Issue Archive
Table of Contents
CLINICAL GUIDELINES
Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update
Bohlius et al’s Clinical Guideline on erythropoiesis-stimulating agents (ESAs) in patients with cancer reviews the evidence and revises previous recommendations. It is based on the findings of an expert panel convened by the American Society of Clinical Oncology and the American Society of Hematology. ESAs, including biosimilars, may be offered to patients with chemotherapy-associated anemia when this chemotherapy is being given with a noncurative intent. However, these same agents should not be offered to patients with chemotherapy-associated anemia whose cancer treatment is curative in intent. In addition, in the appropriate patients, adding iron to ESAs may improve anemia and reduce the need for blood transfusion, whether or not these patients have iron-deficiency anemia. These recommendations provide critical guidance for managing anemia, a common side effect for patients of cancer treatment.
EXCEPTIONAL CASE REPORT
Characterization of a cryptic IGH/CCND1 rearrangement in a case of mantle cell lymphoma with negative CCND1 FISH studies
Clinical Trials & Observations
REVIEW ARTICLE
IMMUNOBIOLOGY AND IMMUNOTHERAPY
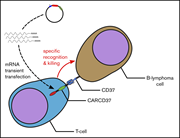
Preclinical development of CD37CAR T-cell therapy for treatment of B-cell lymphoma
Chimeric antigen receptor (CAR) T-cell therapy directed against CD19 has proven to be a novel approach to the treatment of patients with B-cell malignancies. However, novel targets are clearly needed. Köksal and colleagues explored CAR T cells directed against CD37, another cell surface protein highly expressed on B-cell malignancies, in preclinical models.
LYMPHOID NEOPLASIA
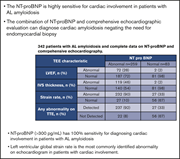
Comparison of different techniques to identify cardiac involvement in immunoglobulin light chain (AL) amyloidosis
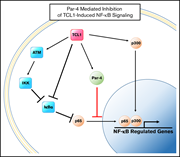
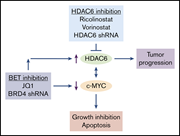
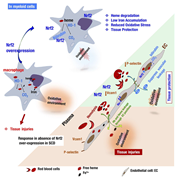
Par-4 overexpression impedes leukemogenesis in the Eµ-TCL1 leukemia model through downregulation of NF-κB signaling
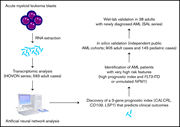
MYELOID NEOPLASIA
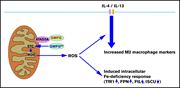
PHAGOCYTES, GRANULOCYTES, AND MYELOPOIESIS
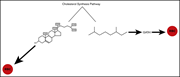
RED CELLS, IRON, AND ERYTHROPOIESIS
THROMBOSIS AND HEMOSTASIS
TRANSPLANTATION
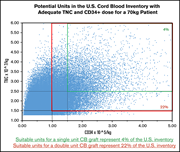
CD34+ cell content of 126 341 cord blood units in the US inventory: implications for transplantation and banking
Clinical Trials & Observations
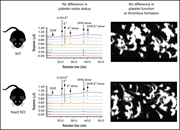
Prophylactic oral NAC reduced poor hematopoietic reconstitution by improving endothelial cells after haploidentical transplantation
Clinical Trials & Observations
-
Cover Image
Cover Image
![issue cover]()
COVER FIGURE
Knockdown of glia maturation factor-γ in macrophages increases transferrin receptor 1 protein levels (green). See the article by Aerbajinai et al. - PDF Icon Front MatterFront Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Advertisement intended for health care professionals