Issue Archive
Table of Contents
BLOOD COMMENTARIES
HOW I TREAT
How I treat acute myeloid leukemia in the era of new drugs
Many new drugs for the treatment of acute myeloid leukemia have emerged over the past 2 years. DiNardo and Wei use 3 cases to illustrate how availability of these new agents is driving a reappraisal of the treatment decision-making process and providing a practical guide for choosing appropriate tailored therapies.
CLINICAL TRIALS AND OBSERVATIONS
Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial
Clinical Trials & Observations
Steroids are conventional frontline therapy for acute graft-versus-host disease (GVHD) but induce substantial toxicity. This randomized multicenter comparison of sirolimus vs prednisone for treatment of standard-risk acute GVHD demonstrates similar response rates at 28 days. Sirolimus therapy was associated with reduced steroid use and improved quality of life.
LYMPHOID NEOPLASIA
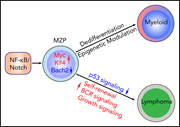
Coactivation of NF-κB and Notch signaling is sufficient to induce B-cell transformation and enables B-myeloid conversion
The authors investigate the effect of combined activated NF-κB and Notch signaling, a combination that has been observed in subsets of B-cell lymphoma. They demonstrate in a mouse model that the combined activation induces lymphoma and further links the combination to secondary myeloid leukemia, a rare but observed phenomenon arising in patients with lymphoma.
Targeting chronic NFAT activation with calcineurin inhibitors in diffuse large B-cell lymphoma
Activated B-cell–like diffuse large B-cell lymphoma (ABC-DLBCL) is more aggressive than its germinal center B-cell–like counterpart. Bucher et al demonstrate that DLBCL displays calcineurin-dependent constitutive NFAT activation and, in preclinical models, calcineurin inhibitors are uniquely toxic to ABC-DLBCL.
MYELOID NEOPLASIA
Defective interaction of mutant calreticulin and SOCE in megakaryocytes from patients with myeloproliferative neoplasms
About 25% of myeloproliferative neoplasms are caused by mutant calreticulin (CALR), which has been demonstrated to stimulate megakaryocyte proliferation by ligand-independent binding to the thrombopoietin receptor. Di Buduo and colleagues report that mutant CALR also shows loss of binding to calcium-regulatory proteins, leading to constitutive increase in intracellular calcium, further increasing proliferation of megakaryocytes, and contributing to their prominence as a clinical feature.
THROMBOSIS AND HEMOSTASIS
Identification of amino acid residues that are crucial for FXIII-A intersubunit interactions and stability
Factor XIII (FXIII) stabilizes the fibrin clot and circulates as a tetramer. The authors identify the critical residues for interaction of FXIII subunits, mutations in which have been reported to be associated with congenital FXIII deficiency.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Amino acid residues of the coagulation factor XIII-A2 intersubunit interface involved in intersubunit interactions. The amino acid residues shown as ball-and-stick models of FXIII-A monomer 1 (yellow) maintain salt bridges with amino acid residues of the opposite FXIII-A monomer 2 (gray). See the article by Li et al on page 145.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals





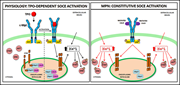


A role for NFAT signaling in ABC-DLBCL