, and barrier permeability (I) was determined in HUVECs as described earlier. (J-M) In vivo cytoprotective effects of APC-released EVs. EVs were isolated from the supernatant medium of bEND.3 cells that were treated with a control vehicle or APC and an equal number of EVs (2 × 108) were administered to wild-type mice (C57BL/6J, between 8 and 12 weeks old, male and female equally distributed) via the tail vein. In additional experimental groups, mice were administered with APC-EVs that were preincubated with polyclonal antibodies against APC (APC B-Ab; 100 μg/mL) or control IgG (100 μg/mL) for 1 hour. Four hours later, mice were given an intraperitoneal injection of LPS (5 mg/kg). Twelve hours after the administration of LPS, blood was obtained from the mice, and the levels of TNF-α (J) and IL-6 (K) in the plasma were measured. In a subset of the same group of mice, vascular leakage into the heart (L) and liver (M) was evaluated, as described in our earlier study.5 All aforementioned in vitro experiments were repeated independently at least 3 times. Six to 8 mice per group were used for in vivo studies. Data are shown as mean ± standard error of the mean. Statistical significance among multiple groups was analyzed by a 1-way analysis of variance followed by the Tukey post hoc test. Statistical significance between the 2 groups was calculated by using the Mann-Whitney U test; ∗P < .05; ∗∗∗P < .001; and ∗∗∗∗P < .0001. ns, not statistically significant.
APC-released EVs promote anti-inflammation and endothelial barrier protection. Primary cultures of human umbilical vein endothelial cells (HUVEC, passages between 3 to 6) were grown to confluency in endothelial basal growth medium (EBM-2; Lonza) in 6-well culture plates. When cells reached confluency, they were washed once with a serum-free EBM-2 medium (lacks growth factors and serum) before they were subjected to experimental treatments. (A) APC increases EV release from endothelial cells. HUVECs grown in 6-well culture plates were treated with a control vehicle (Control) or APC (100 nM) in 1 ml of serum-free EBM-2 medium for 8 hours. EVs were isolated from the culture supernatant media, as described previously.4 Briefly, the supernatant media was centrifugated at 2500g for 10 minutes at 4°C to sediment cells and cell debris. The pellet was suspended in 1 mL Hanks' balanced salt solution (HBSS) and centrifugated at 21 000g for 60 minutes at 4°C. The supernatant was discarded, and the pellet was resuspended in 1 mL of HBSS and the EV suspension was recentrifugated for 60 minutes at 4°C. The aforementioned step was repeated once more, and the final EV pellet was resuspended in 1 mL HBSS. EV count was quantified by nanoparticle tracking analysis (NTA) by Nano Sight NS300 (Malvern Panalytical). (B) Protein content–based EV characterization. Based on MISEV 2018 guidelines,8 EV nature and the degree of purity of EV preparation was analyzed by the presence of transmembrane proteins associated to plasma membrane (CD63, CD31, and EPCR), cytosolic proteins recovered in EVs (HSP70 and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), potential components of non-EV coisolated proteins (albumin), or soluble proteins associated with intracellular compartments other than plasma membrane/endosomes (calnexin) by immunoblot analysis. EVs isolated from an equal volume of conditioned media of endothelial cells treated with a control vehicle (control) or APC were used for the immunoblot analysis. Cell extracts of the same treatments were used as controls. (C) Levels of APC associated with the EVs. APC levels associated with control vehicle- or APC-released EVs were measured by chromogenic assay. (D-E) APC-released EVs confer anti-inflammatory phenotype ro recipient cells. THP-1 cells were incubated with a control vehicle (Control) or EVs derived from HUVECs treated with a control vehicle (Con EVs) or APC (APC EVs) for 4 hours in equal numbers (2 × 108; cells-to-EV ratio, 1:100). After the cells were washed to remove the free EVs, they were challenged with LPS (200 ng/mL). After 12 hours, the levels of proinflammatory cytokines, tumor necrosis factor α (TNF-α) (D) and interleukin-6 (IL-6) (E), in the supernatant medium were determined by enzyme-linked immunosorbent assay. (F) APC-released EVs protect aganist endothelial barrier disruption. HUVECs grown to confluence in transwells were incubated with a control vehicle (Control) or EVs (2 × 108) released from HUVECs treated with control vehicle (Con EVs) or APC (APC EVs) for 4 hours. After 4 hours, the monolayer was washed twice and challenged with LPS (200 ng/mL). Barrier permeability was measured 12 hours after the addition of LPS by endothelial barrier permeability in vitro assay using Evans blue–labeled bovine serum albumin. The barrier permeability (optical denisty readings) observed in cells treated with LPS that were not exposed to EVs were taken as 100%. (G-I) Cytoprotective effects of APC-EVs are independent of EVs’ bound APC. THP-1 cells or naïve HUVECs were preincubated with EPCR-blocking antibody (EPCR B-Ab; JRK1494; 100 μg/mL) or nonblocking control antibody (EPCR NB-Ab; JRK1500; 100 μg/mL) for 1 hour before the addition of control EVs or APC-EVs. In a subset, EVs were preincubated with anti-APC polyclonal antibodies (APC B-Ab; 100 μg/mL) or control immunoglobulin G (IgG; 100 μg/mL) for 1 hour followed by the fusion with recipient THP-1 cells and naïve HUVECs. EVs-fused recipient cells were challenged with LPS, and the release of TNF-α (G) and IL-6 (H) from THP-1 was measured by enzyme-linked immunosorbent assay, and barrier permeability (I) was determined in HUVECs as described earlier. (J-M) In vivo cytoprotective effects of APC-released EVs. EVs were isolated from the supernatant medium of bEND.3 cells that were treated with a control vehicle or APC and an equal number of EVs (2 × 108) were administered to wild-type mice (C57BL/6J, between 8 and 12 weeks old, male and female equally distributed) via the tail vein. In additional experimental groups, mice were administered with APC-EVs that were preincubated with polyclonal antibodies against APC (APC B-Ab; 100 μg/mL) or control IgG (100 μg/mL) for 1 hour. Four hours later, mice were given an intraperitoneal injection of LPS (5 mg/kg). Twelve hours after the administration of LPS, blood was obtained from the mice, and the levels of TNF-α (J) and IL-6 (K) in the plasma were measured. In a subset of the same group of mice, vascular leakage into the heart (L) and liver (M) was evaluated, as described in our earlier study.5 All aforementioned in vitro experiments were repeated independently at least 3 times. Six to 8 mice per group were used for in vivo studies. Data are shown as mean ± standard error of the mean. Statistical significance among multiple groups was analyzed by a 1-way analysis of variance followed by the Tukey post hoc test. Statistical significance between the 2 groups was calculated by using the Mann-Whitney U test; ∗P < .05; ∗∗∗P < .001; and ∗∗∗∗P < .0001. ns, not statistically significant.
APC-released EVs markedly suppressed lipopolysaccharide (LPS)-induced enhanced expression of proinflammatory cytokines in monocytic THP-1 cells (Figure 1D-E) and endothelial barrier disruption (Figure 1F), whereas control EVs had no significant effect. Because an earlier study suggested that APC bound to the EVs was responsible for the APC-released EV-mediated cytoprotective effects in target cells through EPCR-dependent activation of PAR1,3 we reexamined this possibility carefully. APC-released EVs were pretreated with APC proteolytic activity-neutralizing polyclonal antibodies or recipient cells were preincubated with EPCR-blocking monoclonal antibodies. Neither APC-neutralizing antibodies nor EPCR-blocking antibodies failed to attenuate the anti-inflammatory or barrier-protective effects of APC-released EVs (Figure 1G-I). When APC was used as a positive control, APC’s cytoprotective effects were completely prevented upon the pretreatment of APC with APC antibodies or blocking APC binding to EPCR by EPCR-blocking antibodies (Figure 1G-I). We observed similar cytoprotective effects with APC-released EVs isolated by size exclusion chromatography (IZON qEV2 35 nm; Izon Science; optimal recovery range, 35 to 350 nm; data not shown).
In in vivo studies, administration of APC-released EVs from murine endothelial cells to wild-type mice (C57BL/6J) significantly reduced LPS-induced increase in plasma proinflammatory cytokines (Figure 1J-K) and vascular leakage into vital tissues (Figure 1L-M), as analyzed in our recent study.5 Control EVs did not significantly affect LPS-induced elaboration of inflammatory cytokines or barrier disruption in mice. Neutralization of bound APC activity by preincubating EVs with APC antibodies did not affect the cytoprotective effects of APC-released EVs (Figure 1J-M). The aforementioned findings strongly suggest that the components of APC-released EVs rather than the EV-bound APC mediate the cytoprotective effects.
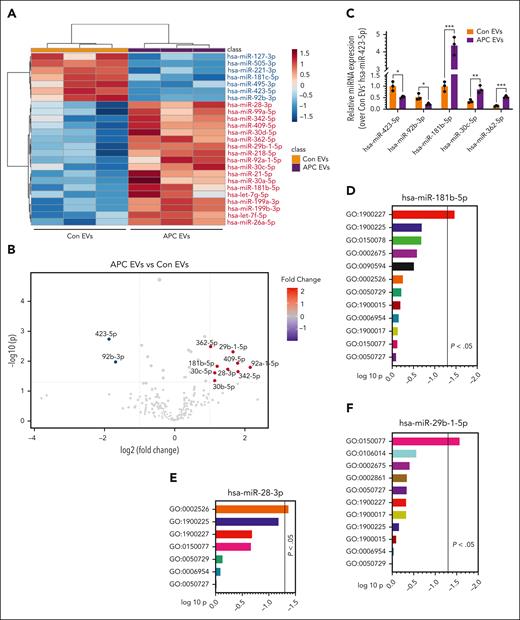
EVs are well-known for carrying miRs between the cells,10,11 which often play a crucial role in regulating inflammation12 and vascular permeability.13 Therefore, in further studies, we subjected both control- and APC-released EVs for miR analysis by deep sequencing to obtain clues on the potential driving factors behind APC-released EVs' anti-inflammatory and barrier-protective responses. The sequencing data identified 234 different miRs in control EVs and APC-released EVs, including 42 differentially expressed miRs in APC-released EVs compared with control EVs (Figure 2A). Among them, 9 were significantly upregulated by twofold or more, whereas 2 were significantly downregulated by twofold or more (Figure 2B). The upregulated miRs were miR-181b-5p, miR-28-3p, miR-29b-1-5p, miR-362-5p, miR-409-5p, miR-92a-1-5p, miR-30c-5p, miR-342-5p, and miR-30b-5p; the downregulated miRs were miR-423-5p and miR-92b-3p. Among the upregulated miRs, except miR-92a-1-5p, all others appear to be anti-inflammatory,14-21 miR-181b-5p being the most abundant among them. miR-92a-1-5p was shown to be associated with tumorigenesis.22 Both the downregulated miRs, miR-423-5p and miR-92b-3p, were shown to be proinflammatory.23-25 We validated the expression of select miRs that were shown to be either upregulated or downregulated in the APC-released EVs as compared with control EVs in omics by real-time polymerase chain reaction (Figure 2C). It is interesting to note that none of the miRs that were differentially expressed in FVIIa-released EVs in our earlier study5 were found to be differentially expressed in the APC-released EVs.
APC-released EVs are enriched with anti-inflammatory miRs. EVs isolated from HUVECs after treatment with a control vehicle or APC (100 nM) for 8 hours were quantified by NTA, and an equal number of EVs (1 × 109) were subjected to miR analysis by deep sequencing (UT Southwestern Core). (A) From the sequencing data, the top 25 most abundant and differentially expressed miRs are shown in the heat map. (B) Group comparison of scaled expression data set using nonparametric test visualized by volcano plot showing differentially expressed miRs between the APC-released EVs and control EVs with an adjusted P value < .05 and fold change of >2.0. (C) Validation of differentially expressed miRs between control EVs and APC-released EVs identified by deep sequencing. EVs isolated from HUVECs after challenging with a control vehicle or APC were subjected to miR isolation by mirVana miRNA isolation kit. The relative abundance of hsa-miR-423-5p, hsa-miR-92b-3p, hsa-miR-181b-5p, hsa-miR-30c-5p, and hsa-miR-362-5p were validated by quantitative real-time polymerase chain reaction. (D-F) GO enrichment analysis of inflammation-related target genes of differentially upregulated miRs such as hsa-miR-181b-5p (D), hsa-miR-28-3p (E), and hsa-miR-29b-1-5p (F) influencing various proinflammatory pathways (denoted in figures as “GO” identification number) (GO:1900227, Positive Regulation of NLRP3 Inflammasome Complex Assembly; GO:1900225, Regulation of NLRP3 Inflammasome Complex Assembly; GO:0150078, Positive Regulation of Neuroinflammatory Response; GO:0002675, Positive Regulation of Acute Inflammatory Response; GO:0090594, Inflammatory Response to Wounding; GO:0002526, Acute Inflammatory Response; GO:0050729, Positive Regulation of Inflammatory Response; GO:1900015, Regulation of Cytokine Production Involved in Inflammatory Response; GO:0006954, Inflammatory Response; GO:1900017, Positive Regulation of Cytokine Production Involved in Inflammatory Response; GO:0150077, Regulation of Neuroinflammatory Response; GO:0050727, Regulation of Inflammatory Response; GO:0106014, Regulation of Inflammatory Response to Wounding; and GO:0002861, Regulation of Inflammatory Response to Antigenic Stimulus). ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
APC-released EVs are enriched with anti-inflammatory miRs. EVs isolated from HUVECs after treatment with a control vehicle or APC (100 nM) for 8 hours were quantified by NTA, and an equal number of EVs (1 × 109) were subjected to miR analysis by deep sequencing (UT Southwestern Core). (A) From the sequencing data, the top 25 most abundant and differentially expressed miRs are shown in the heat map. (B) Group comparison of scaled expression data set using nonparametric test visualized by volcano plot showing differentially expressed miRs between the APC-released EVs and control EVs with an adjusted P value < .05 and fold change of >2.0. (C) Validation of differentially expressed miRs between control EVs and APC-released EVs identified by deep sequencing. EVs isolated from HUVECs after challenging with a control vehicle or APC were subjected to miR isolation by mirVana miRNA isolation kit. The relative abundance of hsa-miR-423-5p, hsa-miR-92b-3p, hsa-miR-181b-5p, hsa-miR-30c-5p, and hsa-miR-362-5p were validated by quantitative real-time polymerase chain reaction. (D-F) GO enrichment analysis of inflammation-related target genes of differentially upregulated miRs such as hsa-miR-181b-5p (D), hsa-miR-28-3p (E), and hsa-miR-29b-1-5p (F) influencing various proinflammatory pathways (denoted in figures as “GO” identification number) (GO:1900227, Positive Regulation of NLRP3 Inflammasome Complex Assembly; GO:1900225, Regulation of NLRP3 Inflammasome Complex Assembly; GO:0150078, Positive Regulation of Neuroinflammatory Response; GO:0002675, Positive Regulation of Acute Inflammatory Response; GO:0090594, Inflammatory Response to Wounding; GO:0002526, Acute Inflammatory Response; GO:0050729, Positive Regulation of Inflammatory Response; GO:1900015, Regulation of Cytokine Production Involved in Inflammatory Response; GO:0006954, Inflammatory Response; GO:1900017, Positive Regulation of Cytokine Production Involved in Inflammatory Response; GO:0150077, Regulation of Neuroinflammatory Response; GO:0050727, Regulation of Inflammatory Response; GO:0106014, Regulation of Inflammatory Response to Wounding; and GO:0002861, Regulation of Inflammatory Response to Antigenic Stimulus). ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Next, based on the target gene prediction through miRDB, a microRNA target prediction database, the potential inflammation-related target genes of miRs, which were differentially expressed in the APC-released EVs, were subjected to gene ontology (GO) enrichment analysis. The gene set enrichment analysis tool, Enirchr, was subsequently used to functionally annotate and score GO modules by performing overrepresentation analysis. Although both upregulated and downregulated miRs were found to be associated with influencing inflammatory pathways in the GO analysis, not all of them reached statistical significance. Among differentially upregulated miRs, miR-181b-5p, miR-28-3p, and miR-29b-1-5p were shown to influence various proinflammatory pathways, and at least 1 pathway reached statistical significance for each of the miRs (Figure 2D-F). Earlier studies suggested that the anti-inflammatory potential of miR-181b-5p, miR-28-3p, and miR-29b-1-5p. miR-181b-5p overexpression was shown to downregulate tumor necrosis factor α–induced expression of intercellular adhesion molecule 1 and vascular cell adhesion protein 1 in rat pulmonary arterial endothelial cells.14 miR-181b-5p was also shown to target importin-α3, thereby downregulating the NF-κB signaling pathway in endothelial cells.26 miR-28-3p was shown to attenuate circular RNA cerebellar degeneration–related protein 1 antisense RNA-induced apoptosis and inflammation in hippocampal neurons via targeting tumor necrosis factor receptor–associated factor 3.17 miR-29b-1-5p overexpression was shown to attenuate ischemia/reperfusion–induced proinflammation in a rat model via targeting metadherin.18 Our GO analysis data shown in Figure 2D-F suggest that miR-181b-5p targets the positive regulation of NLRP3 inflammasome complex assembly (GO:1900227); miR-28-3p targets the acute inflammatory response (GO:0002526); and miR-29b-1-5p targets the regulation of neuroinflammatory response. It is likely that 1 or more of the miRs in APC-released EVs, either individually or in concert with other miRs, could be responsible for the cytoprotective effects of APC-released EVs.
In summary, our present data show that the cytoprotective effects of APC-released EVs were independent of APC bound to the EVs, and that APC-released EVs carry distinct anti-inflammatory miRNAs in their cargo. Identification of the specific miRs behind APC-EVs’ cytoprotective responses will require additional studies, which may open a new therapeutic window in the treatment of inflammation-associated disorders.
Acknowledgments
The authors thank Charles Esmon, Oklahoma Medical Research Foundation, for providing antibodies against endothelial protein C receptor and activated protein C. The authors acknowledge the contribution of UT Southwestern Core facilities in performing deep sequencing.
This work was supported, partly, by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (HL124055 and HL169255) and endowment funds from The Dr. and Mrs. James Vaughn Professorship in Biomedical Research (L.V.M.R.). K.D. received the Judith Graham Pool Postdoctoral Fellowship Award from the National Hemophilia Foundation.
Authorship
Contribution: K.D. conducted the in vitro studies and analyzed the data; S.K. performed the animal studies; T.M. performed the miR sequencing analysis and prepared the data; L.V.M.R. conceived, designed the research, and wrote the manuscript; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, Department of Cellular and Molecular Biology, The University of Texas Health Science Center at Tyler, 11937 US Highway 271, Tyler, TX 75708; email: vijay.rao@uthct.edu.
References
Author notes
Data reported in this manuscript and the protocols used in the study will be provided to any qualified investigator upon email request to the corresponding author, L. Vijaya Mohan Rao (vijay.rao@uthct.edu), after consultation with the relevant author.


![APC-released EVs promote anti-inflammation and endothelial barrier protection. Primary cultures of human umbilical vein endothelial cells (HUVEC, passages between 3 to 6) were grown to confluency in endothelial basal growth medium (EBM-2; Lonza) in 6-well culture plates. When cells reached confluency, they were washed once with a serum-free EBM-2 medium (lacks growth factors and serum) before they were subjected to experimental treatments. (A) APC increases EV release from endothelial cells. HUVECs grown in 6-well culture plates were treated with a control vehicle (Control) or APC (100 nM) in 1 ml of serum-free EBM-2 medium for 8 hours. EVs were isolated from the culture supernatant media, as described previously.4 Briefly, the supernatant media was centrifugated at 2500g for 10 minutes at 4°C to sediment cells and cell debris. The pellet was suspended in 1 mL Hanks' balanced salt solution (HBSS) and centrifugated at 21 000g for 60 minutes at 4°C. The supernatant was discarded, and the pellet was resuspended in 1 mL of HBSS and the EV suspension was recentrifugated for 60 minutes at 4°C. The aforementioned step was repeated once more, and the final EV pellet was resuspended in 1 mL HBSS. EV count was quantified by nanoparticle tracking analysis (NTA) by Nano Sight NS300 (Malvern Panalytical). (B) Protein content–based EV characterization. Based on MISEV 2018 guidelines,8 EV nature and the degree of purity of EV preparation was analyzed by the presence of transmembrane proteins associated to plasma membrane (CD63, CD31, and EPCR), cytosolic proteins recovered in EVs (HSP70 and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]), potential components of non-EV coisolated proteins (albumin), or soluble proteins associated with intracellular compartments other than plasma membrane/endosomes (calnexin) by immunoblot analysis. EVs isolated from an equal volume of conditioned media of endothelial cells treated with a control vehicle (control) or APC were used for the immunoblot analysis. Cell extracts of the same treatments were used as controls. (C) Levels of APC associated with the EVs. APC levels associated with control vehicle- or APC-released EVs were measured by chromogenic assay. (D-E) APC-released EVs confer anti-inflammatory phenotype ro recipient cells. THP-1 cells were incubated with a control vehicle (Control) or EVs derived from HUVECs treated with a control vehicle (Con EVs) or APC (APC EVs) for 4 hours in equal numbers (2 × 108; cells-to-EV ratio, 1:100). After the cells were washed to remove the free EVs, they were challenged with LPS (200 ng/mL). After 12 hours, the levels of proinflammatory cytokines, tumor necrosis factor α (TNF-α) (D) and interleukin-6 (IL-6) (E), in the supernatant medium were determined by enzyme-linked immunosorbent assay. (F) APC-released EVs protect aganist endothelial barrier disruption. HUVECs grown to confluence in transwells were incubated with a control vehicle (Control) or EVs (2 × 108) released from HUVECs treated with control vehicle (Con EVs) or APC (APC EVs) for 4 hours. After 4 hours, the monolayer was washed twice and challenged with LPS (200 ng/mL). Barrier permeability was measured 12 hours after the addition of LPS by endothelial barrier permeability in vitro assay using Evans blue–labeled bovine serum albumin. The barrier permeability (optical denisty readings) observed in cells treated with LPS that were not exposed to EVs were taken as 100%. (G-I) Cytoprotective effects of APC-EVs are independent of EVs’ bound APC. THP-1 cells or naïve HUVECs were preincubated with EPCR-blocking antibody (EPCR B-Ab; JRK1494; 100 μg/mL) or nonblocking control antibody (EPCR NB-Ab; JRK1500; 100 μg/mL) for 1 hour before the addition of control EVs or APC-EVs. In a subset, EVs were preincubated with anti-APC polyclonal antibodies (APC B-Ab; 100 μg/mL) or control immunoglobulin G (IgG; 100 μg/mL) for 1 hour followed by the fusion with recipient THP-1 cells and naïve HUVECs. EVs-fused recipient cells were challenged with LPS, and the release of TNF-α (G) and IL-6 (H) from THP-1 was measured by enzyme-linked immunosorbent assay, and barrier permeability (I) was determined in HUVECs as described earlier. (J-M) In vivo cytoprotective effects of APC-released EVs. EVs were isolated from the supernatant medium of bEND.3 cells that were treated with a control vehicle or APC and an equal number of EVs (2 × 108) were administered to wild-type mice (C57BL/6J, between 8 and 12 weeks old, male and female equally distributed) via the tail vein. In additional experimental groups, mice were administered with APC-EVs that were preincubated with polyclonal antibodies against APC (APC B-Ab; 100 μg/mL) or control IgG (100 μg/mL) for 1 hour. Four hours later, mice were given an intraperitoneal injection of LPS (5 mg/kg). Twelve hours after the administration of LPS, blood was obtained from the mice, and the levels of TNF-α (J) and IL-6 (K) in the plasma were measured. In a subset of the same group of mice, vascular leakage into the heart (L) and liver (M) was evaluated, as described in our earlier study.5 All aforementioned in vitro experiments were repeated independently at least 3 times. Six to 8 mice per group were used for in vivo studies. Data are shown as mean ± standard error of the mean. Statistical significance among multiple groups was analyzed by a 1-way analysis of variance followed by the Tukey post hoc test. Statistical significance between the 2 groups was calculated by using the Mann-Whitney U test; ∗P < .05; ∗∗∗P < .001; and ∗∗∗∗P < .0001. ns, not statistically significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/16/10.1182_blood.2023023518/2/m_blood_bld-2023-023518-gr1b.jpeg?Expires=1765901233&Signature=xAhYH~my4BMs7kUpq48MwUvNBSYOGE3KHeaukidCIrq5TIqiM8fLafq7UCg9lpI1DrlW2m-GcbXd5IMWse9cZNSI9zg28QgAhmuG959cCOikVJNqcG1rQT-Os3WCu9jNruP9pYtd177PBghIVJTbHhAMsIjrPQIkzLplDDPNOGYoLZ1w4iNeNXo5Ed8DRXKtXfB7W8GgB5YvAkbS4iACEqRvkQn0SOBao41N6m~MlABt9g~lVKX1Nu~TNYxbZxJIiWyA8MJLpJ~h1C-jUQKNc~r40hNclZJgEQ1SqfuwdBxWPeKeRuYGSQquncHii4R-7m81whQ34jx9M6YaqVPu9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal