Abstract
The proteasomal pathway of protein degradation involves 2 discrete steps: ubiquitination and degradation. Here, we evaluated the effects of inhibiting the ubiquitination pathway at the level of the ubiquitin-activating enzyme UBA1 (E1). By immunoblotting, leukemia cell lines and primary patient samples had increased protein ubiquitination. Therefore, we examined the effects of genetic and chemical inhibition of the E1 enzyme. Knockdown of E1 decreased the abundance of ubiquitinated proteins in leukemia and myeloma cells and induced cell death. To further investigate effects of E1 inhibition in malignancy, we discovered a novel small molecule inhibitor, 3,5-dioxopyrazolidine compound, 1-(3-chloro-4-fluorophenyl)-4-[(5-nitro-2-furyl)methylene]-3,5-pyrazolidinedione (PYZD-4409). PYZD-4409 induced cell death in malignant cells and preferentially inhibited the clonogenic growth of primary acute myeloid leukemia cells compared with normal hematopoietic cells. Mechanistically, genetic or chemical inhibition of E1 increased expression of E1 stress markers. Moreover, BI-1 overexpression blocked cell death after E1 inhibition, suggesting ER stress is functionally important for cell death after E1 inhibition. Finally, in a mouse model of leukemia, intraperitoneal administration of PYZD-4409 decreased tumor weight and volume compared with control without untoward toxicity. Thus, our work highlights the E1 enzyme as a novel target for the treatment of hematologic malignancies.
Introduction
Protein ubiquitination and degradation by the proteasome is the major route by which cells rid themselves of excess proteins. Blocking protein degradation by inhibiting this pathway at the level of the proteasome is cytotoxic to malignant cells and is an effective clinical strategy to improve the outcome of patients with malignancies such as multiple myeloma and mantle cell lymphoma.1,2 Although the effects of proteasome inhibition in malignant cells have been extensively characterized, the consequences of blocking protein degradation by inhibiting the early steps of protein ubiquitination are less well understood in malignant cells but might be analogous to proteasome inhibition. Here, we used chemical and genetic approaches to investigate inhibition of protein ubiquitination in malignant and normal cells in vitro and in vivo.
Ubiquitination is a multistep enzymatic cascade in which ubiquitin is conjugated to target proteins.3 In the first step of this cascade, the ubiquitin-activating enzyme UBA1 (E1) uses ATP to adenylate and then bind a ubiquitin molecule. Subsequently, a second ubiquitin molecule is then adenylated and bound to a different site of the same E1 enzyme. The E1 enzyme then transfers a ubiquitin molecule to the ubiquitin-conjugating enzyme E2. In the final step, the E2 enzyme transfers the ubiquitin to the target protein with the help of the ubiquitin ligase E3, resulting in ubiquitination of the target proteins with chains of 4 or more ubiquitins linked through Lysine-48 (K48) of ubiquitin. K48-polyubiquitinated proteins are then recognized, unfolded, and degraded by the proteasome enzyme complex.4 Through this pathway, the cell rids itself of excess and misfolded proteins and regulates biologic processes, including cellular proliferation.5 In addition to marking proteins for degradation, recent reports have noted that monoubiquitination of proteins or polyubiquitination by linking ubiquitins via their K63 residues does not promote proteasomal degradation but rather regulates processes such as receptor internalization,6 endocytosis,7 transcription,8 and DNA repair.9 The specificity of the ubiquitination pathway is achieved at the level of the E2 and E3 enzymes where more than 30 E2s and 300 E3s have been identified to date. In contrast, only 2 ubiquitin E1 enzymes, UBA1 and UBA6, have been identified to date, of which UBA1 is the predominant isoform in the protein degradation pathway.
Here, we demonstrated that primary leukemia cells have increased activity of the ubiquitination pathway. We also demonstrated that genetic and chemical inhibition of the E1 enzyme induced cell death in malignant cells preferentially over normal cells. Moreover, inhibition of the E1 enzyme delayed tumor growth in a mouse model of leukemia. E1 inhibition caused cell death by eliciting endoplasmic reticulum (ER) stress and an unfolded protein response. Thus, inhibition of the E1 enzyme is a novel target for the treatment of hematologic malignancies.
Methods
Reagents
The compounds 1-(3-chloro-4-fluorophenyl)-4-[(5-nitro-2-furyl)methylene]-3,5-pyrazolidinedione (PYZD-4409; CAS no. 423148-78-1; molecular weight, 352) and 4-(2-furylmethylene)-1-(4-methylphenyl)-3,5-pyrazolidinedione (PYZDmut; CAS no. 418804-46-3; MW 268) were purchased from Chembridge and the University Health Network's chemistry facility (Shanghai, China) and stored in 100% DMSO at −20°C. Histopaque-1077 was obtained from Sigma-Aldrich. Alamar Blue and Trypan Blue were purchased from Invitrogen. 3-(4,5-Dimethyl-thiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was obtained from Promega. 7-Amino-4-methylcoumarin (AMC)–conjugated fluorogenic proteasome substrates Suc-LLVY-AMC, Z-LLE-AMC, and Boc-LRR-AMC were purchased from EMD Biosciences. Glutathione S-transferase (GST)–tagged human recombinant E1 (UBA1) and human recombinant fluorescin-N-ubiquitin were obtained from BostonBiochem. HIS-tagged E1 and HIS-tagged E2 were purified in house. Fluorogenic pyrophosphate assay kit and ubiquitin were purchased from Invitrogen and Sigma-Aldrich, respectively. Antibodies to ubiquitin, E1, p53, Cdc34, and ATF-4 were obtained from Santa Cruz Biotechnology; cyclin D3, phospho-JNK (c-Jun terminal kinase), phospho-p38, and phospho-PERK (PKR [RNA-dependent protein kinase]–like ER kinase) were obtained from Cell Signaling Technology; CHOP (C/EBP homology protein) was obtained from Affinity BioReagents; and GRP78 was from Sigma-Aldrich.
Cell culture
K562, NB4, THP1, and U937 leukemia and DU-145, HT-29, OVCAR-3, PPC-1, and T-47D solid tumor cell lines were grown in RPMI 1640. KMS11, LP1, OCI-My5, and U266 myeloma cell lines were cultured in IMDM. HT1080-neo, HT1080–BI-1, and HeLa cell lines were grown in DMEM (high glucose). OCI-AML2 and OCI-AML5 leukemia and MDA 468 and FaDu solid tumor cell lines were maintained in α-MEM. All culture media were supplemented with 10% fetal bovine serum (Hyclone), 100 IU/mL penicillin G, and 100 μg/mL streptomycin. Primary leukemia cells and granulocyte colony-stimulating factor–mobilized mononuclear peripheral blood stem cells (PBSCs) were isolated by Histopaque-1077–gradient density centrifugation of samples from whole blood samples obtained from patients with acute myeloid leukemia (AML) or healthy volunteers donating stem cells for allotransplantation. The mean percentage of CD34+ in such samples obtained during the time frame of our current study was 2.3%. The collection and use of human tissue for this study was approved by the local ethics review board (University Health Network, Toronto, ON).
E1 silencing by lentiviral-delivered RNAi
Construction of hairpin-pLKO.1 vectors (carrying a puromycin antibiotic resistance gene) containing short hairpin RNA (shRNA) sequences and production of shRNA viruses has been described in detail.10 The shRNAs targeting the E1 coding sequence (UBA1, Accession no. NM_003334) are as follows: 5′-P-CCGGCTCCAACTTCTCCGACTACATCTCGAGATGTAGTCGGAGAAGTTGGAGTTTTT-3′; 5′-CCGGCCACTGCCTTCTACCTTGTTTCTCGAGAAACAAGGTAGAAGGCAGTGGTTTTT-3′; 5′-CCGGGCACAAATTAGAGATCACCATCTCGAGATGGTGATCTCTAATTTGTGCTTTTT-3′; 5′-CCGGCCTGGGATGTCACGAAGTTAACTCGAGTTAACTTCGTGACATCCCAGGTTTTT-3′; 5′-CCGGGTGCTATGGTTTCTATGGTTACTCGAGTAACCATAGAAACCATAGCACTTTTT-3′. Lentiviral infections were performed essentially as described.10 Briefly, cells (1-2 × 105) in suspension culture were spun down and resuspended in 1 mL of media containing protamine sulfate (5 μg/mL), and then 1 mL of the virus was added, followed by overnight incubation (37°C, 5% CO2) without removing the virus. The next day, cells were diluted in fresh media, plated in 96-well plates (1-5 × 103 cells/well), and incubated in culture media in the presence or absence of puromycin (1-2 μg/mL) for increasing times, followed by cell viability assays.
Cell death assay
Cell growth and viability was determined by the Alamar Blue and MTS assays, according to the manufacturers' instructions and as previously described.11,12 Cell viability was also assessed by the Trypan Blue dye exclusion assay by mixing aliquots of cells with the dye and counting nonstained (viable) cells and with Annexin V-FITC/PI staining (Biovision Research Products) and flow cytometry according to the manufacturer's instructions.
E1 enzymatic assays
To determine IC50, recombinant His6-tagged E1 (1μM) was incubated with the His6-tagged E2 (10μM) and ubiquitin (20μM) with increasing amounts of PYZD-4409 in a 50-μL reaction buffer containing 50mM Tris-HCl, pH 7, 5mM MgCl2, and 250μM ATP in a 96-well plate. An inorganic pyrophosphate detection reagent (50 μL) was added to each well immediately, and fluorescence signals were read over 30 minutes in a microplate reader (Gemini; Molecular Device) set at excitation 530 nm and emission 567 nm.
To determine the E1 enzymatic activity in vitro, GST-tagged E1 and fluorescein-tagged ubiquitin (1μM) were incubated with or without increasing concentrations of PYZD-4409 or PYZDmut in 10 μL of the above-mentioned reaction buffer for 30 minutes at 37°C. The products were then fractionated on 4% to 20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Formation of E1-Ub conjugate was assessed by visualization of fluorescent signals with the use of a Typhoon variable imager (Amersham Biosciences).
To determine the effect of PYZD-4409 on E2 loading, His6-tagged E1 (0.5μM), His6-tagged E2 (5μM), and ubiquitin (5μM) were incubated with increasing concentrations of PYZD-4409 or PYZDmut. The products were resolved on nonreducing 4% to 20% gradient SDS-PAGE followed by immunoblotting with anti-His antibody and fluorescent dye–labeled secondary antibody, and fluorescent bands were detected by a gel imager (LICOR).
Colony formation assays
Colony formation assays were performed essentially as described.13 Briefly, freshly isolated primary AML or normal hematopoietic cells (PBSCs) were treated with PYZD-4409 or buffer control for 24 hours. After treatment, cells were washed and then plated by equal volume into duplicate 35-mm dishes (Nunclon) in a final volume of 1 mL per dish in MethoCult GF H4434 medium (StemCell Technologies). After incubating the dishes for 7 days (AML) or 2 weeks (normal hematopoietic cells) at 37°C with 5% CO2 and 95% humidity, numbers of colonies were counted on an inverted microscope. To confirm the type or lineage of cells in the colonies, cells were picked and stained with May-Grünwald-Giemsa as previously described.14,15 In this assay, a plating efficiency of 0.1% was routinely achieved for both AML and PBSC samples.
Western blot analysis
Cells were washed with phosphate-buffered saline, lysed in 1× SDS sample buffer (60mM Tris-Cl, pH 6.8, 2% SDS, 10% glycerol), heated to 95°C for 5 minutes, and stored at −20°C. Protein concentrations were determined with the use of a DC Protein Assay kit (Bio-Rad). Subsequently, lysates were supplemented with 5% β-mercaptoethanol, heated to 95°C for 5 minutes, fractionated on 10% SDS–polyacrylamide gels, and transferred to nitrocellulose membranes with a gel transfer module (Criterion Blotter; Bio-Rad). For nonreducing conditions, lysates were heated to 95°C for 5 minutes without supplementation of β-mercaptoethanol. Hybridization to antibodies was performed following manufacturers' instructions, and signals were visualized with the use of an enhanced chemiluminescence kit (West Pico Reagent; Pierce Chemical).
20S proteasome assays
PBSCs and primary AML cells were washed in phosphate-buffered saline and lysed in buffer containing 50mM HEPES (pH 7.5), 150mM NaCl, 1% Triton X-100, and 2mM ATP. Proteasome chymotrypsin-like, caspase-like, and trypsin-like activities were determined by incubating cell lysates at 37°C for 1 hour with fluorogenic AMC-conjugated substrates Suc-LLVY-AMC, Z-LLE-AMC, and Boc-LRR-AMC, respectively. After incubation, free AMC was measured with a fluorescent spectrophotometric plate reader (SpectraMax M5; Molecular Devices) as described.16
Quantitative reverse transcriptase PCR
Real-time quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) assays were performed essentially as described.12,17 The cDNAs, encoding GRP78, HSP70, and 18S rRNA, were amplified with the following primer pairs: GRP78, 5′-TAG CGT ATG GTG CTG CTG TC-3′ (forward) and 5′-TTT GTC AGG GGT CTT TCA CC-3′ (reverse); HSP70, 5′-CAA GAT CAC CAT CAC CAA CG-3′ (forward) and 5′-GCT CAA ACT CGT CCT TCT CG-3′ (reverse); 18S rRNA, 5′-AGG AAT TGA CGG AAG GGC AC-3′ (forward) and 5′-GGA CAT CTA AGG GCA TCA CA-3′ (reverse). Relative mRNA expression was determined with the ΔΔCT normalization as previously described.12
Tumor xenografts
MDAY-D2 murine leukemia cells (1 × 105) were injected subcutaneously into male severe combined immunodeficient (SCID) mice. The next day, animals were treated with PYZD-4409 at 10 mg/kg in saline by intraperitoneal injection or buffer control alone. Treatment was repeated once every other day over 8 days. Tumor growth was monitored at least once every other day by external calipers after tumors were palpable. At 16 days after tumor inoculation, mice were killed by CO2 asphyxiation, and tumors were excised and weighed. All animal studies were carried out according to the regulations of the Canadian Council on Animal Care and with the approval of the local ethics review board.
Results
Ubiquitination pathway activity is increased in malignant cell lines and primary patient samples compared with normal hematopoietic cells
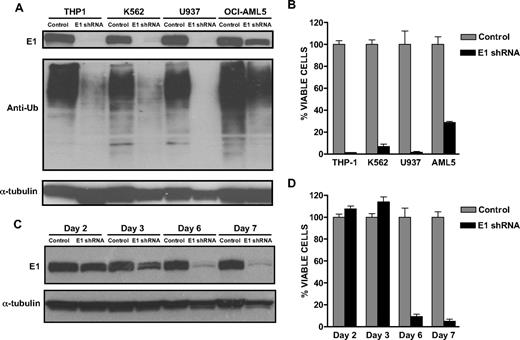
The proteasomal pathway of protein degradation involves 2 discrete steps: ubiquitination and degradation. To examine and compare this pathway in malignant and normal hematopoietic cells, we measured levels of ubiquitinated proteins in lysates from leukemia cell lines and primary AML patient samples as well as normal hematopoietic cells. By immunoblotting, levels of ubiquitinated proteins were significantly increased in the malignant cell lines and primary samples compared with normal hematopoietic cells (Figure 1A). Increased levels of ubiquitinated proteins in the malignant cells could be due to increased activity of the ubiquitination enzymes or to decreased activity of proteasomal enzymes. To distinguish between these possibilities, we measured the activity of the proteasomal enzymes and demonstrated that the leukemia cell lines and primary AML patient samples had increased chymotrypsin-like, caspase-like, and trypsin-like enzymatic activity compared with normal hematopoietic cells (Figure 1B). Therefore, the increased abundance of ubiquitinated proteins in leukemia cells is not due to impaired degradation of ubiquitinated proteins. Rather, leukemia cells have increased activity of the ubiquitination pathway. However, the increased activity of the pathway was not due to a greater abundance of the E1 enzyme, because levels of the E1 protein did not differ between the malignant and normal cells by immunoblotting (data not shown). Thus, these results suggest that the E1 enzyme is more actively used in malignant cells and may be a potential therapeutic target.
The activity of the ubiquitination pathway is increased in malignant cell lines and primary patient samples. (A) Total cellular proteins were isolated from leukemia cell lines, primary AML patient samples, and normal hematopoietic cells (PBSCs). Equal amounts of protein were analyzed by SDS-PAGE followed by immunoblotting with anti-ubiquitin, anti–α-tubulin, or anti-GAPDH antibodies (the latter as protein loading control). (B) Proteasome chymotrypsin-like (CT-L), caspase-like (C-L), and trypsin-like (T-L) activities were measured in primary AML (n = 12), primary normal hematopoietic cells (n = 6), and leukemia cell lines as described in “20S proteasome assays” with the use of cell lysates that were prepared from the samples that included those used for immunoblotting assays in panel A. Data represent the mean fold increase ± SD activity compared with the PBSC normal controls.
The activity of the ubiquitination pathway is increased in malignant cell lines and primary patient samples. (A) Total cellular proteins were isolated from leukemia cell lines, primary AML patient samples, and normal hematopoietic cells (PBSCs). Equal amounts of protein were analyzed by SDS-PAGE followed by immunoblotting with anti-ubiquitin, anti–α-tubulin, or anti-GAPDH antibodies (the latter as protein loading control). (B) Proteasome chymotrypsin-like (CT-L), caspase-like (C-L), and trypsin-like (T-L) activities were measured in primary AML (n = 12), primary normal hematopoietic cells (n = 6), and leukemia cell lines as described in “20S proteasome assays” with the use of cell lysates that were prepared from the samples that included those used for immunoblotting assays in panel A. Data represent the mean fold increase ± SD activity compared with the PBSC normal controls.
Knockdown of E1 protein induces cell death in leukemia and myeloma cell lines
The E1 is the initiating enzyme in the ubiquitination cascade, and its inhibition disrupts protein ubiquitination. Because the activity of the ubiquitination pathway was increased in malignant cells, we used a genetic approach to explore the effects of down-regulating the E1 protein in malignant cell lines. THP1, K562, U937, and OCI-AML5 leukemia cell lines were infected with E1 enzyme shRNA or control sequences in a lentiviral vector. Knockdown of E1 protein was confirmed by immunoblotting (Figure 2A). Knockdown of the E1 decreased the abundance of ubiquitinated proteins in the infected cells, showing a functional effect from inhibiting this target (Figure 2A). We then evaluated the effect of knocking down the E1 on cell viability. Knockdown of the E1 protein inhibited cell growth and viability as measured by the MTS assay 6 or 7 days after infection (Figure 2B). We also demonstrated that knockdown of the target was temporally associated with cell death (Figure 2C,D). Finally, cell death was confirmed by Trypan Blue and Annexin V/PI staining. Similar results were observed in LP1 and My-5 myeloma cell lines (data not shown). Therefore, inhibition of the E1 is cytotoxic to malignant cells.
Knockdown of the E1 enzyme induces cell death in malignant cells. (A) THP1, K562, U937, and OCI-AML5 leukemia cells were infected with an E1 shRNA lentiviral vector or control sequences, and populations of infected cells were selected. Total cellular proteins were isolated and analyzed by SDS-PAGE followed by immunoblotting with the use of anti-E1, anti-ubiquitin, and anti–α-tubulin antibodies. (B) Cells infected with an E1 shRNA lentiviral vector or control sequences were seeded 24 hours after infection into 96-well plates (5 × 103 cells/well) in the presence of puromycin to select for infected cells. Seven days after seeding, cell growth and viability were assessed by the MTS assay. Data represent the mean percentage ± SD of viable cells relative to cells infected with control sequences (n = 3). A representative experiment is shown. (C) K562 leukemia cells were infected with an E1 shRNA lentiviral vector or control sequences, and populations of infected cells were harvested at increasing times after infection. Total cellular proteins were isolated and analyzed by SDS-PAGE followed by immunoblotting with the use of anti-E1 and anti–α-tubulin antibodies. (D) Cells infected with an E1 shRNA lentiviral vector or control sequences were seeded 24 hours after infection into 96-well plates (5 × 103 cells/well) in the presence of puromycin to select for infected cells. At increasing times after seeding, cell growth and viability were assessed by the MTS assay. Data represent the mean percentage ± SD of viable cells relative to cells infected with control sequences (n = 3). A representative experiment is shown.
Knockdown of the E1 enzyme induces cell death in malignant cells. (A) THP1, K562, U937, and OCI-AML5 leukemia cells were infected with an E1 shRNA lentiviral vector or control sequences, and populations of infected cells were selected. Total cellular proteins were isolated and analyzed by SDS-PAGE followed by immunoblotting with the use of anti-E1, anti-ubiquitin, and anti–α-tubulin antibodies. (B) Cells infected with an E1 shRNA lentiviral vector or control sequences were seeded 24 hours after infection into 96-well plates (5 × 103 cells/well) in the presence of puromycin to select for infected cells. Seven days after seeding, cell growth and viability were assessed by the MTS assay. Data represent the mean percentage ± SD of viable cells relative to cells infected with control sequences (n = 3). A representative experiment is shown. (C) K562 leukemia cells were infected with an E1 shRNA lentiviral vector or control sequences, and populations of infected cells were harvested at increasing times after infection. Total cellular proteins were isolated and analyzed by SDS-PAGE followed by immunoblotting with the use of anti-E1 and anti–α-tubulin antibodies. (D) Cells infected with an E1 shRNA lentiviral vector or control sequences were seeded 24 hours after infection into 96-well plates (5 × 103 cells/well) in the presence of puromycin to select for infected cells. At increasing times after seeding, cell growth and viability were assessed by the MTS assay. Data represent the mean percentage ± SD of viable cells relative to cells infected with control sequences (n = 3). A representative experiment is shown.
The small molecule PYZD-4409 inhibits the E1 enzyme
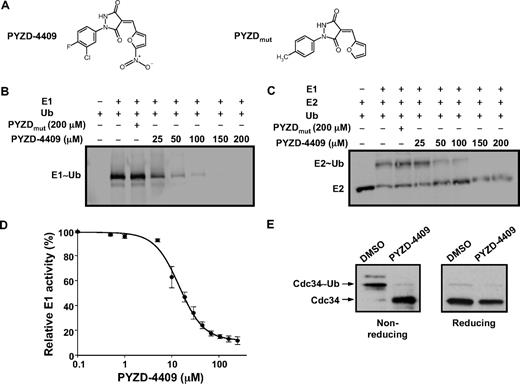
To further investigate the effects of E1 inhibition in malignant and normal cells in vitro and in vivo, we sought to identify a small molecule E1 inhibitor. Therefore, we screened a focused chemical library based on the pyrazolidine pharmacophore because the pyrazolidine compound 4-[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester was recently shown to inhibit this enzyme.18 From this screen, we identified the novel 3,5-dioxopyrazolidine compound, PYZD-4409 (Figure 3A). PYZD-4409 inhibited the ATP-dependent activation of ubiquitin (Figure 3B) and subsequent transfer of the activated ubiquitin from the E1 to the common human E2 enzyme UBE2E2 (Figure 3C) in a gel-based assay. In contrast, the structurally related compound PYZDmut (Figure 3A) had no effect on the E1 activity (Figure 3B-C). The IC50 of inhibition was estimated to be 20μM in a cell-free enzymatic assay (Figure 3D). Suggesting specificity of PYZD-4409 for the E1 enzyme, the compound had no effect on unrelated enzymes such as α-Mannosidase II glycosylation enzyme or Luciferase at concentrations up to 100μM (data not shown). It also did not inhibit the summo E1, Uba2, at concentrations up to 100μM (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, our screen identified a novel chemical inhibitor of the E1 enzyme.
PYZD-4409 inhibits the E1 enzyme. (A) Chemical structure of the E1 inhibitor PYZD-4409 and the inactive control PYZDmut. (B) GST-tagged human E1 (0.5μM) and fluorescein-labeled ubiquitin (1μM) were coincubated with increasing concentrations of PYZD-4409 or PYZDmut for 30 minutes and resolved on SDS-PAGE under nonreducing conditions. Formation of E1-Ub conjugates were assessed by visualization of fluorescent signals using a gel imager. (C) GST-tagged human E1 (1μM), His6-tagged human E2 (UbcH5A; 5μM), ubiquitin (20μM), and ATP (1mM) were coincubated with or without increasing concentrations of PYZD-4409 or PYZDmut for 30 minutes at 30°C. The reactions were then fractionated on 4% to 20% gradient SDS-PAGE followed by immunoblotting with anti-His antibodies and fluorescent dye–labeled secondary antibodies. Fluorescent signals were detected with an infrared imaging system. (D) Recombinant His-tagged human E1 (1μM) was incubated with the His-tagged human UbcH5A E2 enzyme (10μM), ubiquitin (20μM), and ATP (1mM) in the presence of increasing concentrations of PYZD-4409 for 30 minutes at 30°C. Inorganic pyrophosphate resulting from ATP hydrolysis in E1-catalyzed ubiquitin activation was quantified with the use of a fluorogenic pyrophosphate assay kit and a fluorescence microplate reader as described in “E1 enzymatic assays.” Data represent the mean percentage ± SD of E1 enzyme activity compared with buffer-treated controls (n = 3). A representative experiment is shown. (E) K562 cells were treated with PYZD-4409 (50μM) for 4 hours. Cell lysates were heated in either nonreducing or reducing SDS-PAGE sample buffer and fractionated on 10% SDS-PAGE, followed by immunoblotting with antibodies against the E2 protein cdc34.
PYZD-4409 inhibits the E1 enzyme. (A) Chemical structure of the E1 inhibitor PYZD-4409 and the inactive control PYZDmut. (B) GST-tagged human E1 (0.5μM) and fluorescein-labeled ubiquitin (1μM) were coincubated with increasing concentrations of PYZD-4409 or PYZDmut for 30 minutes and resolved on SDS-PAGE under nonreducing conditions. Formation of E1-Ub conjugates were assessed by visualization of fluorescent signals using a gel imager. (C) GST-tagged human E1 (1μM), His6-tagged human E2 (UbcH5A; 5μM), ubiquitin (20μM), and ATP (1mM) were coincubated with or without increasing concentrations of PYZD-4409 or PYZDmut for 30 minutes at 30°C. The reactions were then fractionated on 4% to 20% gradient SDS-PAGE followed by immunoblotting with anti-His antibodies and fluorescent dye–labeled secondary antibodies. Fluorescent signals were detected with an infrared imaging system. (D) Recombinant His-tagged human E1 (1μM) was incubated with the His-tagged human UbcH5A E2 enzyme (10μM), ubiquitin (20μM), and ATP (1mM) in the presence of increasing concentrations of PYZD-4409 for 30 minutes at 30°C. Inorganic pyrophosphate resulting from ATP hydrolysis in E1-catalyzed ubiquitin activation was quantified with the use of a fluorogenic pyrophosphate assay kit and a fluorescence microplate reader as described in “E1 enzymatic assays.” Data represent the mean percentage ± SD of E1 enzyme activity compared with buffer-treated controls (n = 3). A representative experiment is shown. (E) K562 cells were treated with PYZD-4409 (50μM) for 4 hours. Cell lysates were heated in either nonreducing or reducing SDS-PAGE sample buffer and fractionated on 10% SDS-PAGE, followed by immunoblotting with antibodies against the E2 protein cdc34.
To determine whether PYZD-4409 could inhibit the E1 enzyme activity in cultured cells, we treated K562 leukemia cells with PYZD-4409 and measured E1-mediated loading of ubiquitin onto the E2 enzyme Cdc34 similar to that previously described.19 After 4 hours of incubation, PYZD-4409, but not the structurally related control compound PYZDmut, blocked the E1-dependent conjugation of ubiquitin to the E2 enzyme cdc34 (Figure 3E). Thus, PYZD-4409 inhibits the E1 enzyme in cell-free and cell-based assays and can serve as a probe to interrogate the effects of E1 inhibition.
PYZD-4409 induces cell death in hematologic malignant cell lines and primary patient samples preferentially over normal hematopoietic cells
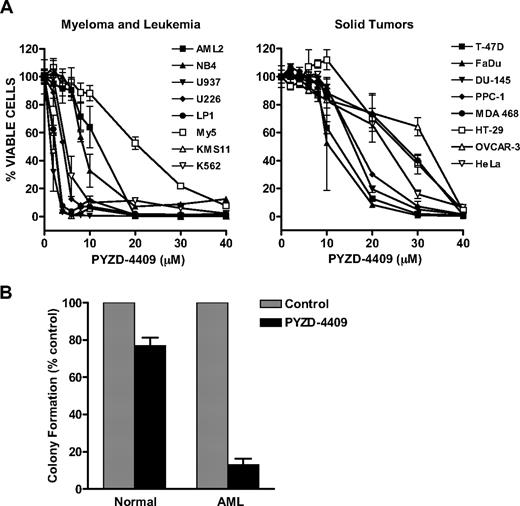
Given the ability of PYZD-4409 to inhibit the E1 enzyme activity in cell-free assays and in cultured cells, we examined the effects of inhibiting the E1 on the growth of malignant and nonmalignant cell lines and primary patient samples. To evaluate the cytotoxicity of PYZD-4409, a panel of leukemia, multiple myeloma, and solid tumor cell lines were treated with increasing concentrations of PYZD-4409. Cell viability was measured 72 hours after incubation by the Alamar Blue assay. In 5 of 8 leukemia and myeloma cell lines, PYZD-4409 induced cell death with a LD50 less than 10μM (Figure 4A). Myeloma cell lines were particularly sensitive to E1 inhibition because PYZD-4409 induced cell death with 3 of 4 myeloma cell lines (ie, LP1, KMS11, and U226) having an LD50 of 3μM or less. In contrast, solid tumor cell lines were less sensitive with an LD50 of approximately 15 to 20μM (Figure 4A). Cell death was confirmed by Trypan Blue staining (supplementary Figure 2). Supporting a mechanism of cell death linked to inhibition of the E1 enzyme, PYZDmut was not cytotoxic at concentrations up to 50μM (data not shown).
Inhibition of the E1 enzyme with PYZD-4409 preferentially induces cell death in malignant cell lines and primary AML cells over normal hematopoietic cells. (A) Myeloma, leukemia, and solid tumor cell lines were treated with increasing concentrations of PYZD-4409 for 72 hours. After incubation, cell growth and viability was measured by the Alamar Blue assay. Data represent the mean percentage ± SD of viable cells relative to control. Representative experiments are shown. (B) Mononuclear cells from patients with AML (n = 2) and the PBSCs of donors for allotransplantation (n = 4) were treated with PYZD-4409 (10μM) for 24 hours and then plated in duplicate for clonogenic growth in media containing 1% methylcellulose and cytokines. The numbers of colonies were counted after incubation for 7 days (AML) or 2 weeks (normal). Data represent the mean percentage ± SD of colonies relative to buffer-treated controls.
Inhibition of the E1 enzyme with PYZD-4409 preferentially induces cell death in malignant cell lines and primary AML cells over normal hematopoietic cells. (A) Myeloma, leukemia, and solid tumor cell lines were treated with increasing concentrations of PYZD-4409 for 72 hours. After incubation, cell growth and viability was measured by the Alamar Blue assay. Data represent the mean percentage ± SD of viable cells relative to control. Representative experiments are shown. (B) Mononuclear cells from patients with AML (n = 2) and the PBSCs of donors for allotransplantation (n = 4) were treated with PYZD-4409 (10μM) for 24 hours and then plated in duplicate for clonogenic growth in media containing 1% methylcellulose and cytokines. The numbers of colonies were counted after incubation for 7 days (AML) or 2 weeks (normal). Data represent the mean percentage ± SD of colonies relative to buffer-treated controls.
We also assessed the effects of PYZD-4409 on the viability of primary AML cells and normal hematopoietic cells. AML cells obtained from patients with leukemia and normal hematopoietic cells obtained from peripheral blood of volunteers donating peripheral blood stem cells for allotransplantation were treated with PYZD-4409 and then plated in clonogenic growth assays. PYZD-4409 inhibited the clonogenic growth of AML cells but did not reduce the growth of normal hematopoietic cells (Figure 4B). Thus, inhibition of the E1 enzyme is preferentially cytotoxic to malignant cells over normal hematopoietic cells.
PYZD-4409 blocks degradation of the short-lived proteins p53 and cyclin D3
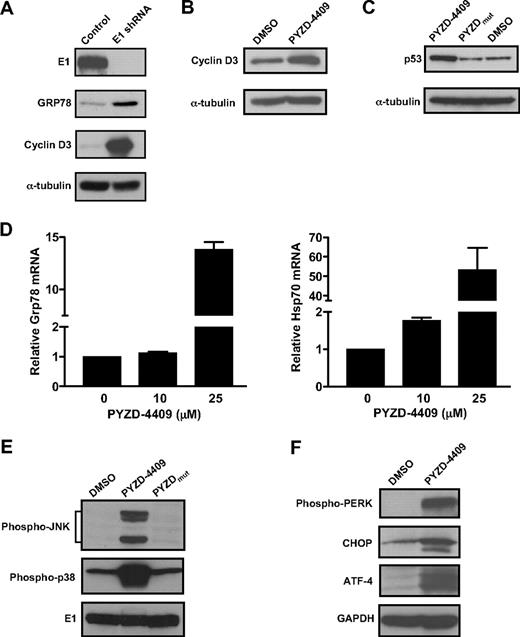
Inhibition of the E1 enzyme may increase the abundance of short half-life proteins that are normally degraded by ubiquitination. Therefore, we tested the effects of genetic and chemical inhibition of the E1 enzyme on levels of short half-life proteins that are regulated by ubiquitination and proteasomal degradation. Knockdown of the E1 protein with shRNA increased levels of cyclin D3 (Figure 5A). Likewise, inhibition of the E1 with PYZD-4409 increased total amounts of cyclin D3 and p53 (Figure 5B-C). However, increased levels of p53 were not sufficient to explain the effects of E1 inhibition because HCT116 cells with wild-type or null p53 were equally sensitive to the effects of PYZD-4409 (data not shown). Similar to the effects observed with proteasome inhibition, E1 inhibition with PYZD-4409 also inhibited tumor necrosis factor-α–induced activation of nuclear factor-κB (data not shown).
E1 inhibition increases short half-life proteins and induces ER stress. (A) K562 cells were infected with an E1 shRNA lentiviral vector or control sequences and selected as described in Figure 2. Total cell lysates were prepared and analyzed by SDS-PAGE immunoblotting with anti-E1, anti-GRP78, anti-cyclin D3, and anti–α-tubulin antibodies. (B) K562 cells were treated with PYZD-4409 or PYZDmut (50μM) for 4 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti-cyclin D3 and anti–α-tubulin antibodies. (C) HCT116 cells were treated with PYZD-4409 or PYZDmut (50μM) for 2 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti-p53 and anti–α-tubulin antibodies. (D) K562 cells were treated with PYZD-4409 at the concentrations indicated for 24 hours, and total cellular RNA was isolated. GRP78 and HSP70 mRNA expression was measured relative to 18S RNA by real-time RT-PCR. Data points represent the mean ± SD fold increase of GRP78 and HSP70/18S expression relative to controls (ΔΔCT normalization). (E) K562 cells were treated with PYZD-4409 (50μM), PYZDmut (50μM), or DMSO for 2.5 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti–phospho-JNK, phospho-p38 mitogen-activated protein kinase, and anti-E1. The E1 serves as a protein loading control. (F) MDAY-D2 cells were treated with PYZD-4409 (10μM) or DMSO for 24 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti–phospho-PERK, anti-CHOP, anti–ATF-4 and antitubulin antibodies.
E1 inhibition increases short half-life proteins and induces ER stress. (A) K562 cells were infected with an E1 shRNA lentiviral vector or control sequences and selected as described in Figure 2. Total cell lysates were prepared and analyzed by SDS-PAGE immunoblotting with anti-E1, anti-GRP78, anti-cyclin D3, and anti–α-tubulin antibodies. (B) K562 cells were treated with PYZD-4409 or PYZDmut (50μM) for 4 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti-cyclin D3 and anti–α-tubulin antibodies. (C) HCT116 cells were treated with PYZD-4409 or PYZDmut (50μM) for 2 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti-p53 and anti–α-tubulin antibodies. (D) K562 cells were treated with PYZD-4409 at the concentrations indicated for 24 hours, and total cellular RNA was isolated. GRP78 and HSP70 mRNA expression was measured relative to 18S RNA by real-time RT-PCR. Data points represent the mean ± SD fold increase of GRP78 and HSP70/18S expression relative to controls (ΔΔCT normalization). (E) K562 cells were treated with PYZD-4409 (50μM), PYZDmut (50μM), or DMSO for 2.5 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti–phospho-JNK, phospho-p38 mitogen-activated protein kinase, and anti-E1. The E1 serves as a protein loading control. (F) MDAY-D2 cells were treated with PYZD-4409 (10μM) or DMSO for 24 hours. After incubation, total cellular proteins were isolated and analyzed by SDS-PAGE immunoblotting with anti–phospho-PERK, anti-CHOP, anti–ATF-4 and antitubulin antibodies.
PYZD-4409–induced cell death is associated with ER stress
Blocking protein degradation at the level of the proteasome leads to accumulation of intracellular protein that elicits ER stress and an unfolded protein response.20 Therefore, we evaluated whether inhibition of the E1 induced ER stress and whether such effects were functionally important for cell death. Knockdown of the E1 with shRNA increased the abundance of the ER stress and unfolded protein response marker Grp78 (Figure 5A). Likewise, PYZD-4409 but not PYZDmut significantly increased both mRNA and protein levels of Grp78 and Hsp70 (Figure 5D; data not shown). In addition, PYZD-4409 but not PYZDmut increased levels of phospho-JNK and phospho-p38 mitogen-activated protein kinase, which have also been linked to ER stress and the unfolded protein response (Figure 5E).21 Finally, we demonstrated that PYZD-4409 also increased expression of other ER stress markers, including phospho-PERK, ATF4, and CHOP (Figure 5F).
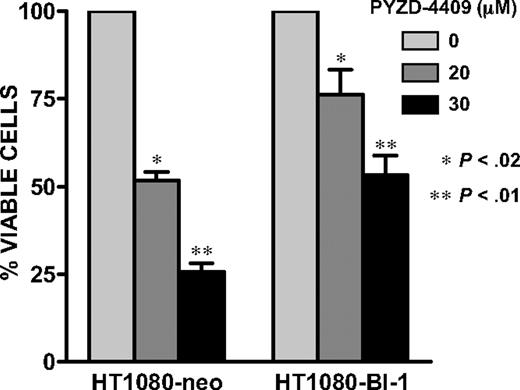
To determine whether the ER stress resulting from E1 inhibition was functionally important for cell death, HT1080 cells overexpressing BI-1 that protects cells from ER stress without affecting cell death induction via the tumor necrosis factor/Fas receptor (extrinsic) or mitochondria-initiated (intrinsic) cell death pathways22 or empty vector were treated with increasing concentrations of PYZD-4409. Overexpression of BI-1 inhibited PYZD-4409–induced cell death (Figure 6). Thus, ER stress may be functionally important for cell death induced by E1 inhibition.
BI-1 overexpression inhibited PYZD-4409–induced cell death. HT1080–BI-1 and HT1080-neo cells (3 × 103) were seeded overnight in 96-well plates. The next day, cells were treated with increasing concentrations of PYZD-4409 for 24 hours. Cell growth and viability was determined by the Alamar Blue assay. Data represent the mean percentage ± SD of viable cells relative to control (n = 3). A representative experiment is shown.
BI-1 overexpression inhibited PYZD-4409–induced cell death. HT1080–BI-1 and HT1080-neo cells (3 × 103) were seeded overnight in 96-well plates. The next day, cells were treated with increasing concentrations of PYZD-4409 for 24 hours. Cell growth and viability was determined by the Alamar Blue assay. Data represent the mean percentage ± SD of viable cells relative to control (n = 3). A representative experiment is shown.
Inhibition of E1 enzyme delays tumor growth in a mouse model of leukemia
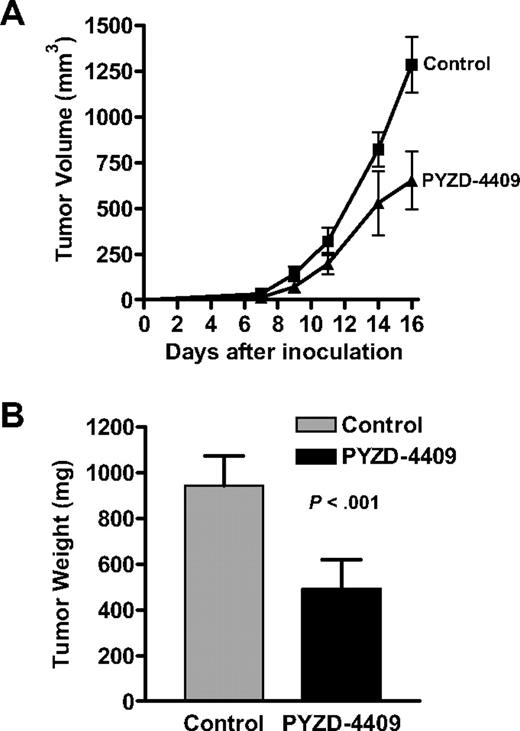
In cultured cells, inhibition of the E1 enzyme preferentially induced cell death in malignant cells over normal cells. Therefore, we explored the effects of E1 inhibition in a mouse model of leukemia. SCID mice were injected subcutaneously with MDAY-D2 murine leukemia cells and then treated with PYZD-4409 (10 mg/kg) or buffer control intraperitoneally daily on alternate days over 8 days. Sixteen days after tumor implantation, the mice were killed, and the tumors excised and weighed. Compared with control-treated mice, treatment with PYZD-4409 delayed tumor growth and decreased tumor weight (Figure 7) without untoward toxicity. However, we could not detect increases in the ER stress markers phospho-PERK, Grp78, and CHOP by immunoblotting the tumors. Thus, inhibition of the E1 can achieve an antitumor effect in vivo.
PYZD-4409 delays tumor growth in mouse model of leukemia. (A) MDAY-D2 murine leukemia cells (1 × 105) were injected subcutaneously into male SCID mice (n = 20). Starting the next day, animals were treated with PYZD-4409 (10 mg/kg; n = 10) in saline by intraperitoneal injection or vehicle alone (n = 10) once every other day over 8 days. Tumor growth was monitored at least every other day by external calipers. (B) At 16 days after tumor injection, mice were killed, and tumors were excised and weighed. Data represent the mean tumor weight ± SD. A representative experiment is shown.
PYZD-4409 delays tumor growth in mouse model of leukemia. (A) MDAY-D2 murine leukemia cells (1 × 105) were injected subcutaneously into male SCID mice (n = 20). Starting the next day, animals were treated with PYZD-4409 (10 mg/kg; n = 10) in saline by intraperitoneal injection or vehicle alone (n = 10) once every other day over 8 days. Tumor growth was monitored at least every other day by external calipers. (B) At 16 days after tumor injection, mice were killed, and tumors were excised and weighed. Data represent the mean tumor weight ± SD. A representative experiment is shown.
Discussion
The proteasomal pathway of protein degradation involves 2 discrete steps: ubiquitination and degradation. Inhibition of this pathway with proteasome inhibitors is a well-established strategy for the treatment of malignancies such as multiple myeloma and mantle cell lymphoma. Here, we evaluated the effects of inhibiting the ubiquitination pathway in malignant cells genetically and chemically at the level of the E1 enzyme.
We demonstrated that leukemia cell lines and primary patient samples have increased levels of ubiquitinated proteins and that this increase could not be explained by a reduction in protein degradation. To the best of our knowledge, the activity of the ubiquitination cascade has not been previously compared between normal and malignant hematopoietic cells. Potentially, the increased ubiquitination activity may reflect the higher metabolic rate required to support the growth of malignant cells. Because levels of the E1 protein did not differ between normal and malignant cells, it suggests that the E1 enzyme is more actively used in malignant cells. Thus, these observations suggest that malignant cells are more dependent on E1 activity and provide a rationale for targeting the E1 enzyme as an anticancer strategy.
Our studies also showed that malignant cells have increased activity of the proteasomal enzymes. This result is in keeping with prior studies that have shown increased abundance of proteasomal enzymes in malignant cells compared with normal cells,23,24 thereby providing a biologic rationale for the development of proteasome inhibitors as anticancer agents.
Given that malignant cells may be more dependent on the E1 enzyme activity compared with normal cells, we examined genetic and chemical inhibition of this target. Knockdown of the E1 protein with shRNA induced cell death in malignant cell lines. However, genetic knockout of the E1 protein was technically difficult in primary cells and mouse models of malignancy. Therefore, we developed PYZD-4409 as a chemical inhibitor of the E1 enzyme to better understand the effects of E1 inhibition in malignancy. PYZD-4409 inhibited the activity of the E1 enzyme in cell-free systems and also blocked the E1 activity when added to cultured cells. PYZD-4409 is similar in structure to 4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester (PYR-41) that was recently identified as an E1 inhibitor in cell-free and cell-based assays.18 However, that study did not fully address the preclinical activity of E1 inhibition by evaluating E1 inhibition in primary cells or in vivo. That study also did not explore the effects of E1 inhibition on ER stress. Nonetheless, the results with PYR-41 support our observations that PYZD-4409 inhibits the E1 enzyme. Despite the similarities in structures, we cannot exclude that PYZD-4409 has additional targets beyond the E1 enzyme that may contribute to its cytotoxic effects. Although a useful chemical probe, the micromolar potency of PYZD-4409 is suboptimal and efforts are under way to identify chemical E1 inhibitors with improved potency.
Inhibiting the E1 enzyme increased the abundance of short half-life proteins such as p53. However, cell death after E1 inhibition did not appear specifically dependent on the accumulation of a single protein such as p53. Rather, we demonstrated that E1 inhibition induced cell death through a mechanism linked to ER stress, suggesting a more global effect because of the general accumulation of intracellular proteins. The specific effectors linking E1 inhibition to ER stress are uncertain and will be explored in future studies. However, there are probably similarities to proteasome inhibition. Several studies have observed ER stress and unfolded protein response after proteasome inhibition, but the mechanism is not fully understood. Potentially, the accumulation of excess proteins in the cytoplasm inhibits retrograde protein translocation from the ER to the cytoplasm,25 leading to ER stress and the unfolded protein response.
BI-1 is a protein localized to the ER membrane and its overexpression protects cells from death by ER stress signals but not stimuli of the death receptor pathway of caspase activation.22 Our studies have shown that overexpression of BI-1 protected cells against PYZD-4409–induced cell death. These results coupled with the increase in ER stress proteins supports a mechanism of cell death linked to ER stress. However, additional studies to confirm this observation would be important. We note that these studies are challenging because altering ER stress proteins by overexpression of the mutant eIFα, eIF2α-S51A, that is resistant to phosphorylation or knockdown of PERK can increase cell death in response to ER stress signals.26 In the absence of these confirmatory studies, however, we must acknowledge that cell death after E1 inhibition may also have mechanisms beyond ER stress. Further studies are also required to understand fully how ER stress after E1 inhibition leads to cell death.
Proteins that are ubiquitinated with K48-linked chains are specifically recognized by the 26S proteasome and subjected to degradation. In contrast, proteins that are tagged with a single ubiquitin residue (monoubiquitination) or polyubiquitin chains formed by linking ubiquitin molecules through K-63 residues (K-63 ubiquitination) do not mark the protein for degradation. Rather, K-63 polyubiquitination or monoubiquitination alters protein localization and function. In our study, the effects of E1 inhibition that we observed appeared related to the accumulation of excess proteins. However, we cannot exclude that inhibition of the E1 enzyme also leads to more subtle changes related to inhibition of monoubiquitination and K-63 polyubiquitination.
Although the cellular effects of E1 inhibition and proteasome inhibition appear similar, the difference in targets suggests that E1 inhibition may overcome some forms of resistance to proteasome inhibitors. For example, K562 cells that overexpress the β5 binding proteasome subunits are resistant to the proteasome inhibitor bortezomib.27,28 Yet, these cells remain fully sensitive to genetic or chemical inhibition of the E1 enzyme. Thus, E1 inhibitors could be potentially useful therapeutic agents for some patients who are resistant to proteasome inhibitors.
In summary, we demonstrated that malignant cell lines and primary patient samples have increased activity of the ubiquitination pathway and that blocking this pathway with chemical or genetic inhibitors of the E1 enzyme induces ER stress and is preferentially cytotoxic to malignant cells. Thus, this work highlights the E1 enzyme as a new therapeutic target for the treatment of malignancy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The International Myeloma Foundation, The Leukemia & Lymphoma Society, the Ontario Ministry of Health and Long Term Care (OMOHLTC), and the National Institutes of Health (AG-015393; J.C.R.).
A.D.S. is a Clinical Research Scholar of the Leukemia & Lymphoma Society.
The views expressed do not necessarily reflect those of the OMOHLTC.
National Institutes of Health
Authorship
Contribution: G.W.X. designed research, analyzed data, performed research and wrote the paper, M.A., T.E.W., D.W., N.M., X.M., M.G., M.S., X.L., R.B.Z., R.H., X.M., and M.V. designed research, analyzed data, and performed research; D.R., J.M., R.A.B., and S.D.-P., analyzed data and supervised research; J.C.R. provided critical reagents; and A.D.S. designed research, supervised research, analyzed data, and wrote the paper. All authors reviewed and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron D. Schimmer, Princess Margaret Hospital, Rm 9-516, 610 University Ave, Toronto, ON, Canada M5G 2M9; e-mail: aaron.schimmer@utoronto.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal