Visual Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative option for patients with high-risk myelodysplastic syndromes (MDS). Advances in conditioning regimens and supportive measures have reduced treatment-related mortality and increased the role of transplantation, leading to more patients undergoing HSCT. However, posttransplant relapse of MDS remains a leading cause of morbidity and mortality for this procedure, necessitating expert management and ongoing results analysis. In this article, we review treatment options and our institutional approaches to managing MDS relapse after HSCT, using illustrative clinical cases that exemplify different clinical manifestations and management of relapse. We address areas of controversy relating to conditioning regimen intensity, chemotherapeutic bridging, and donor selection. In addition, we discuss future directions for advancing the field, including (1) the need for prospective clinical trials separating MDS from acute myeloid leukemia and focusing on posttransplant relapse, as well as (2) the validation of measurable residual disease methodologies to guide timely interventions.
Introduction
Myelodysplastic syndromes (MDS) are the most frequently diagnosed myeloid malignancies in older individuals in the United States.1 MDS present as a heterogeneous spectrum of diseases characterized by dysfunctional hematopoiesis, cytopenia, and risk of transformation to acute myeloid leukemia (AML).2 MDS are characterized by recurrent genetic aberrations that define biological behavior and prognosis3 and affect therapeutic interventions.4,5 Several prognostic classifications have been developed, including the International Prognostic Scoring System (IPSS), the World Health Organization (WHO) Classification–based prognostic scoring system, the revised IPSS (IPSS-R), and the molecular IPSS (IPSS-M).3,6-8 These scoring systems are useful for prognostication, clinical trial design, and the customization of treatment strategies. These strategies encompass observation, biologic treatments, chemotherapy (particularly with hypomethylating agents [HMAs]), and allogeneic hematopoietic stem cell transplantation (HSCT), dependent on the patient’s clinical risk and performance status.
Over the past 2 decades, HMAs have been the mainstay of treatment of higher-risk (HR) disease.9,10 HMAs, however, are not curative.11,12 HSCT stands as the only proven option with curative potential, with data showing improved overall and relapse-free survival (RFS) across all age groups.13,14 However, despite its curative potential, relapse remains frequent, with an incidence of 15% to 60%.15,16 Although advances in conditioning regimens, graft-versus-host disease (GVHD) management, and infection control have reduced treatment-related mortality (TRM),17 relapse remains a challenge, and a standard treatment approach has yet to be established. In this review, using illustrative clinical cases, we explore strategies aimed at reducing posttransplant relapse, encompassing choice of pretransplant conditioning regimens, chemotherapeutic bridging approaches, donor selection, and posttransplant interventions.
Pretransplant considerations
What are disease- and patient-related considerations that affect the initial approach to transplantation in patients with MDS?
Disease related
Patients with lower- and intermediate-risk MDS (defined by prognostic classification) are generally managed with the goal of improving cytopenia and quality of life. In patients with HR-MDS, the aim is typically to delay or prevent leukemic transformation, thereby prolonging life. Noting that both the WHO and the International Consensus Classification have recently updated morphologic classifications of patients with MDS,18,19 data support an approach that adjusts timing of transplantation to disease stage: patients with low- and intermediate-1 risk disease (according to IPSS-R) benefit from delaying transplantation, whereas patients with intermediate-2 and high-risk disease benefit from early transplantation.20,21 With the addition of molecular parameters as prognostication tools,3 this approach can be further refined. Certain mutations are known to increase the risk of posttransplant relapse such as RAS-pathway mutations,22RUNX1, ASXL1,23,24 and TP53.22,25 Such mutations may reinforce the decision to proceed to an early transplant, go with higher-intensity conditioning regimens, or include such patients in clinical trials looking at high-risk disease and posttransplant maintenance strategies.2 Germ line predisposition is also an important consideration. A recent position paper from the European Society for Blood and Marrow Transplantation (EBMT) recommended germ line testing for all pediatric patients and adults aged <50 years diagnosed with MDS. The panel also advised, when selecting a donor, avoiding family members with germ line mutations predisposing to hematologic malignancies, particularly those with dominant inheritance patterns.26
Our approach: in practice, patients with high-risk genetic mutations and other high-risk features should be referred for transplantation without delay. Those with high disease burden, including a myeloblast count of >10%, may benefit from bridging therapies, although data are not without controversy.27 Patients diagnosed at a younger age (aged <50 years), those with additional cancer diagnoses or first degree relatives with myeloid malignancies or bone marrow failure disorders, and those with a clinical suspicion for a genetic predisposition syndrome, should undergo testing for germ line predispositions.26,28
Patient related
“Fitness” is a term often used interchangeably with “age,” and many patients are assumed ineligible for HSCT because of their age, without proper evaluation.29 A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of 1080 patients who received transplantation with reduced intensity conditioning (RIC) showed age per se had no impact on TRM, relapse, or overall survival (OS) when adjusted for the hematopoietic cell transplantation comorbidity index (HCT-CI).16 Similarly, Lim et al analyzed 1333 patients with MDS aged >50 years from the EBMT.15 Multivariate analysis identified RIC, unrelated donor, and advanced disease stage at transplantation as independent predictors of TRM. Advanced disease stage was the only variable associated with inferior OS.15 Data from the Bone Marrow Transplant Clinical Trials Network (BMT CTN) show significant RFS and OS advantage in patients aged 50 to 75 years, with intermediate-2 or high-risk MDS, who had an HLA-matched donor and underwent RIC, compared with those who received best supportive care or HMAs.13 Benefit was consistent across all age groups.
Several other studies have demonstrated that relying solely on HCT-CI for outcome prediction is inadequate. A more comprehensive approach, incorporating geriatric30,31 and frailty assessments,32 is warranted, especially in older patients.
Our approach: HSCT should be considered in HR-MDS, irrespective of chronological age. It is essential to perform a comprehensive evaluation that includes not only the HCT-CI but also geriatric and frailty assessments. Factors such as performance status, psychological well-being, and support system are important considerations.
Posttransplant relapse scoring tools
Although IPSS prognostic tools are informative when predicting overall outcomes after diagnosis, they are less reliable when projecting stem cell transplant outcomes.33 Several scoring tools tried to better predict posttransplant clinical outcomes, including relapse, using patient- and disease-related variables. A CIBMTR analysis of 2133 patients with MDS came up with a transplant-specific scoring system that used age, performance status, peripheral blood blasts, cytogenetics, and platelet count.34 Increased score was associated with increased relapse, and this tool was superior to IPSS-R in predicting survival outcomes after transplantation.34 Other noteworthy scoring tools were the transplantation risk index33 and the EBMT transplant-specific risk score.35 The transplantation risk index used age, IPSS-R, cytogenetics, refractoriness to chemotherapy, and the HCT-CI to predict relapse and OS after transplantation33 whereas the EBMT risk score used age, performance status, cytogenetics, blast count, platelet count, recipient cytomegalovirus status, and donor relation to predict relapse, RFS, TRM, and OS.35
Our approach: in practice, no tool is prioritized over the other. These tools serve to guide management decisions rather than dictating them.
Is there a role for induction therapy in patients with MDS eligible for transplantation?
The optimal timing of transplantation is controversial. In patients with high disease burden, cytoreduction before HSCT has been recommended, based on data suggesting decreased relapse.36,37 Achieving a complete remission (CR) before transplantation has been associated with improved post-HSCT outcomes.38,39 Data from the VERONA trial (NCT02942290), a phase 3 study of an azacitidine and venetoclax combination in newly diagnosed HR-MDS, showed great promise, with ∼30% of treated patients achieving CR and 39.3% receiving transplantation after study.40 HMAs have been widely used as a bridge to HSCT. Standard AML-type induction chemotherapy (IC) has also been used as a bridging modality, without a consistent advantage in terms of relapse prevention or OS, that would justify the added toxicity.41,42 Although better tolerated than IC, HMAs are often associated with prolonged myelosuppression and increased risk of infection and organ toxicities.14 Particularly in patients who are frail,43 this may lead to abandoning the plan for transplantation.
Our approach: in practice, we initiate HMAs while recipient evaluation and donor search are ongoing; this process may take 6 to 8 weeks in most academic centers. Individuals with myeloblast counts of >10% may benefit from additional cytoreductive therapies.27
Role of MRD in MDS
There is mounting evidence that measurable residual disease (MRD) at the time of transplantation is a powerful predictor of posttransplant outcomes. The relevance of MRD in AML management44 has not yet been fully replicated in MDS. However, the most recent iteration of the International Working Group 2023 response criteria for HR-MDS recommends that MRD status be added as an exploratory end point and be included as a response category.45 With the emergence of high-sensitivity next-generation sequencing (NGS) and other technological advances, such as single-cell sequencing and droplet digital polymerase chain reaction (ddPCR), MRD assessment is being incorporated into trial design and clinical practice.46 Nakamura et al investigated the predictive value of ddPCR for posttransplant relapse in patients with MDS or AML. Increasing circulating tumor DNA between the first and third months after transplantation proved a sensitive predictor of disease relapse, demonstrating the prognostic utility of circulating tumor DNA in MDS.47
Additional studies are evaluating the relevance of pretransplantation MRD for informing choice of conditioning.48,49 In an analysis of 287 patients with MDS or secondary AML, Festuccia et al coined the term “minimal identifiable disease” (MID) to describe patients with cytogenetic abnormalities or evidence of disease by multiparameter flow cytometry. Results suggested that patients with MID by cytogenetics, experienced a high post-HSCT relapse rate if conditioned with RIC but, if conditioned with myeloablative conditioning (MAC) regimens, experienced a relapse rate similar to those who were MID negative.50 Similarly, Dillon et al examined 48 MDS preconditioning samples from the BMT CTN0901 study. MRD positivity, by targeted DNA detection, was associated with higher relapse rates in patients undergoing RIC than in those undergoing MAC.51 These findings suggest that MRD determination can help inform the choice of conditioning.
Once properly validated, various methodologies (NGS, ddPCR, multiparameter flow cytometry, and CD34+ donor chimerism analysis) should help guide posttransplant (or even preemptive) interventions aimed at reducing posttransplant relapse.52,53 Using several methodologies in conjunction (for example NGS and ddPCR) may further enhance detection.
Our approach: MRD testing is beginning to be incorporated into clinical practice and should be integral to clinical trial design, serving as an end point.
Peritransplant considerations
What is the optimal conditioning intensity?
Historically, MAC was the preferred choice for myeloid malignancies. Retrospective studies, and a recent prospective trial, demonstrated improved OS and RFS in the MAC cohort compared with patients who received RIC conditioning, despite higher TRM.54 However, data from the RICMAC trial by the EBMT group, comparing RIC with MAC in patients with MDS showed no difference in 2-year RFS and OS.55 Even within RIC regimens, differences in relapse have been described. A CIBMTR analysis showed that fludarabine with busulfan was linked to higher relapse rates and poorer OS than fludarabine with melphalan, in older patients with MDS.56 In the absence of conclusive data, providers tend to tailor conditioning regimen to patient and disease characteristics. Patients who are “fit” typically receive MAC with the goal of maximizing cure rates, particularly in patients with aggressive disease biology (high-risk mutations, increased blasts, and chemoresistance). Of note, the dichotomous classification of MAC/RIC has its limitations because the intensity of conditioning regimens likely vary on a continuum. A more refined classification of regimen intensity was the transplant conditioning intensity score that identified an intermediate class, distinct from MAC and RIC. The transplant conditioning intensity score was significantly better than the conventional RIC/MAC classification at predicting TRM and relapse but has only been tested in AML and not yet validated in MDS.57
Our approach: in older patients who are “less fit,” and those with lower risk disease, our choice remains a RIC regimen, because lower TRM would outweigh the risk of increased relapse rates. In younger, or “fit” patients, especially those with a high disease burden or high-risk clinical/molecular features, intensifying the conditioning regimen might be warranted. Another consideration is the choice of donor. Generally, less intensive regimens are used for conditioning in HLA-haploidentical transplantation, with the frequent incorporation of post-transplant cyclophosphamide (PTCy).58
Is an HLA-matched sibling still the best donor source?
Because patients with MDS tend to be older, they are unlikely to have a suitable matched sibling.59 HLA-matched unrelated donors (MUDs) are an attractive option, particularly as evidence suggests that younger (unrelated) donors provide a survival and relapse advantage over older HLA–matched sibling donors (MSDs).60,61 A suitable unrelated donor can be identified, often expeditiously, for 70% to 75% of White patients,62 although the odds of this for other ethnic groups are lower. A CIBMTR analysis of 701 patients with MDS who underwent HSCT indicated that those who received transplantation from MSDs had the best RFS and OS, followed by those who received transplantation from MUDs. Both cohorts had better outcomes than patients who received transplantation from HLA-mismatched unrelated donors (MMUDs).63 For many patients, especially those of non-White backgrounds, it is often challenging to identify a MUD. For those patients, HLA-haploidentical donors and PTCy–based GVHD prophylaxis are increasingly being used.64 In a CIBMTR analysis of 603 patients with MDS undergoing RIC and PTCy, haploidentical HSCTs had higher rates of relapse but a lower incidence of 2-year mortality from chronic GVHD than recipients of MUD transplantations, with no difference in OS.65 In a large EBMT study, HSCT from HLA-haploidentical donors and PTCy showed superior RFS and OS compared with MMUD and umbilical cord blood (UCB) transplantations whereas relapse rates were comparable between donors.66 There is an ongoing debate regarding potential higher relapse rates after haploidentical HSCT.67 UCB is not our first choice either, given slow engraftment and delayed immune reconstitution.68 In a CIBMTR analysis that looked at UCB transplantation, TRM and relapse rates at 3 years were 40% and 32%, respectively. Of note, RIC was associated with increased rates of relapse across IPSS-R scores.69
Our approach: currently, our preferred donor remains an HLA-matched sibling or unrelated volunteer. Based on recent data,60 we favor a younger MUD over an older MSD, although a definitive age cutoff has not been established. Additional data are needed to compare MMUDs and haploidentical donors.
Posttransplant considerations: management of posttransplant relapse
Case 1: cytogenetic/molecular relapse and declining donor chimerism
A 75-year-old woman was diagnosed with MDS with 2% blasts (MDS-LB, WHO5) and normal cytogenetics. NGS revealed TET2, ASXL1, and RUNX1 mutations (IPSS-R and IPSS-M categories were 3.5 [intermediate risk] and 0.86 [high risk], respectively). She was requiring 1- to 3-monthly red blood cell transfusions and her disease had failed to respond to erythroid stimulating agents. She received 4 cycles of oral decitabine/cedazuridine, and 2 cycles of azacytidine-venetoclax (14 days) combination. Marrow biopsy was hypocellular with 10% to 15% blasts, indicating progression. She underwent an HSCT from a male MUD after RIC with fludarabine and cyclophosphamide. Day-60 whole-blood short tandem repeat chimerism analysis revealed 100% donor cells. Day-100 bone marrow biopsy, however, showed 40% XX recipient cells. TET2, ASXL1, and RUNX1 mutations were detected. GVHD prophylaxis with tacrolimus was tapered off over 2 weeks and a marrow biopsy, 1 month later, showed complete cellular and molecular remissions. Shortly thereafter, the patient returned with skin GVHD (40% of her body surface area) confirmed by biopsy. The rash responded to topical steroids and the patient remains in CR at 6 months.
Tapering of immunosuppressive therapy
Tapering of immunosuppression is aimed at reactivating donor T cells to enhance their graft-versus-leukemia (GVL) effect. This approach may lead to durable remissions but is often associated with GVHD.70 In addition, complete responses are rare (6.6%) and typically limited to patients with low disease burden recognized early after transplantation.71 Other treatment options that harness GVL activity include a second HSCT or donor lymphocyte infusion (DLI) (Figure 1).72 However, patients may not be eligible for these strategies because of advanced age, comorbid conditions, poor recovery after transplantation, or the presence of GVHD. This leaves tapering of immunosuppressive therapy as the preferred approach. Our patient in Case 1, responded very well to tapering of immunosuppressive therapy despite a flair of skin GVHD. Given advanced age, and her personal preference, we elected to hold off any further interventions aside from close monitoring.
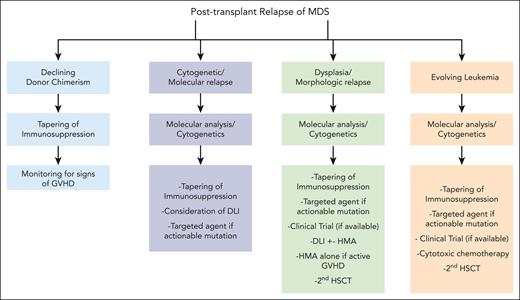
General approach to reduce and treat posttransplantation relapse of MDS.
Our approach: when relapse is suspected, enhancement of donor alloreactivity through withdrawal of immunosuppressive medications while monitoring for GVHD, should be attempted. Factors needing consideration when tapering immunosuppression include timing of relapse, baseline dose, number and type of immunosuppressive agents, and presence/stage of acute GVHD. Mild GVHD would be acceptable in this setting. Tapering immunosuppression can induce prolonged remissions and restoration of full donor chimerism, especially when relapse is molecular, cytogenetic, or reflected in declining donor chimerism.
Case 2: dysplasia/morphologic relapse
A 73-year-old woman with MDS (MDS-IB2, 10%-15%), normal cytogenetics, and a RUNX1 mutation (IPSS-R and IPSS-M categories were 4.5 [high risk] and 1.73 [very high risk], respectively) was treated with azacytidine-venetoclax and achieved CR after 2 cycles. She underwent HSCT after RIC with fludarabine and cyclophosphamide, from a MUD. GVHD prophylaxis was discontinued by day 100. However, at 4 months, she presented with anemia and neutropenia. Bone marrow was hypercellular with trilineage dysplasia, 15% to 20% blasts, RUNX1 mutation, and normal cytogenetics. She received 2 cycles of azacytidine (75 mg/m2 per day for 7 days) and 3 DLIs at escalating doses of 1 × 106, 1 × 107, and 1 × 108 at 6-week intervals. She experienced transient diarrhea treated with supportive management. Her blood counts normalized, and her subsequent marrow examination showed complete morphologic and molecular remissions. At 6 months after the last DLI infusion, the patient remains in remission.
DLI with or without HMAs
After transplantation, HMAs increase cytotoxic as well as regulatory T cells, thus enhancing GVL effects and possibly attenuating GVHD.73 DLI can also enhance GVL effects and induce prolonged remissions by activating cytotoxic T cells but at the risk of triggering GVHD.74 DLI administration is, therefore, not advisable in the setting of active GVHD. DLI has been very effective in patients who had received transplantation for chronic myeloid leukemia72 but has been less effective in other disease entities.75 Several studies have shown improved efficacy of DLI when preceded by cytotoxic therapy, especially with hematologic/clinical relapse73,76 as demonstrated by Case 2, whereas in the setting of molecular relapse77 or declining donor chimerism (with evidence of disease relapse),78 DLI alone might be considered. Although dysplasia after HSCT may be related to drug exposure, toxicity, and inflammation, dysplasia in Case 2 was thought to be secondary to underlying MDS, given its association with decreased counts, increased marrow blasts and cellularity, and reappearance of the RUNX1 mutation.
Our approach: DLI is most effective with lower disease burden or when relapse is evident only by molecular analysis with persistent donor T-cell engraftment. The optimal DLI (CD3) T-cell dose has not been firmly established and clinical practice is largely guided by empirical data and institutional experience. Initial doses may start at 1 × 106 cells per kg and may be escalated up to 1 × 108 cells per kg, given at 1- to 2-month intervals with 5- to 10-fold increases in CD3 T-cell doses, provided there is no GVHD. This escalation can be repeated up to 3 times.79 Of note, greater HLA disparity increases the risk for GVHD, making starting doses of <1 × 106 cells per kg preferable.80
Case 3: relapse with evolving leukemia
A 56-year-old man was diagnosed with MDS-IB2 (10%-15% myeloblasts). Cytogenetics showed 46, XX, del(12)[13]/46, XX[7], and NGS identified U2AF1, ASXL1, and CEBBP (IPSS-R and IPSS-M were 6 [high risk] and 2.69 [very high risk], respectively). After 4 cycles of decitabine, and only a slight decrease in marrow blasts, he underwent HSCT from an MSD after RIC (busulfan and fludarabine). He achieved a molecular remission. Blood counts at 7 months showed progressive pancytopenia. A marrow biopsy revealed 30% myeloblasts, consistent with AML. Reinduction was attempted with clofarabine and cytarabine81 followed by MAC and a second transplantation from his HLA-haploidentical son. On transplant day, he developed abdominal pain and hyperbilirubinemia consistent with veno-occlusive disease (VOD)/sinusoidal obstruction syndrome. He died on day 7 after HSCT.
Second HSCT
Relapse after transplantation suggests disease resistance to cytotoxic therapy and evasion of donor immunity. In such cases, an aggressive approach is warranted and consideration of a second HSCT is especially relevant when relapse is accompanied by elevated disease burden, increasing blast count, or evolving leukemia. Management of patients who relapse with leukemia is driven by the same principles that guide primary AML management and includes disease control with IC followed by a consolidative HSCT. Studies have linked receipt of cellular therapy (DLI or HSCT) with improved survival82,83 but toxicities associated with this approach need thorough consideration. Case 3 illustrates the potential morbidity and mortality associated with a second transplantation, in a patient with a relatively short remission (<12 months),84 even in a young and fit individual with no preexisting end-organ dysfunction. The significant toxicity seen could be attributed to the relatively brief interval separating the 2 transplantations (7 months), making the cumulative effects of intensive chemotherapy more pronounced. Although treatment of GVHD and infectious complications have improved, advances in VOD85 management have lagged, and mortality rates are still very high,86 especially in patients who have been exposed to prior allo-HSCT.87,88
Our approach: the key step in deciding on a second transplantation is selecting appropriate candidates, considering factors such as performance status, the presence/absence of active GVHD, and the timing of relapse relative to the first transplantation. Our preference is to proceed with RIC to minimize TRM, including VOD,89,90 regardless of the conditioning intensity of the first transplantation. Regarding the selection of a donor, 1 mechanism contributing to immune evasion and relapse is HLA loss. Opting for an alternative donor might improve outcomes.91 However, data have not consistently demonstrated an advantage in choosing a different donor for the second HSCT to enhance GVL effects,82,92 despite it being the preferred approach in many transplant centers.
Case 4: relapse with targetable mutation
A 69-year-old man was diagnosed with MDS with multilineage dysplasia and no increased blasts (MDS-LB). Cytogenetics showed 47 XY, +6 [5]/46 XY [15], and NGS identified U2AF1 and IDH2 (IPSS-R and IPSS-M categories were 4 [intermediate risk] and 1.56 [very high risk], respectively). He proceeded directly to HSCT from a MUD after RIC (fludarabine, cyclophosphamide, and low-dose total body irradiation [200 cGy]). Posttransplant course was complicated by acute GVHD of the gut, which resolved with a 3-week course of oral steroids. At 4 months, he had worsening anemia severe neutropenia and declining donor chimerism. A marrow biopsy showed mild dysplasia and persistence of the U2AF1 and IDH2 mutations. He was started on azacytidine (75 mg/m2 per day for 7 days of a 28-day cycle) and the isocitrate dehydrogenase enzyme 2 (IDH2) inhibitor enasidenib (100 mg orally, daily). After 4 cycles, he achieved complete molecular remission with 100% donor chimerism in the blood and marrow. Azacytidine was continued for 2 more cycles. One year after transplantation, the patient remains on maintenance enasidenib with no evidence of disease.
Targeted agents
Ivosidenib was approved by the US Food and Drug Administration for the treatment of adult patients with relapsed/refractory IDH1-mutated AML66 and MDS.93 Similarly, enasidenib, an IDH2 inhibitor was US Food and Drug Administration approved for relapsed/refractory IDH2-mutated AML.94. Enasidenib is currently not approved for IDH2-mutated MDS but results of early-phase trials are promising in de novo and relapsed/refractory disease.95-97 After HSCT, their use as maintenance strategies is being investigated in ongoing trials for IDH1-mutated (NCT03564821 and NCT03839771) and IDH2-mutated (NCT03515512, NCT03839771, NCT04522895, and NCT03728335) myeloid malignancies. It is worth noting that FLT3 mutations are exceptionally rare in MDS,98 with no ongoing trials investigating FLT3 inhibition in this setting.
Our approach: there are currently no prospective clinical trials looking into the use of targeted agents in patients with MDS who have relapsed after HSCT. If available, that would have been our preferred course of action in Case 4. Otherwise, dependent upon the mutation, we recommend a targeted agent with an established safety profile, before initiating cytotoxic or cellular therapies, given their wide-ranging toxicities. Combining HMA with enasidenib, as in Case 4, could be a consideration, inspired by early-phase studies demonstrating tolerability and efficacy of this combination in patients who are HMA naïve and/or HMA refractory.99,100 The general approach to managing posttransplant relapse of MDS is outlined in Figure 1.
Future directions
Peritransplant strategies
Novel peritransplant strategies in MDS focus on conditioning regimens and GVHD. JSP191, a humanized monoclonal antibody targeting CD117, showed promise in eradicating MDS/leukemic stem cells101 and was combined with nonmyeloablative conditioning in older adults with HR-MDS (NCT04429191). Preliminary results showed safety of the combination.
Considerable work has already been done on PTCy for GVHD prophylaxis. This strategy was initially limited to HLA-haploidentical transplantation58 but its TRM benefits have extended across different donor types.102-104 Concerned that suppressing GVHD might reduce GVL, and increase relapse rates,105 a prospective study compared PTCy-based GVHD prophylaxis with conventional tacrolimus-based regimens.64 PTCy was associated with lower rates of chronic GVHD and reduced relapse.64 Results from BMT CTN1703 also showed no increase in relapse rates between the standard tacrolimus-based prophylaxis group and the PTCy group, with the latter showing improved GVHD-free, relapse-free survival (GRFS).106 Antithymocyte globulin has been commonly used as GVHD prophylaxis in patients undergoing HSCT and has consistently demonstrated improved GRFS.107,108 The increased rates of GVHD seen with PTCy when peripheral blood instead of marrow stem cells were used led to strategies combining antithymocyte globulin and PTCy. The combination led to improved GRFS and minimized toxicities in patients of advanced age or with cardiac comorbidities.109
Posttransplant strategies
Maintenance therapy after transplantation has decreased the incidence of relapse in several hematological malignancies.110 Although common practice in acute lymphoblastic leukemia and AML, this strategy is still being investigated in MDS. HMA maintenance trials after transplantation have yielded inconsistent results regarding relapse prevention.111 The largest trial that randomized azacitidine maintenance vs observation after transplantation in high-risk AML and MDS, showed no difference in RFS between the 2 arms.112 Nonetheless, with the availability of oral formulations of HMAs, these agents deserve consideration, especially because they have little negative impact on quality of life. After the promising results of an early trial showing safety of oral azacitidine (CC-486) maintenance after transplantation,113 a phase 3 trial of CC-486 vs placebo is currently underway (NCT04173533).
Other maintenance strategies being investigated (discussed earlier) include targeted agents such as IDH1 (NCT03564821) and IDH2 inhibitors (NCT03515512). The p53 activator APR-246 used in combination with azacitidine in TP53-mutated MDS has generated some positive signals (NCT03931291) but requires additional study. Cellular therapy–based approaches currently under investigation include the use of chimeric antigen receptor T cells expressing the CD123 antigen upregulated in MDS stem cells.114 The UltraPRGN-3006 chimeric antigen receptor T cells that coexpress membrane-bound interleukin-15 and CD33 are being evaluated in a phase 1 clinical trial in HR-MDS refractory to HMAs; patients who relapsed >3 months after transplantation are included (NCT03927261). Other cellular strategies include the isolation of Wilms tumor antigen 1–specific T-cell receptors from HLA-A2+ normal donors, inserting them into donor CD8+ T cells and infusing these cells after transplantation in patients with AML to prevent relapse; RFS was 100% at 44 months.115 This effort is yet to be replicated in MDS. Studies evaluating DLI as a strategy to prevent posttransplant relapse have demonstrated its feasibility in myeloid malignancies. However, varying degrees of associated acute and chronic GVHD116-118 may pose limitations to the tolerability of this approach. Irrespective of the adopted posttransplant strategy, long-term follow-up studies are necessary to ensure improved survival outcomes and quality of life for patients with MDS. Figure 2 outlines different peri- and posttransplant strategies to reduce MDS relapse after transplantation.
Novel strategies to potentially reduce relapse after transplantation for MDS.
Separate criteria and stratification of patients with MDS and AML within HSCT trials
Managing posttransplant relapse in MDS has thus far relied on expert opinion and retrospective analyses, often combining patients with both MDS and AML. This approach makes it challenging to isolate specific factors and draw robust conclusions for each disease entity. MDS and AML have distinct clinical and biological characteristics.119 Even within MDS, significant heterogeneity exists because of different genetic signatures leading to different clinical manifestations. An important strategy that can drive innovation in relapse management is the use of randomized clinical trials that not only separate MDS from AML but limit their investigative scope to the posttransplant course.
Conclusion
Although much work has been done to prevent or treat post-HSCT relapse, to our knowledge, there has not been a comprehensive assessment of methods to approach these clinical issues in patients with MDS. Our discussion provides data based on our institutional practices and a formal review of the literature to help guide therapeutic approaches. Clinical trials are required for many of these unanswered questions. Despite suboptimal progress in nontransplant therapies, we are particularly encouraged by peritransplant and posttransplant advances ranging from optimizing RIC regimens and innovative GVHD strategies, to targeted oral agents, which are increasingly being relied upon in maintenance strategies. More refined molecular techniques such as single-cell analysis, that allow precise disease identification, will further determine the role of MRD in tailoring therapies.
Acknowledgment
All figures were created with BioRender.com.
Authorship
Contribution: H.J.D., P.L.G., and A.M. contributed to idea generation, provided expertise, and were involved in both writing and editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alain Mina, National Institutes of Health, 10 Center Dr, Building 10, Room 6N119A, Bethesda, MD 20892; email: alain.mina@nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal