Abstract
Chronic myeloid leukemia (CML) is treated effectively with tyrosine kinase inhibitors (TKIs); however, 2 key problems remain—the insensitivity of CML stem and progenitor cells to TKIs and the emergence of TKI-resistant BCR-ABL mutations. BCR-ABL activity is associated with increased proteasome activity and proteasome inhibitors (PIs) are cytotoxic against CML cell lines. We demonstrate that bortezomib is antiproliferative and induces apoptosis in chronic phase (CP) CD34+ CML cells at clinically achievable concentrations. We also show that bortezomib targets primitive CML cells, with effects on CD34+38−, long-term culture-initiating (LTC-IC) and nonobese diabetic/severe combined immunodeficient (NOD/SCID) repopulating cells. Bortezomib is not selective for CML cells and induces apoptosis in normal CD34+38− cells. The effects against CML cells are seen when bortezomib is used alone and in combination with dasatinib. Bortezomib causes proteasome but not BCR-ABL inhibition and is also effective in inhibiting proteasome activity and inducing apoptosis in cell lines expressing BCR-ABL mutations, including T315I. By targeting both TKI-insensitive stem and progenitor cells and TKI-resistant BCR-ABL mutations, we believe that bortezomib offers a potential therapeutic option in CML. Because of known toxicities, including myelosuppression, the likely initial clinical application of bortezomib in CML would be in resistant and advanced disease.
Introduction
Chronic myeloid leukemia (CML) is a clonal disorder of hematopoietic stem cells (HSCs). The disease arises as a consequence of a rare mutational event resulting in a reciprocal translocation between the long arms of chromosomes 9 and 22. This creates the chimeric oncogene BCR-ABL with the protein product BCR-ABL, a tyrosine kinase with constitutive activity. BCR-ABL is responsible for the pathogenesis of CML1,2 and therefore provides a target for therapy.
The current recommended first-line therapy for patients with chronic phase (CP) CML is imatinib mesylate (IM, Glivec; Novartis Pharma),3 a rationally designed tyrosine kinase inhibitor (TKI) with impressive efficacy. The majority of newly diagnosed patients with CP CML will achieve a complete cytogenetic response (CCyR) with IM.4 However, it is accepted that IM induces a state of minimal residual disease (MRD) rather than cure. Evidence for this is provided from clinical studies in which the number of patients sustaining a CCyR (63%) is fewer than the number achieving MDR (82%).4 Furthermore, BCR-ABL transcripts remain detectable in the majority of patients,5 and responding patients who discontinue IM are likely to suffer recrudescence of disease.6 It is likely that CML HSCs are the source of MRD. A primitive population of viable CML HSCs can be isolated from patients with a CCyR after IM treatment for up to 5 years.7,8 CML HSCs are relatively resistant to IM, even at concentrations higher than those achieved in vivo9 and are capable of reconstituting disease in mice.10,11 In addition to MRD, IM resistance is well documented with mechanisms including BCR-ABL mutations affecting IM binding, amplification of BCR-ABL, and reduction in effective intracellular concentrations of the drug by altered drug efflux or influx.12-15
More potent TKIs have been developed and include dasatinib (Sprycel; Bristol-Myers Squibb) and nilotinib (Tasigna; Novartis Pharma).16,17 These drugs show clinical benefit in IM-resistant CML. However, key problems remain: the primitive CML HSC is insensitive in vitro to dasatinib, nilotinib, and other TKIs18-20 ; and none of the TKIs inhibit the T315I BCR-ABL mutation. In view of these limitations, we have explored other strategies with the aim of targeting the CML HSC and cells expressing BCR-ABL mutations.
The proteasome is an intracellular organelle providing a targeted mechanism for protein degradation via 3 catalytic specificities—chymotrypsin-like (CT-L), trypsin-like (T-L), and post–glutamyl hydrolytic (PG). Through this protein degradation it is vital for cell cycling, adhesion, proliferation, and apoptosis. Cancer cells, including CML cells, have relatively increased proteasomal activity.21,22 This has been exploited by proteasome inhibitor (PI) therapy with antiproliferative and apoptotic effects demonstrated in a number of malignant cell types.23 Bortezomib is a reversible and specific inhibitor of CT-L activity and is licensed for clinical use in mantle cell lymphoma and multiple myeloma. However, benefit is seen in other solid organ and hematologic malignancies.24-28
The mechanism by which bortezomib induces apoptosis and inhibits proliferation has not been fully elucidated, though may involve nuclear factor κB,29,30 cyclin-dependent kinase inhibitors,31,32 apoptotic mediators including BCL-2 family members,33-37 and generation of reactive oxygen species.38 A number of in vitro studies of PI in CML have been published. These studies used BCR-ABL+ cell lines both sensitive and resistant to IM and demonstrated antiproliferative and apoptotic effects.39-41 In addition, the PI Z-Ile-Glu(OtBu)-Ala-Leucinal selectively inhibits colony formation by CD34+ BCR-ABL+ progenitor cells.41 Despite promising in vitro effects, there are little in vivo data. A phase 2 study has been published in abstract form, in which bortezomib was used in 7 IM-refractory or IM-intolerant patients; however, no cytogenetic responses were seen and no survival data were presented.42
Here we aimed to establish the effects of bortezomib in CD34-enriched samples taken from patients with CP CML at diagnosis. We performed standard in vitro assays and demonstrate induction of apoptosis and inhibition of proliferation by bortezomib, with an effect on colony formation after long-term culture. In vivo evidence of an impairment of CML HSC function is provided with relatively poor engraftment of bortezomib-treated patient-derived CD34+ CML cells. With a view to clinical application of our findings, we also assessed effects in cell lines expressing BCR-ABL mutations (with various degrees of TKI resistance) and considered synergy of bortezomib in combination with dasatinib.
Methods
Reagents
Dasatinib was stored as aliquots of 10 mg/mL in dimethyl sulphoxide and bortezomib (Millennium Pharma) as aliquots of 1 mg/mL in 0.9% NaCl. Both drugs were stored at −20°C and diluted in phosphate-buffered saline immediately before use.
Cell lines
Ba/F3 cells transfected with p210 and mutated (T315I, H396P, M351T) BCR-ABL (a gift from Dr Brian Druker, Howard Hughes Medical Institute, Portland, OR) were cultured (humidified incubator at 37°C and 5% CO2) in RPMI-1640 medium (Sigma-Aldrich) supplemented with l-glutamine (2 mM), 10% fetal bovine serum, penicillin (105 units/mL), and streptomycin (100 mg/L; all from Invitrogen Ltd). Parental Ba/F3 (BCR-ABL−) cells were cultured in similar conditions with the addition of recombinant murine interleukin-3 (IL-3, 10 ng/mL; Peprotech).
Patient samples
Patient samples were leukapheresis products taken at time of diagnosis with CP CML, with informed consent in accordance with the Declaration of Helsinki and approval of the National Health Service Greater Glasgow Institutional Review Board. The CD34+ population was enriched using CliniMACS (Miltenyi Biotec) according to standard protocols, before storage as aliquots at −150°C. For particular experiments, CML CD34+ samples were stained with CD34-allophycocyanin and CD38–fluorescein isothiocyanate (FITC; BD Biosciences) and sorted using a FACSAria (BD Biosciences) to obtain the CD34+38− population. CML CD34+ samples were cultured (humidified incubator at 37°C and 5% CO2) in serum-free media (SFM) consisting of Iscove modified Dulbecco medium (Sigma-Aldrich) supplemented with serum substitute (bovine serum albumin/insulin/transferrin, BIT9500; StemCell Technologies), 2mM l-glutamine, 105 units/mL penicillin, 100 mg/mL streptomycin, 0.1mM 2-mercaptoethanol, and 0.8 μg/mL low-density lipoprotein (all from Sigma-Aldrich). SFM was supplemented with a 5 growth factor (+5GF) cocktail of 20 ng/mL recombinant human IL-3 (rhIL-3), 20 ng/mL rhIL-6, 100 ng/mL rhFlt3-ligand, 100 ng/mL rh stem cell factor (all from StemCell Technologies) and 20 ng/mL rh granulocyte colony-stimulating factor (Chugai Pharma Europe) except where indicated (−5GF). “Normal CD34+” samples were CD34+-enriched non-CML leukapheresis products maintained and used as described for CML CD34+ samples.
Cell counting and apoptosis assays
After retrieval from −150°C aliquot and an overnight incubation, cells were seeded in 24-well plates at 2.5 × 105 cells/mL before drug exposure. After treatment, aliquots were removed and counted by trypan blue (Sigma-Aldrich) exclusion in triplicate. The remaining sample was then stained with annexin V–FITC and 7-amino-actinomycin D (7-AAD, Via-Probe solution; both from BD Biosciences) according to the manufacturer's recommendations. Flow cytometry (FACSCalibur; BD Biosciences) enabled distinction of viable cells (annexin V–FITC−, 7-AAD−) from those in early (annexin V–FITC+, 7-AAD−) or late apoptosis (annexin V–FITC+, 7-AAD+). All individual samples were analyzed as a minimum in duplicate. Except where documented, all results are expressed as a mean plus or minus SEM. Active caspase-3 was exploited as a marker for cells undergoing apoptosis. We used the FIX and PERM kit (Caltag Laboratories) before intracellular staining with anti–caspase-3-PE (BD Biosciences) according to the manufacturer's recommendations.
For combination assays, CML CD34+ cells were treated simultaneously with bortezomib and dasatinib with a viable cell count established after 72-hour incubation. A checker square of drug concentrations was used to enable assessment of different fixed ratios of drug combinations.
Western blotting
After 24-hour culture in experimental conditions CML CD34+ cells (1 × 106 per sample) were resuspended in standard lysis buffer (50mM Tris [tris(hydroxymethyl)aminomethane], pH 7.5, 150mM NaCl, 5mM ethylenediaminetetraacetic acid [all from Sigma-Aldrich], and 1% Nonidet P-40 substitute [Roche Diagnostics]) containing a protease inhibitor cocktail (1 Complete mini-tab/10 mL of lysis buffer; Roche Diagnostics). For sodium dodecyl sulfate gel separation and semidry electrophoretic transfer, a Mini-PROTEAN and Trans-blot SD cell were used (both from Bio-Rad Laboratories). Ten-microgram samples (BSA Protein Assay Kit; Pierce) were loaded using a Laemmli sample buffer, with a molecular weight marker and separated on a 4% to 15% gradient gel (Bio-Rad Laboratories). After transfer onto a nitrocellulose membrane and exposure to a blocking solution (10% low-fat dried milk in Tris-buffered saline Tween 20), the membrane was incubated overnight at 4°C with the primary antibody of interest (anti–pan-actin, antiubiquitin, antiphosphorylated Crkl (pCrkl), anti-poly(ADP-ribose polymerase) [PARP]; all from Cell Signaling Technology and at 1:1000 dilution). Membranes were labeled with the appropriate horseradish peroxidase–labeled secondary antibody (anti–mouse, anti–rabbit; both from Cell Signaling Technology and at 1:3000 dilution). After a series of washes in Tris-buffered saline Tween 20 and a short incubation with a chemiluminescence solution (ECL+; GE Healthcare Ltd), the images were developed onto photographic film (Kodak X-OMAT 1000). The membranes were then stripped of bound antibody using Re-Blot solution (Upstate) according to the manufacturer's recommendation.
Proteasome extraction and activity assay
The method for proteasome extraction and activity assay has previously been described.22 Cells (107) were cultured in experimental conditions, washed in phosphate-buffered saline, and resuspended in fresh adenosine triphosphate/dithiothreitol lysis buffer. After sonication and centrifugation, the supernatant was collected and protein concentration calculated as previously described. Proteasome subunit activity was detected as release of 7-amido-4-methylcoumarin (AMC) from the fluorogenic substrates N-Succ-LLVY-AMC, Z-ARR-AMC, and Z-LLE-AMC (all from Sigma-Aldrich; used to measure CT-L, T-L, and PG activities, respectively). Excitation and emission wavelengths of 380 nm and 460 nm, respectively, were analyzed, using a dual-monochromator, multidetection microplate reader (SpectraMax M5). Fluorescence readings (arbitrary fluorescence units [AFUs]) were taken every 5 minutes for 35 minutes starting immediately after addition of the fluorogenic substrate. The linear rise in AFU per unit of time determined the rate of fluorescence release (AFU/minute). This technique was unsuitable for CML CD34+ cells because of the large numbers of cells required.
CFSE tracking of cell division
CD34+ CML cells were removed from storage in liquid nitrogen and exposed to 1μM carboxy-fluorescein diacetate succinimidyl diester (CFSE; Invitrogen). This was considered day 0. After overnight incubation (day 1), cells were cultured (SFM+5GF) for 72 hours (day 3) in the following conditions: untreated; 5, 10, or 20nM bortezomib; 150nM dasatinib; and 100 ng/mL demecolcine (Colcemid; Sigma-Aldrich). Final analysis at day 3 was by viable cell counting and flow cytometry with anti–CD34-PE and propidium iodide (both from BD Biosciences). The use of demecolcine as an agent arresting cell-cycle progression aided identification of the undivided population and geometric calculation of subsequent division peaks.
Long-term culture-initiating cell assay
CD34+ CML cells were cultured (SFM+5GF) for 24 hours in the following conditions: untreated; 10nM dasatinib; 10nM bortezomib; and both 10nM dasatinib and 10nM bortezomib. Cells were then overlaid on a pre-established, irradiated (80 Gy) stromal layer (M2-10B and S1/S1 fibroblasts) in myeloid long-term culture medium (Myelocult; StemCell Technologies). Cultures were maintained for 5 weeks with weekly half-medium changes. At this time, cells were harvested, counted, and transferred to Methocult media (StemCell Technologies) for colony-forming assays. Colonies were counted by light microscopy after 2-week culture.
Engraftment of treated cells in mice
CML CD34+ cells (2 × 106 cells/mouse) were exposed to 4 different experimental conditions (untreated, bortezomib [10/20nM] or dasatinib [10/150nM] alone, and bortezomib and dasatinib in combination) for 24 or 72 hours in SFM+5GF. The cells were then harvested, washed, and transplanted via tail vein injection into sublethally irradiated (300 cGy), 8-week-old NOD.Cg-Prkdcscid IL2rgtm1Wjl /SzJ mice (NSG mice; The Jackson Laboratory). Mice were killed after 6 weeks and marrow contents of femurs, spleen cells, and blood cells were obtained at necropsy. To assess human cell engraftment, cells were labeled with anti–human CD45 antibody and analyzed by flow cytometry. To assess engraftment of BCR-ABL+ cells, BCR-ABL mRNA levels in enriched human CD45+ cells were evaluated by quantitative polymerase chain reaction (Q-PCR). To quantify the frequency of BCR-ABL+ cells within the engrafted human CD45+ cells, double-fusion fluorescent in situ hybridization (D-FISH) was performed as previously described.18
Software and statistical analysis
GraphPad Prism Version 4 was used to draw graphs and charts, calculate descriptive statistics, predict dose-response curves, and perform statistical analysis (Student t test). Proteasome activity assay data were analyzed with SpectraMax Pro 5. Synergistic effects were predicted by CalcuSyn (Version 2.0; Biosoft). This program derives a combination index at a set drug ratio using a median-effect method of Chou and Talalay.43 A combination index of less than 1 indicates synergism; more than 1, antagonism; and 1, additive effect. Flow cytometry data were analyzed by CellQuest Pro software (BD Biosciences).
Results
Bortezomib is antiproliferative and induces apoptosis in CML CD34+ patient samples
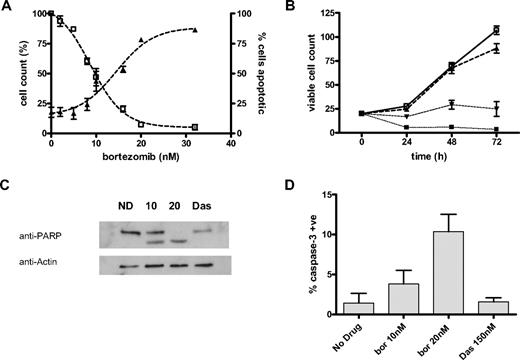
CML CD34+ cells were cultured in SFM+5GF in the presence and absence of bortezomib (Figure 1A). At each time point, the viable cell count and percentage of apoptotic cells were calculated. The effect of bortezomib did not vary significantly between different patient samples and the results were therefore pooled with mean plus or minus SEM established and dose-response curves predicted. The median inhibitory concentration (IC50) was 8.8 plus or minus 0.7nM (n = 5) at 24 hours, 7.6 plus or minus 0.4nM (n = 3) at 48 hours, and 5.7 plus or minus 1.6nM (n = 7) at 72 hours. The effect of bortezomib on the expansion of the CML CD34+ cell population in SFM+5GF can be seen in Figure 1B. Samples (n = 3) were seeded and analyzed at 24, 48, and 72 hours as described. CML CD34+ cells (n = 1) were also analyzed for the effects of bortezomib on cell viability in SFM−5GF with the IC50 7.89 plus or minus 1.07nM (24 hours), 3.54 plus or minus 1.41nM (48 hours), and 4.13 plus or minus 0.18nM (72 hours). Furthermore, apoptosis was demonstrated with bortezomib exposure at 24-hour incubation by cleavage of the caspase substrate PARP (Figure 1C) shown with Western blot and increased detection of intracellular activated caspase-3 by flow cytometry (Figure 1D). Dasatinib 150nM did not induce significant apoptosis at this time point by either method.
Induction of apoptosis in CML cells. (A) CML CD34+ cells (n = 5) were analyzed at 24 hours of drug exposure. The viable cell count (□) is expressed as a percentage of untreated (cell count %). The percentage of cells staining positive for 7-AAD (▴) is expressed on the y-axis (% cells apoptotic). Results represent the mean ± SEM with predicted dose-response curves. (B) CML CD34+ cells (n = 3) cultured in SFM+5GF and the viable cell count (× 104 cells/mL) established at each time point as described. Results illustrated are untreated (□), and treated with 4nM (▴), 8nM (▾), and 16nM (■) bortezomib and are expressed as mean ± SEM with connecting lines. (C) Representative example of Western blot of CML CD34+ cells untreated (ND) or exposed to 10 or 20nM bortezomib (10,20) or 150nM dasatinib (Das) for 24 hours. The lower band represents the apoptosis-related PARP cleavage product. (D) CML CD34+ cells (n = 3) cultured for 24 hours in SFM+5GF and analyzed by flow cytometry to assess the percentage of cells with detectable active caspase-3 (mean ± SEM).
Induction of apoptosis in CML cells. (A) CML CD34+ cells (n = 5) were analyzed at 24 hours of drug exposure. The viable cell count (□) is expressed as a percentage of untreated (cell count %). The percentage of cells staining positive for 7-AAD (▴) is expressed on the y-axis (% cells apoptotic). Results represent the mean ± SEM with predicted dose-response curves. (B) CML CD34+ cells (n = 3) cultured in SFM+5GF and the viable cell count (× 104 cells/mL) established at each time point as described. Results illustrated are untreated (□), and treated with 4nM (▴), 8nM (▾), and 16nM (■) bortezomib and are expressed as mean ± SEM with connecting lines. (C) Representative example of Western blot of CML CD34+ cells untreated (ND) or exposed to 10 or 20nM bortezomib (10,20) or 150nM dasatinib (Das) for 24 hours. The lower band represents the apoptosis-related PARP cleavage product. (D) CML CD34+ cells (n = 3) cultured for 24 hours in SFM+5GF and analyzed by flow cytometry to assess the percentage of cells with detectable active caspase-3 (mean ± SEM).
Bortezomib inhibits proteasome activity but does not affect BCR-ABL activity in CML CD34+ patient samples
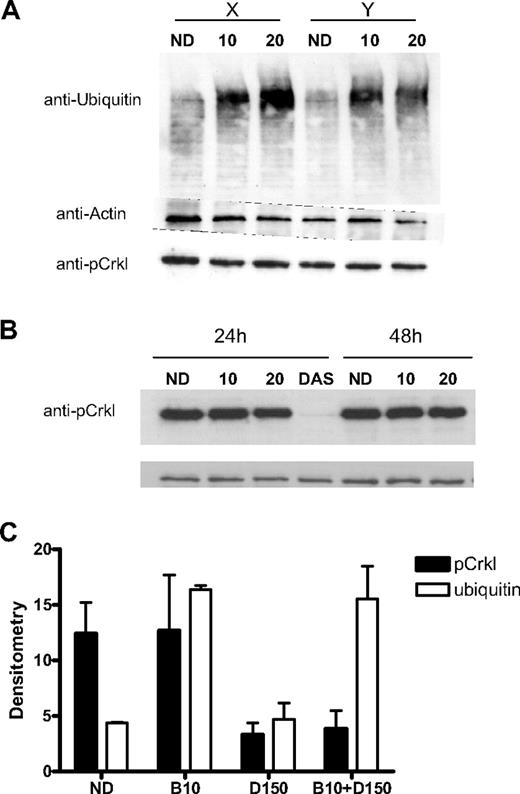
Evidence for proteasome inhibition in CML CD34+ cells after 24-hour exposure to bortezomib was provided by the accumulation of polyubiquitinated proteins with Western blot. This effect was not seen with dasatinib 150nM alone (Figure 2A,C). We used the presence of the adaptor protein pCrkl as a specific marker of BCR-ABL kinase activity. The detection of pCrkl by Western blot after 24- and 48-hour bortezomib and 24-hour dasatinib treatment is seen in Figure 2B. Despite significant effects on cell viability, there was minimal effect on BCR-ABL activity with either 10nM bortezomib (97% ± 23% vs untreated cells) or 20nM bortezomib (73% ± 5%) in contrast to dasatinib 150nM (23% ± 10%)—results from 24-hour drug exposure, expressed as densitometry readings (n = 2) using the ratio of pCrkl-actin bands shown as a percentage of the untreated sample (mean ± SD).
Effect of bortezomib on proteasome and BCR-ABL activity. (A) CML CD34+ samples (n = 2) labeled X and Y were treated for 24 hours with bortezomib 10 or 20nM (10,20) and compared with an untreated control (ND). Accumulation of polyubiquitinated proteins of various molecular weights was seen. Protein loading was assessed using antiactin antibody. The presence of significant BCR-ABL activity represented by the pCrkl band can be seen in all samples. (B) A representative CML CD34+ sample treated with bortezomib 10 or 20nM (10, 20) or dasatinib 150nM (DAS) for 24 to 48 hours and compared with untreated control (ND). The presence of the pCrkl band demonstrates the significant residual BCR-ABL kinase activity in PI-treated samples compared with the abrogated activity in the TKI-treated sample. (C) Densitometry of pCrkl (■) and ubiquitin (□) relative to protein loading control for 3 samples is shown after bortezomib 10nM (B10), dasatinib 150nM (D150), and combination treatment (mean ± SEM).
Effect of bortezomib on proteasome and BCR-ABL activity. (A) CML CD34+ samples (n = 2) labeled X and Y were treated for 24 hours with bortezomib 10 or 20nM (10,20) and compared with an untreated control (ND). Accumulation of polyubiquitinated proteins of various molecular weights was seen. Protein loading was assessed using antiactin antibody. The presence of significant BCR-ABL activity represented by the pCrkl band can be seen in all samples. (B) A representative CML CD34+ sample treated with bortezomib 10 or 20nM (10, 20) or dasatinib 150nM (DAS) for 24 to 48 hours and compared with untreated control (ND). The presence of the pCrkl band demonstrates the significant residual BCR-ABL kinase activity in PI-treated samples compared with the abrogated activity in the TKI-treated sample. (C) Densitometry of pCrkl (■) and ubiquitin (□) relative to protein loading control for 3 samples is shown after bortezomib 10nM (B10), dasatinib 150nM (D150), and combination treatment (mean ± SEM).
Bortezomib is antiproliferative and induces apoptosis in the CML CD34+38− population, although with minimal selectivity compared with equivalent non-CML cells
CD34+38− cells were enriched from 3 CML patient samples. They were cultured in SFM+5GF with bortezomib and viable cell counts taken at 24 hours of drug exposure (Figure 3A). The IC50 at 24 hours was 8.93 plus or minus 1.42nM. This was similar to the IC50 of the unsorted CML CD34+ cells. To illustrate this consistent effect on different cell populations, CML CD34+38− and CML CD34+38+ cells (n = 3) were exposed to bortezomib for 24 hours and effects compared (Figure 3B). The IC50 of bortezomib with CD34+38+ cells (8.18 ± 0.64nM) was not significantly different from the CD34+38− population. Similar experiments were performed in duplicate using 2 non-CML samples. At 24 hours of drug exposure, the IC50 was 10.18 plus or minus 1.85nM (Figure 3C), which was not significantly higher for CML CD34+38− cells. Although it is disappointing that bortezomib is nonselective and induces apoptosis in both CML and normal CD34+38− cells, this is the case for many agents currently used to treat hematologic malignancies.
Effect of bortezomib on CD34+38− and undivided CML cells. (A) CD34+38− (n = 3) cells were cultured in SFM+5GF and analyzed at 24 hours. The viable cell count (□) is expressed as a percentage of untreated cells (cell count %). The percentage of cells staining positive for 7-AAD (▴) is expressed on the y-axis (% cells apoptotic). (B) The effect of bortezomib on CD34+38− (■) and CD34+38+ (□) cells (n = 3) was compared. The viable cell count is expressed as in panel A. (C) CD34+38− (n = 2) cells from non-CML samples were cultured in SFM+5GF and analyzed at 24 hours. The viable cell count (□) and percentage of apoptotic cells (▴) are derived as in panel A. (D) CD34+ CFSE-stained CML cells were cultured in SFM+5GF and analyzed by flow cytometry at 72 hours. Cell division peaks were calculated relative to demecolcine control. The percentage of residual viable cells in each division peak is shown for untreated (■), or treated with 150nM dasatinib ( ), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.
), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.
Effect of bortezomib on CD34+38− and undivided CML cells. (A) CD34+38− (n = 3) cells were cultured in SFM+5GF and analyzed at 24 hours. The viable cell count (□) is expressed as a percentage of untreated cells (cell count %). The percentage of cells staining positive for 7-AAD (▴) is expressed on the y-axis (% cells apoptotic). (B) The effect of bortezomib on CD34+38− (■) and CD34+38+ (□) cells (n = 3) was compared. The viable cell count is expressed as in panel A. (C) CD34+38− (n = 2) cells from non-CML samples were cultured in SFM+5GF and analyzed at 24 hours. The viable cell count (□) and percentage of apoptotic cells (▴) are derived as in panel A. (D) CD34+ CFSE-stained CML cells were cultured in SFM+5GF and analyzed by flow cytometry at 72 hours. Cell division peaks were calculated relative to demecolcine control. The percentage of residual viable cells in each division peak is shown for untreated (■), or treated with 150nM dasatinib ( ), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.
), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.
The effect of bortezomib on differentiation and division of 3 different CD34+ CML patient samples cultured in SFM+5GF was assessed by flow cytometry with CFSE, anti–CD34-PE, and propidium iodide staining. After 72-hour culture, the majority of remaining viable (propidium iodide–treated) and untreated CD34+ cells had undergone 3 or more divisions. With bortezomib or dasatinib exposure, residual viable cell numbers were reduced (in line with results from Figure 1A) and those cells surviving had undergone relatively few divisions (Figure 3D). To compare between untreated and drug-treated cells, the total number of viable, undivided, and therefore quiescent cells remaining in culture, a recovery calculation was made (Figure 3E-F). This was based on viable cell count, CFSE, CD34, and propidium iodide staining. By this technique, it was seen that the total recovery of cells was reduced relative to control in all conditions; however, the recovery of quiescent cells was not affected. This assay is able to quantify the numbers of CD34+ and undivided/quiescent CD34+ cells remaining after drug exposures but is unable to assess function in cells exposed to dasatinib versus bortezomib. This has been assessed using long-term culture-initiating cell [LTC-IC] and engraftment of NOD-SCID mice.
Bortezomib reduces long-term colony formation and inhibits engraftment of BCR-ABL+ cells in mice
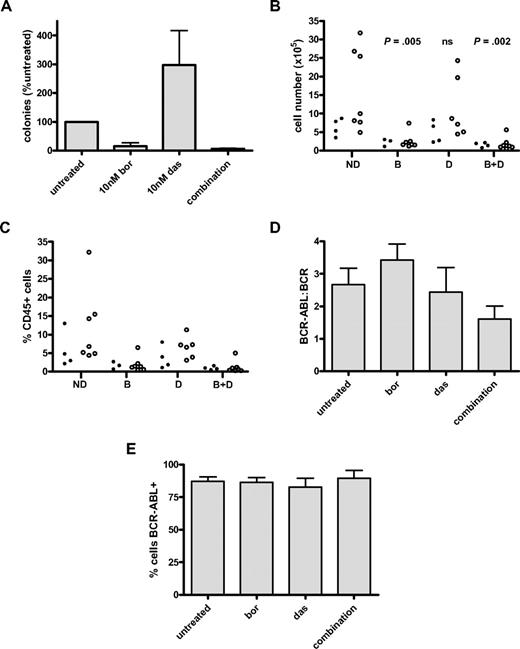
We used 2 established techniques to assess the effect of bortezomib on stem cell function—LTC-IC and engraftment of treated cells in NSG mice. For LTC-IC, CD34+ CML cells were exposed to experimental conditions and cultured for 5 weeks in the presence of stromal cells. Colony formation was then measured after 2 more weeks of culture. Two different CML samples were treated in this way (in duplicate), with results shown in Figure 4A. Relative to untreated cells, colony formation was reduced in the bortezomib-treated arms (bortezomib alone: 15.5% ± 11.7%, combination: 6.7% ± 2%). It is important to note that the apparent increase in colonies with dasatinib alone (298.6% ± 119.0%) is not statistically significant because only 2 CML samples were assayed. However, over 24-hour culture in the presence of 5GF, the stem cells present in the no-drug control may have undergone 1 or more divisions and thereby lost their LTC-IC potential, leading to a lower number of colonies in the untreated control, compared with the number of colonies present at baseline (ie, before the 24-hour culture). Furthermore, the presence of dasatinib, which is antiproliferative without inducing apoptosis, will have prevented stem cell division over the 24-hour culture (as we have published before for 72-hour cultures44 ). Taken together, the culture conditions are likely to reduce the colony number in the no-treatment arm and to therefore produce an apparent increase in colonies for dasatinib.
Effect of bortezomib on long-term colony formation and engraftment of BCR-ABL+ cells. (A) Results of LTC-IC assay in CD34+ CML (n = 2 CML samples each performed in duplicate). Results are expressed as percentage of number of colonies counted after culture of untreated cells (mean ± SEM). Cells were exposed to drug for 24 hours before long-term culture with 10nM bortezomib and 10nM dasatinib. Statistical analysis was not performed because only 2 samples were assayed. The apparent increase in colonies after dasatinib exposure is not thought to be significant. (B) Engraftment of treated CD34+ CML cells in sublethally irradiated mice. In the first experiment, 1 CML sample was exposed to no drug, bortezomib 10nM, dasatinib 10nM, or the combination of bortezomib 10nM with dasatinib 10nM for 72 hours and then injected into 4 mice per arm (ie, 16 mice; ●). In the second and third experiments, CML samples (1 per experiment) were exposed to no drug, bortezomib 20nM, dasatinib 150nM, or the combination of bortezomib 20nM with dasatinib 150nM for 24 hours and then injected into 4 mice per arm per experiment (ie, 32 mice; ○). The total number of human CD45+ cells isolated from BM at 6 weeks is shown with statistical comparison of experimental arms in comparison with untreated control. (C) The data generated from panel B are expressed as percentage of human CD45+ cells isolated from mouse BM at 6 weeks. (D) Human CD45+ cells from panel B underwent analysis by Q-PCR to determine BCR-ABL/BCR ratio. Because engraftment levels showed similar trends within each arm for the 3 CML samples assayed, the data were pooled for each treatment arm across all 3 experiments. (E) For each mouse, the percentage of CD45+ cells that is BCR-ABL+ by D-FISH is shown.
Effect of bortezomib on long-term colony formation and engraftment of BCR-ABL+ cells. (A) Results of LTC-IC assay in CD34+ CML (n = 2 CML samples each performed in duplicate). Results are expressed as percentage of number of colonies counted after culture of untreated cells (mean ± SEM). Cells were exposed to drug for 24 hours before long-term culture with 10nM bortezomib and 10nM dasatinib. Statistical analysis was not performed because only 2 samples were assayed. The apparent increase in colonies after dasatinib exposure is not thought to be significant. (B) Engraftment of treated CD34+ CML cells in sublethally irradiated mice. In the first experiment, 1 CML sample was exposed to no drug, bortezomib 10nM, dasatinib 10nM, or the combination of bortezomib 10nM with dasatinib 10nM for 72 hours and then injected into 4 mice per arm (ie, 16 mice; ●). In the second and third experiments, CML samples (1 per experiment) were exposed to no drug, bortezomib 20nM, dasatinib 150nM, or the combination of bortezomib 20nM with dasatinib 150nM for 24 hours and then injected into 4 mice per arm per experiment (ie, 32 mice; ○). The total number of human CD45+ cells isolated from BM at 6 weeks is shown with statistical comparison of experimental arms in comparison with untreated control. (C) The data generated from panel B are expressed as percentage of human CD45+ cells isolated from mouse BM at 6 weeks. (D) Human CD45+ cells from panel B underwent analysis by Q-PCR to determine BCR-ABL/BCR ratio. Because engraftment levels showed similar trends within each arm for the 3 CML samples assayed, the data were pooled for each treatment arm across all 3 experiments. (E) For each mouse, the percentage of CD45+ cells that is BCR-ABL+ by D-FISH is shown.
To investigate the potential in vivo significance of this, CML CD34+ cells taken from 3 different patients and each exposed to 4 different experimental conditions (no-drug control, bortezomib, dasatinib, and the combination) were injected into sublethally irradiated NSG mice (4 mice per arm × 3 experiments = 48 mice). Engraftment of human CD45+ cells was assessed at 6 weeks in surviving mice in peripheral blood, bone marrow (BM), and spleen. Mean plus or minus SEM CD45+ cell engraftment of BM at 6 weeks was 12.18 plus or minus 5.63 (untreated), 2.33 plus or minus 0.39 (bortezomib), 9.28 plus or minus 3.44 (dasatinib), and 1.63 plus or minus 0.18 (combined) × 10 cells. Standard deviation values were 12.7, 2.4, 8.8, and 1.6 × 105 cells, respectively. Data from BM are shown (Figure 4B). Drug exposures were for either 24 (Figure 4B open circle) or 72 (filled circle) hours. Cells treated with bortezomib showed markedly reduced levels of engraftment, with statistical significance (2-tailed t test) achieved when comparing untreated cells with bortezomib alone (P = .005) or in combination with dasatinib (P = .002). No significant difference was seen between untreated control and cells treated with dasatinib alone. The percentage of human CD45 cells obtained from mouse BM at 6 weeks is shown in Figure 4C. BCR-ABL remained detectable by Q-PCR in those cells recovered from mice that underwent transplant (Figure 4D). Although there was a trend to lower BCR-ABL/BCR ratios in the combination arm, this did not achieve statistical significance (P = .114 vs no-drug control). Because transcript numbers per cell can vary, we performed D-FISH on the purified human CD45+ cells from each treatment arm. A high proportion of engrafted human cells were BCR-ABL+, confirming that this technique does indeed detect leukemic HSCs (Figure 4E).
Dasatinib and bortezomib act synergistically against CML CD34+ cells in vitro
CML CD34+ cells (n = 3) were cultured in SFM+5GF and exposed simultaneously to a combination of bortezomib and dasatinib. Cell counts and flow cytometry were performed at 72 hours to assess the effects on cell viability. This time point was chosen as preliminary experiments demonstrated that earlier time points underestimated cell death induced by dasatinib. Dasatinib was chosen for these experiments as a potent and clinically available TKI that our group has previously shown to inhibit BCR-ABL more strongly than IM in CML CD34+ cells.18 For the final analysis, 3 different samples were examined in duplicate at comparable fixed ratios of bortezomib-dasatinib. The reduction in viable cell count compared with the untreated sample was converted to a reciprocal “effect” as is conventionally required for data interpretation. The data were analyzed using CalcuSyn and significant synergy was demonstrated in these samples at all 3 drug ratios and at all 3 end points, including lethal dose 50% (LD50), LD75, and LD90 (Table 1; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The likely explanation for our ability to demonstrate significant levels of synergy between bortezomib and dasatinib against bulk CD34+ cells but not against either LTC-IC or NSG engraftment is as follows. Bulk CD34+ cells, although containing CML stem cells, are not highly enriched for stem cell activity. Therefore we see significant effects for dasatinib alone, for bortezomib alone, and for the drugs used in combination. Furthermore, using bulk CD34+ cells we were able to explore multiple drug combination ratios, including relatively low drug concentrations. Importantly, we did not demonstrate antagonism for this drug combination at any ratio. For the functional work (ie, LTC-IC and NSG engraftment), although bulk CD34+ cells were exposed to the single drugs and the combination, only the most primitive stem cells are able to “readout” in these assays. Furthermore, the complex nature and larger cell requirement of these assays meant that we had to select limited time points for drug exposure and were not able to explore the range of drug combination ratios used in the synergism experiments. In particular, the few concentrations of bortezomib selected for these experiments were too high to allow any synergistic effects to be revealed. In this setting, we saw a predominant effect from bortezomib with little/no additional effect from dasatinib. The lack of effect from dasatinib against this very primitive CML stem cell population is expected and has been reported previously by ourselves and others.18,45
Synergistic effect with bortezomib and dasatinib
| Ratio . | Combination index . | r . | ||
|---|---|---|---|---|
| LD50 . | LD75 . | LD90 . | ||
| 2.5:1 | 0.67 ± 0.16 | 0.54 ± 0.16 | 0.53 ± 0.21 | 0.85-0.99 |
| 5:1 | 0.78 ± 0.12 | 0.66 ± 0.13 | 0.64 ± 0.16 | 0.90-0.98 |
| 10:1 | 0.87 ± 0.25 | 0.75 ± 0.22 | 0.69 ± 0.22 | 0.93-0.94 |
| Ratio . | Combination index . | r . | ||
|---|---|---|---|---|
| LD50 . | LD75 . | LD90 . | ||
| 2.5:1 | 0.67 ± 0.16 | 0.54 ± 0.16 | 0.53 ± 0.21 | 0.85-0.99 |
| 5:1 | 0.78 ± 0.12 | 0.66 ± 0.13 | 0.64 ± 0.16 | 0.90-0.98 |
| 10:1 | 0.87 ± 0.25 | 0.75 ± 0.22 | 0.69 ± 0.22 | 0.93-0.94 |
Samples examined by cell counting and flow cytometry to obtain viable cell counts at 72-hour exposure to both drugs, which were added simultaneously at the beginning of the experiment. Drug ratio is bortezomib to dasatinib. Combination index is expressed as mean ± SD from samples (n = 3) examined in duplicate and the r values are expressed as a range. All figures are derived from CalcuSyn software (Biosoft).
Bortezomib induces apoptosis in BCR-ABL+ cell lines including those expressing T315I and basal proteasome activity varies in different BCR-ABL mutants
Ba/F3 cells expressing BCR-ABL were cultured for 24 hours in the presence of bortezomib. Viable cell counts and percentage apoptotic cells were established (Figure 5A-B). IC50 values were 6.16 plus or minus 0.48nM (p210), 10.06 plus or minus 0.51nM (H396P), 10.50 plus or minus 1.08nM (M351T), and 7.86 plus or minus 0.46nM (T315I). The presence of active caspase-3 was demonstrated by intracellular staining with anti–active capase-3–PE and flow cytometry. In all BCR-ABL+ cell lines, there was significantly more antibody bound after exposure to 20nM bortezomib than in untreated controls (all P values ≤ .01); however, there was no statistical difference in this measure of apoptosis induction between the different BCR-ABL mutants. Parental Ba/F3 (BCR-ABL−) cells were cultured with IL-3 supplementation and treated with bortezomib. Bortezomib induced apoptosis with IC50 value of 5.25 plus or minus 1.45nM, consistent with previously documented IL-3–stimulated nuclear factor κB dependence of these cells; therefore, these cells were not deemed a suitable negative control.46
Effect of bortezomib on BCR-ABL+ cell lines. (A) Ba/F3 cells transfected with p210 (□), H396P (▴), M351T (▾) or T315I (●) BCR-ABL and cultured with bortezomib for 24 hours. Viable cell count is expressed as percentage of untreated cells. (B) BCR-ABL+ Ba/F3 cells were cultured either with no drug or treated with bortezomib 20nM for 24 hours and then stained for active caspase-3 as described. A significant increase in anti–active capase-3 staining was seen with bortezomib treatment (P values as shown). There was no significant difference within the untreated or treated groups. (C) Basal proteasome activity in untreated BCR-ABL+ Ba/F3 cells. *Statistical significance was achieved when comparing mutant forms to p210 in PG activity (P < .01 [H396P], P = .013 [M351T]) and CT-L activity (P = .047 [T315I], P = .045 [H396P], P = .038 [M351T]). (D) Residual CT-L activity in BCR-ABL+ Ba/F3 cells after exposure to 20nM bortezomib for 24 hours, expressed as percentage of untreated controls. AFU is arbitrary fluorescence units released per minute, as described. Results in panels A through C represent the mean ± SEM with predicted dose-effect curve in panel A.
Effect of bortezomib on BCR-ABL+ cell lines. (A) Ba/F3 cells transfected with p210 (□), H396P (▴), M351T (▾) or T315I (●) BCR-ABL and cultured with bortezomib for 24 hours. Viable cell count is expressed as percentage of untreated cells. (B) BCR-ABL+ Ba/F3 cells were cultured either with no drug or treated with bortezomib 20nM for 24 hours and then stained for active caspase-3 as described. A significant increase in anti–active capase-3 staining was seen with bortezomib treatment (P values as shown). There was no significant difference within the untreated or treated groups. (C) Basal proteasome activity in untreated BCR-ABL+ Ba/F3 cells. *Statistical significance was achieved when comparing mutant forms to p210 in PG activity (P < .01 [H396P], P = .013 [M351T]) and CT-L activity (P = .047 [T315I], P = .045 [H396P], P = .038 [M351T]). (D) Residual CT-L activity in BCR-ABL+ Ba/F3 cells after exposure to 20nM bortezomib for 24 hours, expressed as percentage of untreated controls. AFU is arbitrary fluorescence units released per minute, as described. Results in panels A through C represent the mean ± SEM with predicted dose-effect curve in panel A.
Linear rises in AFU, consistent with AMC release representing the 3 different proteasome subunit enzyme activities, were detectable in BCR-ABL+ Ba/F3 cells (Figure 5C). Interestingly, there was a significant difference in the basal PG and CT-L activity when p210-Ba/F3 cells were compared with the BCR-ABL+ mutants (Figure 5C), with p210 demonstrating the lowest values. In all 4 cell lines, the CT-L activity was reduced by 36% to 47% with 24-hour exposure to bortezomib 20nM, consistent with the known activity of this drug (Figure 5D). Despite 53% to 64% residual CT-L activity, significant apoptosis was demonstrated with this dose (Figure 5A-B), suggesting a threshold effect.
Discussion
The current recommended treatment for the majority of patients with CP CML is IM. There is accumulating evidence that the majority of patients respond to this drug and achieve the therapeutic milestone of a CCyR. However, it is estimated that in patients with undetectable BCR-ABL transcripts by PCR, up to 106 leukemia cells may remain.47 As a consequence of MRD, it is recommended that TKI treatment be continued indefinitely. The safety of discontinuing IM in those with sustained undetectable BCR-ABL is the focus of a clinical trial (STop IMatinib study); however, preliminary evidence reveals the significant risk of disease relapse.16 The source of MRD in IM-treated patients is presumed to be the CML HSCs. These cells are relatively insensitive to TKI. Furthermore, the development of TKI-resistant BCR-ABL mutations is a cause of primary and secondary treatment failure. In particular, T315I is resistant to all available TKIs, and there is concern that selection pressure may result in this mutation becoming more prevalent.
The proteasome is an attractive target for cancer therapy and there has been expansion of the clinical use of bortezomib, to date the only licensed PI. There is published evidence that BCR-ABL expression is associated with increased proteasome activity; primitive CML cells express higher levels of BCR-ABL than their more mature counterparts and BCR-ABL+ cell lines are susceptible to PI.18,22,39,40 We have extended this work to demonstrate that CD34-enriched samples taken at diagnosis from patients with CP CML undergo apoptosis in SFM±5GF with bortezomib exposure. This effect was replicated in 7 different patient samples and the drug was consistent in its effect. The use and detection of CFSE retention revealed the antiproliferative effect of bortezomib in addition to the marked cell killing as demonstrated by estimation of cell recovery. Bortezomib exposure was associated with an accumulation of polyubiquitinated proteins consistent with inhibition of the proteasome. Despite these effects, bortezomib did not appear to influence BCR-ABL kinase activity as evident by pCrkl detection by Western blot. The IC50 in CML CD34+ cells was comparable with that quoted previously for CML blast crisis cell lines (10-15 and 32nM at 48 hours39,40 ), purified human multiple myeloma cells from BM (2.5-30nM at 48 hours48 ), and CD34-enriched human acute myeloid leukemia cells (5-10nM at 7 days49 ). Pharmacokinetic data are available from a series of myeloma patients treated with 1.3 mg/m2 (standard dose) that would support the claim that such concentrations are easily achieved in vivo.50 The level of proteasome inhibition achieved in the BM may be up to 50% less than that of the peripheral blood, although this claim is based on examination of small numbers of patients.28
We used an established technique to isolate the CD34+38− cells from patient samples. This population contains the primitive and quiescent HSCs that are resistant to current therapies. We demonstrate for the first time that these cells are sensitive to the apoptotic effects of bortezomib at comparable concentrations to those achieved in vivo. These data are in concordance with published observations of the sensitivity of CD34+38− acute myeloid leukemia cells to the PI MG132.51 We also show that bortezomib treatment, either alone or in combination with dasatinib at clinically relevant concentrations, markedly reduces the capacity for long-term colony formation—consistent with an effect on the primitive HSC population. Finally, we demonstrate that bortezomib treatment leads to a significant reduction in the engraftment potential of human CD34+ CML cells. It is important to note that within this reduced population of cells, there remained detectable BCR-ABL. This is consistent with the detection of a small number of undivided and viable cells at 72 hours of drug exposure by CFSE tracking experiments. These results would indicate that although a marked decline in primitive BCR-ABL+ HSC numbers has been achieved, they have not been eradicated after a single exposure to bortezomib. Relevant further experiments to assess the effects of cyclical exposure, consistent with the recommended dosing of this drug, have not yet been performed.
Clinical trials with bortezomib have shown that reversible hematologic toxicity (in particular severe thrombocytopenia) is very common. We show that nonmalignant CD34+38− cells are susceptible to the apoptotic effects of bortezomib, with IC50 values very similar to those of the CML HSCs. We could find no published data of the effects of bortezomib in this specific cell population. Other groups have used, as a control population, nonmalignant CD34+ enriched cells (IC50, 11.3nM by granulocyte-macrophage colony-forming unit and < 20nM by proliferation assays41,49 ) and peripheral blood lymphocytes (∼ 5% apoptosis with 50nM by annexin V staining52 ). The toxicity we demonstrate is concerning; however, the reversible nature of the severe cytopenias seen in patients may reflect an improved recovery of this population relative to malignant cells. It is noted that in phase 1 dose-escalation studies using more intensive regimens, the dose-limiting toxicity was hematologic.53
An established technique to ameliorate toxicity of chemotherapy is to use drug combinations. We speculated that TKIs may provide additional benefits to bortezomib treatment in view of the presence of residual BCR-ABL kinase activity in PI-treated cells. We demonstrate that bortezomib and dasatinib are synergistic in inducing cell death within bulk CD34+ CML cells using concentrations of both drugs that are easily achieved in vivo. Synergism could not be demonstrated in LTC-IC or NSG mouse engraftment. Because one drug of the combination, dasatinib, is ineffective in inducing apoptosis in CD34+38− cells or inhibition of LTC-IC and NSG engraftment, synergism would be unlikely. Furthermore, the limited number of replicates and concentrations used also reduced the chance of showing synergism.
Our results add to the evidence published previously that IM resistance does not appear to influence bortezomib sensitivity. In particular, we show that specific mutations of BCR-ABL are equally sensitive to apoptosis, including T315I. This is of clear relevance as there is no current licensed therapy available for patients with this mutation. Such results can be compared with those generated using the aurora kinase inhibitor MK-0457 and homoharringtonine.54,55 Interestingly, we show for the first time that the proteasome subunit activity may vary among different BCR-ABL mutations and this is not thought to be because of different BCR-ABL protein levels (data not shown). We plan to investigate this further using more specific assays of proteasomal subunit activity applied to TKI-resistant patient samples.
In conclusion, bortezomib is antiproliferative and induces apoptosis in human CML and normal cells and appears to have effects on the primitive stem cell population—believed to be a source of residual disease in TKI-treated patients. Potential toxicities associated with this drug may be bypassed by combining it with dasatinib or alternative TKI. Bortezomib is also effective in the presence of TKI-resistant BCR-ABL mutations. With these 2 key findings, we believe that PI should play a future role in the treatment of patients failing current therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
N.B.H. received full funding from The Leukemia Research Fund, UK. L.C. receives funding from The Leukemia Research Fund, Northern Ireland. R.B. receives support from the National Institutes of Health (R01 CA and HL).
National Institutes of Health
Authorship
Contribution: N.B.H. performed all the experiments (except mouse work, which was performed by B.Z., S.C., and R.B.), analyzed the data, and wrote the paper; F.P., L.C., S.M.A.K., E.K.A., and H.G.J. performed experiments; A.E.I. provided help in experimental design; T.L.H. supervised the project; and all authors commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tessa Holyoake, Professor of Experimental Haematology, Paul O'Gorman Leukaemia Research Centre, Gartnavel General Hospital, 1053 Great Western Rd, Glasgow, G12 0YN, United Kingdom; e-mail: tlh1g@clinmed.gla.ac.uk.

![Figure 3. Effect of bortezomib on CD34+38− and undivided CML cells. (A) CD34+38− (n = 3) cells were cultured in SFM+5GF and analyzed at 24 hours. The viable cell count (□) is expressed as a percentage of untreated cells (cell count %). The percentage of cells staining positive for 7-AAD (▴) is expressed on the y-axis (% cells apoptotic). (B) The effect of bortezomib on CD34+38− (■) and CD34+38+ (□) cells (n = 3) was compared. The viable cell count is expressed as in panel A. (C) CD34+38− (n = 2) cells from non-CML samples were cultured in SFM+5GF and analyzed at 24 hours. The viable cell count (□) and percentage of apoptotic cells (▴) are derived as in panel A. (D) CD34+ CFSE-stained CML cells were cultured in SFM+5GF and analyzed by flow cytometry at 72 hours. Cell division peaks were calculated relative to demecolcine control. The percentage of residual viable cells in each division peak is shown for untreated (■), or treated with 150nM dasatinib (), 10nM bortezomib (▩), and 20nM bortezomib (□). Cell recovery calculations for cells from all division peaks (E) and for undivided cells (F) in untreated and treated cells (bortezomib [bor] and dasatinib [das]). All results represent mean ± SEM with predicted dose-response curves.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/11/10.1182_blood-2008-06-164582/4/m_zh89991049650003.jpeg?Expires=1769082506&Signature=kbOX50GRXXFPr77tcaYNgb2huNKcO9WVscAy7SNtxEu3KX1trCjJrL6ezMZNxLyGqMToaTIjqpVtfJgOzq7AO3YAXUs~VUNc57~4wYysPADgaZIIxG-DTxRuC79XH-pxPIYs7zVRvsNQFkaUaPVaiYxt8n-11f-iiQa~MUfs0U4bBNGXwWkLXl5n0tieGckA87WPIkQitCM0btiYlxklZ1RavapR6emvplz4CeDWC9kFt1ZZ-4CpzG7XU4BwuQJbPnKGRoV6c1Cl2vkETx6sK2Y0Zll~Iwu51lg4q0qgCAYDYp4LNiYmZU0MQ91fXsurZI51njGRTTyzpEJjrStTtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Effect of bortezomib on BCR-ABL+ cell lines. (A) Ba/F3 cells transfected with p210 (□), H396P (▴), M351T (▾) or T315I (●) BCR-ABL and cultured with bortezomib for 24 hours. Viable cell count is expressed as percentage of untreated cells. (B) BCR-ABL+ Ba/F3 cells were cultured either with no drug or treated with bortezomib 20nM for 24 hours and then stained for active caspase-3 as described. A significant increase in anti–active capase-3 staining was seen with bortezomib treatment (P values as shown). There was no significant difference within the untreated or treated groups. (C) Basal proteasome activity in untreated BCR-ABL+ Ba/F3 cells. *Statistical significance was achieved when comparing mutant forms to p210 in PG activity (P < .01 [H396P], P = .013 [M351T]) and CT-L activity (P = .047 [T315I], P = .045 [H396P], P = .038 [M351T]). (D) Residual CT-L activity in BCR-ABL+ Ba/F3 cells after exposure to 20nM bortezomib for 24 hours, expressed as percentage of untreated controls. AFU is arbitrary fluorescence units released per minute, as described. Results in panels A through C represent the mean ± SEM with predicted dose-effect curve in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/11/10.1182_blood-2008-06-164582/4/m_zh89991049650005.jpeg?Expires=1769082506&Signature=hZn3btKXOYA385KcDB3jA0ifqLlhGy9z~XkNNA8yVYIRyLLmCd3m1nHI5FexHPQjTcDdO1YTUWraQ~TAxfbjyzArWKoSoCgxTWLN2i6jAohU~l7ZufaEB4d1ZVmmBsA5JIaFnkZFF7Dqe6HRqFQLefd2pb-ISN5cden3avcGOUM4Xqt5MHHJyE~iTCm5Hkz8s0W~Y4rvAUVz28YtyNr2Z3x559zXtBfQP954y8xF3QllRUeyq~G-D2i4PSUB5VymCXPRqIO9Q~VMzD3LXuFJ~sk~GeNwPJlMuKcgjgcSmGtn-0uhQJQs-KvkaJ-IrkH5NO4T0Rj0O9~WYa1z-B0iqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal