In this issue of Blood, Lynch et al1 reveal that HOXA9 and β-catenin act in concert to maintain hematopoietic stem cells (HSCs) and preserve their multilineage differentiation potential. They found that in HSCs HOXA9 and β-catenin collaborate to promote quiescence and safeguard DNA replication and repair. Loss of HOXA9 or β-catenin in HSCs is permissive due to redundancy, but their codeletion causes hematopoietic dysregulation. Mechanistically, HOXA9 and β-catenin converge to regulate the expression of overlapping target genes, including PRMT1, which is critical for HSC maintenance.
Multilineage hematopoiesis is sustained by self-renewing HSCs and a complex dynamic hierarchy of progenitor cells that give rise to mature blood lineages.2-4 To preserve their integrity under steady-state conditions, HSCs remain quiescent with low metabolic activity.3,4 However, HSCs are required to temporarily exit quiescence to replenish progenitor pools and thereby maintain hematopoietic homeostasis. Moreover, following stress, such as blood loss, infection, or inflammation, HSCs are acutely activated and undergo numerous divisions to sustain hematopoietic regeneration, before returning to the quiescent state.3,4 Given that excessive divisions can cause accumulating DNA damage and result in HSC exhaustion or failure5 and predispose hematopoiesis to leukemogenesis, it is essential that HSCs efficiently safeguard their genomic integrity and activation. Thus, HSCs require precise mechanisms promoting quiescence, and once activated, HSCs must ensure error-free DNA replication. However, mechanisms that coordinate these processes remain elusive.
To address this, Lynch et al focused on HOXA9 and β-catenin (encoded by Ctnnb1), key transcriptional regulators that promote leukemogenesis.6,7 Individual deletion of Hoxa9 or Ctnnb1 has little impact on normal HSCs or their quiescence and DNA replication. Notably, Hoxa9-deficeint HSCs upregulate canonical Wnt/β-catenin signaling, while HSCs lacking Ctnnb1 display activation of HOXA9-mediated transcriptional program, suggesting functional redundancy between HOXA9 and β-catenin. Indeed, deletion of both Hoxa9 and Ctnnb1 in HSCs results in loss of quiescence, defective DNA replication dynamics, DNA damage, and consequent HSC depletion. Thus, Lynch et al reveal an unexpected cross talk between HOXA9 and β-catenin, whereby they function redundantly to maintain HSC dormancy and DNA replication and repair.
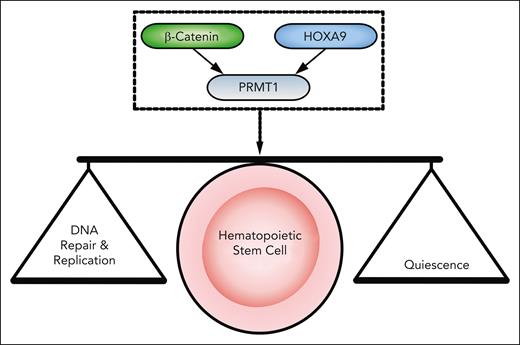
Unraveling mechanisms of HOXA9 and β-catenin function may reveal how HSCs seamlessly switch between quiescent and active states, while ensuring faithful DNA replication and integrity. Lynch et al discovered that HOXA9 and β-catenin co-occupy a subset of loci to regulate transcription of genes required for HSC integrity. Indeed, both HOXA9 and β-catenin are present at the transcription start site of Prmt1 (encoding protein arginine methyltransferase 1 [PRMT1]). Concordantly, combined HOXA9/β-catenin deficiency results in reduced chromatin accessibility at the Prmt1 promoter, resulting in defective Prmt1 expression and dysregulation of arginine histone methylation. Strikingly, Prmt1 deletion phenocopied loss of HOXA9/β-catenin with HSC defects, perturbations in DNA replication dynamics, and transcriptional dysregulation. Furthermore, ectopic PRMT1 expression in HOXA9/β-catenin-deficient HSCs partially restored phenotypic and molecular defects. Therefore, HOXA9 and β-catenin function together to control expression of Prmt1, which protects HSC quiescence by controlling the expression of genes that promote error-free DNA replication during HSC division, thus ensuring HSC integrity (see figure). Finally, given that HOXA9/β-catenin and PRMT1 share some overlapping target genes involved in DNA repair and replication, it possible that PRMT1 reinforces HOXA9/β-catenin-driven transcription to promote DNA replication fork stability and HSC integrity.
The β-Catenin/HOXA9→PRMT1 axis regulates quiescence as well as DNA replication and repair in HSCs. β-catenin and HOXA9 act together to regulate Prmt1 expression. This axis is required to maintain quiescence, expression of DNA repair, and initiation factors in HSCs, thus sustaining HSC integrity.
The β-Catenin/HOXA9→PRMT1 axis regulates quiescence as well as DNA replication and repair in HSCs. β-catenin and HOXA9 act together to regulate Prmt1 expression. This axis is required to maintain quiescence, expression of DNA repair, and initiation factors in HSCs, thus sustaining HSC integrity.
The discovery of the HOXA9/β-catenin→PRMT1 axis represents a significant step toward revealing regulatory mechanisms that control HSCs transitions between dormant and active states. This raises multiple questions. How is this axis orchestrated in HSCs to allow transitions? Do HSCs require transient repression of HOXA9/β-catenin-mediated transcription to exit quiescence and subsequent reactivation upon the entry into S phase to ensure efficient error-free DNA replication? How is this pathway restrained to avoid clonal expansion or transformation? Considering that both HOXA9 and β-catenin are essential for transcriptional activity at specific loci such as Prmt1, what mechanisms ensure the simultaneous presence of these factors on designated loci to regulate gene transcription? How are HOXA9/β-catenin orchestrated during quiescence, cycling, and transition between these states? Addressing these questions will significantly enhance our understanding of how quiescence and DNA replication are coordinated under physiological conditions and following injury in HSCs.
Particularly intriguing is that the HOXA9/β-catenin→PRMT1 axis exhibits a dual role, driving quiescence while promoting DNA replication and repair. Theoretically, DNA replication and repair pathways are required to a greater extent during division than quiescence. Therefore, does the HOXA9/β-catenin→PRMT1 axis maintain quiescence while also priming HSCs with factors required for cell division?
From a translational perspective, HOXA9 and β-catenin are key driver genes in a number of hematopoietic malignancies associated with poor prognosis but have demonstrated poor druggability. PRMT1 has recently emerged as a potential therapeutic target in various cancers, including leukemia,8,9 and clinical trials have attempted to inhibit PRMT1 for therapeutic purposes.10 Considering that Prmt1 deletion in mice causes HSC and progenitor cell depletion, it is of immense importance to validate these findings in human hematopoiesis and identify a therapeutic window in which PRMT1 inactivation compromises cancer without causing significant damage to normal hematopoiesis.
Together, Lynch et al introduce an exciting layer of complexity to our understanding of HOXA9 and β-catenin in HSCs, revealing how their axis with PRMT1 intricately regulates HSC quiescence and DNA integrity upon HSC division.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal