Issue Archive
Table of Contents
BLOOD COMMENTARIES
REVIEW ARTICLE
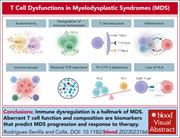
T-cell dysfunctions in myelodysplastic syndromes
In a very timely review, Rodriguez-Sevilla and Colla summarize what we know about T-cell dysfunction in myelodysplastic syndromes and how it contributes to pathogenesis, progression, and treatment failure. The authors also discuss emerging opportunities for T-cell–based therapies, including immune checkpoint inhibitors, chimeric antigen receptor T cells, and bispecific antibodies.
HOW I TREAT
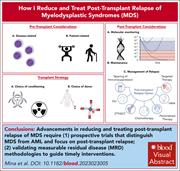
How I reduce and treat posttransplant relapse of MDS
Posttransplant relapse is the major cause of failure when patients undergo allogeneic stem cell transplant as a cure for myelodysplastic syndromes (MDS). Mina and colleagues used 4 clinical cases to exemplify how their practice approach is designed to minimize relapse. The authors complement this with a concise summary of current evidence and identify areas for future research.
CLINICAL TRIALS AND OBSERVATIONS
Emicizumab prophylaxis in infants with hemophilia A (HAVEN 7): primary analysis of a phase 3b open-label trial
Clinical Trials & Observations
Intravenous prophylaxis with factor VIII (FVIII) for infants under 1 year of age with severe hemophilia can be logistically challenging. Emicizumab is given subcutaneously and acts as an immunoglobulin G molecular bridge between activated FIX and FX, thereby substituting for the important role of FVIII. Pipe and colleagues report on the efficacy and safety of emicizumab in 55 infants in a single-arm study, finding high compliance and low bleeding rates with no spontaneous bleeds and no immunogenicity. These data indicate the potential of emicizumab prophylaxis initiated very early in life to reduce the morbidity of severe hemophilia.
HEMATOPOIESIS AND STEM CELLS
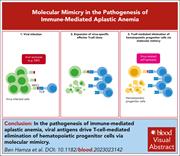
Virus-reactive T cells expanded in aplastic anemia eliminate hematopoietic progenitor cells by molecular mimicry
Acquired aplastic anemia (AA) is recognized as an immune-mediated condition in most patients, but the pathogenic mechanisms remain elusive. Ben Hamza et al demonstrate that T cells from patients with acquired AA target viral antigens which can mimic epitopes presented on hematopoietic stem and progenitor cells (HSPCs). Expansion of these T-cell clones drives AA by elimination of HSPCs. These elegant primary cell data provide direct evidence as to how AA is triggered and why HSPCs are targeted.
LYMPHOID NEOPLASIA
Developmental trajectories and cooperating genomic events define molecular subtypes of BCR::ABL1-positive ALL
Clinical Trials & Observations
Brief Report
Since it was first recognized as an entity, BCR::ABL1-positive acute lymphoblastic leukemia (ALL) has been considered and managed as a homogeneous entity. Bastian and colleagues now report that BCR::ABL1-positive ALL is more heterogeneous than previously thought. Subtypes with distinct transcriptomic and genomic profiles can be identified and have distinct clinical phenotypes.
MYELOID NEOPLASIA
First-hit SETBP1 mutations cause a myeloproliferative disorder with bone marrow fibrosis
SETBP1 is a transcription factor that is mutated in various myeloid neoplasms. Crespiatico and colleagues used a murine model and patient samples to show that SETBP1 mutations act as an early event to drive an aggressive myelofibrosis occurring in the absence of JAK2, MPL, or CALR mutations (so-called triple-negative myelofibrosis). The study provides novel insights into how early mutations can highjack hematopoiesis to drive disease phenotype while at the same time providing a biomarker for some cases of triple-negative myelofibrosis.
THROMBOSIS AND HEMOSTASIS
Type 1 VWD classification revisited: novel insights from combined analysis of the LoVIC and WiN studies
von Willebrand disease (VWD) is the most common inherited bleeding disorder, but diagnosis and accurate subtyping of VWD are complex and have been controversial. Atiq et al analyzed data from 2 large cohort studies to illuminate the natural history of patients with reduced (30-50 IU/dL) von Willebrand factor (VWF) who did not meet the strict definition of type 1 VWD (VWF levels <30 IU/dL). The authors’ data support the hypothesis that low VWF is an age-dependent evolution of type 1 VWD rather than its own discrete clinical entity. This finding reinforces the general principle that bleeding phenotype should be the key determinant of management decisions in patients with VWD.
LETTER TO BLOOD
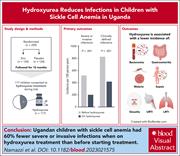
Hydroxyurea reduces infections in children with sickle cell anemia in Uganda
Clinical Trials & Observations
Data from rigorously conducted randomized trials can be repurposed to answer secondary questions. Namazzi and colleagues reanalyzed data from the negative zinc for infection prevention in a sickle cell anemia (SCA) trial conducted in Uganda to address whether hydroxyurea use reduced infections. The authors found that introducing hydroxyurea treatment for SCA was associated with a >60% reduction in severe infections, including malaria and those requiring hospitalization. This report provides definitive evidence that hydroxyurea therapy for SCA also reduces infections.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Reticulin stain of a representative bone marrow section from a patient with triple-negative, SETBP1G870S-positive primary myelofibrosis showing grade 3 fibrosis with an increased network of reticulin fibers and osteomyelosclerosis. See the article by Crespiatico et al on page 1399.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals








A treatment bridge for infants with hemophilia
Clinical Trials & Observations