Key Points
NT-proBNP is highly sensitive for cardiac involvement in patients with systemic immunoglobulin light chain amyloidosis.
The combination of NT-proBNP and echocardiographic evaluation can diagnose cardiac amyloidosis, negating the need for endomyocardial biopsy.
Abstract
We retrospectively reviewed the utility of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and transthoracic echocardiogram (TTE) in diagnosing cardiac involvement in patients with biopsy-proven systemic immunoglobulin light chain amyloidosis seen at the Mayo Clinic between 1 January 2006 and 30 December 2015. We analyzed 2 cohorts: patients undergoing endomyocardial biopsy for suspicion of cardiac involvement (cohort 1) and patients who had serum NT-proBNP and comprehensive echocardiographic evaluation at diagnosis (cohort 2). Of 179 patients undergoing endomyocardial biopsy (cohort 1), 173 (97%) had evidence of amyloid deposition, with 159 having NT-proBNP performed at the time of the procedure. The NT-proBNP was elevated (>300 pg/mL) in all 159 patients (sensitivity, 100%; median NT-proBNP, 4917 pg/mL; range, 355-69 541). The left ventricular ejection fraction, interventricular septal thickness, and strain rate were abnormal in 89/168 (53%), 102/64 (61%) and 92/95 (97%), respectively. Among cohort 2 (n = 342), 259 (76%) had an elevated NT-proBNP, of whom 237 (92%) had an abnormality detected on TTE. Of 83 patients with normal NT-proBNP <300 pg/mL, 27 (33%) had an abnormality on TTE (all with borderline strain rate −18% to −15%). Only 5/27 patients were considered to have possible early cardiac involvement and none had any other diagnostic or classical features of amyloidosis on TTE. The combination of NT-proBNP and comprehensive echocardiographic evaluation can diagnose cardiac amyloidosis negating the need for endomyocardial biopsy. A negative NT-proBNP rules out clinically meaningful cardiac involvement and may obviate the routine use of TTE in patients with a low clinical suspicion of cardiac amyloidosis.
Introduction
Systemic immunoglobulin light chain (AL) amyloidosis is a clonal plasma cell disorder characterized by the deposition of insoluble amyloid fibrils in various organs leading to dysfunction. Cardiac involvement is essential in staging and remains the most important prognostic feature.1 The N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and cardiac troponins are cardiac biomarkers used in screening for cardiac involvement, staging of the disease, and prognostication.2-7 Transthoracic echocardiogram (TTE) is the recommended noninvasive imaging modality to determine cardiac involvement, whereas cardiac magnetic resonance (CMR) is increasingly being used for early disease detection and prognostication.8,9
We sought to identify the nature of the abnormalities in cardiac biomarkers and imaging features in patients with AL amyloidosis and the ability of these investigations to diagnose cardiac involvement.
Methods
We conducted a retrospective review of all patients with biopsy-proven systemic AL amyloidosis seen at the Mayo Clinic between 1 January 2006 and 31 December 2015. We first identified all patients with AL amyloidosis that underwent endomyocardial biopsy for suspicion of cardiac involvement (cohort 1). We then analyzed a consecutive cohort (cohort 2) of patients with biopsy-proven systemic AL amyloidosis seen between 1 December 2010 and 31 December 2015 who had serum NT-proBNP and a comprehensive echocardiographic evaluation at time of diagnosis. An NT-proBNP <300 pg/mL has a high sensitivity and negative predictive value to exclude acute heart failure.10 Therefore, we used this cutoff in our study and used it to determine test positivity.
Demographics, laboratory data, and imaging results were extracted from a prospectively maintained computerized database, and patients’ records were reviewed. The Mayo Clinic institutional review board approved the study. All the patients consented to have their medical records reviewed according to institutional review board practices.
Results
Table 1 describes the baseline characteristics of patients in both cohorts. Among the 179 patients (cohort 1) with an endomyocardial biopsy, 173 (97%) had evidence of amyloid deposition. In this cohort, 159 (89%) patients had NT-proBNP performed at the time of the procedure. The NT-proBNP was elevated in all 159 patients with a median NT-proBNP of 4917 pg/mL (range, 355-69 541). The median left ventricular ejection fraction (LVEF), interventricular septal (IVS) thickness, and strain rate were 54% (range, 10-77), 15 mm (range, 8-30), and −9% (range, −21 to 0), respectively. The LVEF, IVS thickness, and strain rate were abnormal in 89/168 (53%), 102/64 (61%), and 92/95 (97%), respectively. Ninety-five of 173 patients with biopsy-proven cardiac amyloidosis had complete echocardiogram data available on LVEF, IVS thickness, and LV global average strain rate, with 97% (n = 92) presenting with an abnormality in at least 1 of these variables. CMR findings were consistent or suggestive of AL amyloidosis in 38/42 patients, yielding a sensitivity of 90%. Based on this finding, NT-proBNP appears to have 100% sensitivity in recognizing cardiac involvement with amyloidosis, suggesting that a negative NT-proBNP essentially excludes significant cardiac involvement.
Baseline characteristics of the both cohorts
| . | Cohort 1 (n = 179) . | Cohort 2 (n = 342) . |
|---|---|---|
| Age, median (range), y | 62 (35-89) | 64 (32-88) |
| Male, n (%) | 120 (67) | 227 (66) |
| BMPCs, median (IQR), % | 10 (5-16) | 10 (5-17) |
| dFLC, median (IQR), mg/dL | 34.6 (14.4-79.4) | 25.2 (7.9-66.2) |
| iFLC, median (IQR), mg/dL | 34.3 (15.8-80.5) | 26.7 (9.9-67.3) |
| Urine 24-h protein, median (range), g | 0.2 (0-8.3) | 0.8 (0.1-22.8) |
| Creatinine, median (IQR), mg/dL | 1.2 (1-1.5) | 1.02 (0.8-1.5) |
| Creatinine >2 mg/dL, n (%) | 14 (8) | 41 (12) |
| Alkaline phosphatase, median (IQR), U/L | 99 (79-148) | 85 (65-125) |
| NT-proBNP, median (range), pg/mL | 4554 (2 323-10 321) | 1878 (314-5906) |
| Troponin T, median (range), ng/mL | 0.06 (0.03-0.12) | 0.02 (0.01-0.06) |
| Echocardiographic features, n (%) | ||
| LVEF <55% | 89 (51) | 74 (22) |
| LVIS >14 mm | 105 (61) | 121 (35) |
| LV strain rate >−19% | 94 (95) | 259 (76) |
| . | Cohort 1 (n = 179) . | Cohort 2 (n = 342) . |
|---|---|---|
| Age, median (range), y | 62 (35-89) | 64 (32-88) |
| Male, n (%) | 120 (67) | 227 (66) |
| BMPCs, median (IQR), % | 10 (5-16) | 10 (5-17) |
| dFLC, median (IQR), mg/dL | 34.6 (14.4-79.4) | 25.2 (7.9-66.2) |
| iFLC, median (IQR), mg/dL | 34.3 (15.8-80.5) | 26.7 (9.9-67.3) |
| Urine 24-h protein, median (range), g | 0.2 (0-8.3) | 0.8 (0.1-22.8) |
| Creatinine, median (IQR), mg/dL | 1.2 (1-1.5) | 1.02 (0.8-1.5) |
| Creatinine >2 mg/dL, n (%) | 14 (8) | 41 (12) |
| Alkaline phosphatase, median (IQR), U/L | 99 (79-148) | 85 (65-125) |
| NT-proBNP, median (range), pg/mL | 4554 (2 323-10 321) | 1878 (314-5906) |
| Troponin T, median (range), ng/mL | 0.06 (0.03-0.12) | 0.02 (0.01-0.06) |
| Echocardiographic features, n (%) | ||
| LVEF <55% | 89 (51) | 74 (22) |
| LVIS >14 mm | 105 (61) | 121 (35) |
| LV strain rate >−19% | 94 (95) | 259 (76) |
BMPC, bone marrow plasma cell; dFLC, difference between involved and uninvolved free light chains; iFLC, involved free light chain; IQR, interquartile ratio.
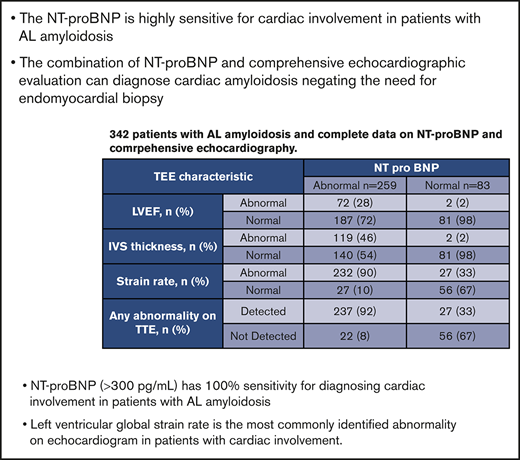
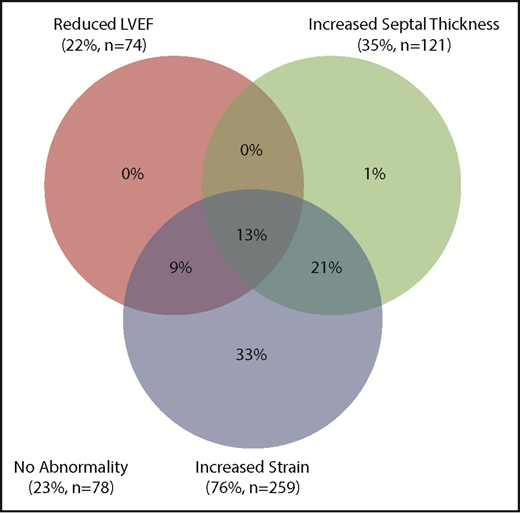
Based on these findings, we studied another cohort of 342 consecutive patients to assess the spectrum of imaging abnormalities in the presence and absence of abnormal NT-proBNP. Table 2 demonstrates the echocardiographic and NT-proBNP results of cohort 2. The median NT-proBNP was 1878 pg/mL (25-48 214); 259 (76%) patients had a positive NT-proBNP (>300), of whom 237 (92%) had an abnormality detected on TTE. The median LVEF, IVS thickness, and strain rate were 63% (22-90), 14 mm (6-25), and −13% (−25 to −3), respectively. The distribution of abnormalities seen on the echocardiogram is summarized in Figure 1. Among this cohort, 41 (12%) patients had an elevated creatinine (>2 mg/dL), of whom 39 had an elevated NT-proBNP. Of these 39 patients, 33 (85%) also had an abnormality detected on echocardiography.
Cohort 2 echocardiographic features and NT-proBNP results
| TEE characteristic . | NT-proBNP . | |
|---|---|---|
| Abnormal (n = 259) . | Normal (n = 83) . | |
| LVEF, n (%) | ||
| Abnormal | 72 (28) | 2 (2) |
| Normal | 187 (72) | 81 (98) |
| IVS thickness, n (%) | ||
| Abnormal | 119 (46) | 2 (2) |
| Normal | 140 (54) | 81 (98) |
| Strain rate, n (%) | ||
| Abnormal | 232 (90) | 27 (33) |
| Normal | 27 (10) | 56 (67) |
| Any abnormality on TTE, n (%) | ||
| Detected | 237 (92) | 27 (33) |
| Not Detected | 22 (8) | 56 (67) |
| TEE characteristic . | NT-proBNP . | |
|---|---|---|
| Abnormal (n = 259) . | Normal (n = 83) . | |
| LVEF, n (%) | ||
| Abnormal | 72 (28) | 2 (2) |
| Normal | 187 (72) | 81 (98) |
| IVS thickness, n (%) | ||
| Abnormal | 119 (46) | 2 (2) |
| Normal | 140 (54) | 81 (98) |
| Strain rate, n (%) | ||
| Abnormal | 232 (90) | 27 (33) |
| Normal | 27 (10) | 56 (67) |
| Any abnormality on TTE, n (%) | ||
| Detected | 237 (92) | 27 (33) |
| Not Detected | 22 (8) | 56 (67) |
Cutoffs for abnormal findings: LVEF <55%, IVS thickness >14 mm, and strain rate ≥−18.
Eighty-three patients had a normal NT-proBNP, of whom 27 (33%) had an abnormality in either LVEF, IVS thickness, or strain rate; 19/27 had a borderline reduced strain rate between −17 and −18, whereas the remaining 8 patients had a strain between −14% and −15%. Only 5/27 patients were considered to have possible early cardiac involvement, but none had any other diagnostic or classical features of amyloidosis on TTE. At last follow-up (median, 49 months), none of these 5 patients were diagnosed with progressive cardiac involvement, although 2 have died without a clear cause.
Discussion
Determination of cardiac involvement in systemic AL amyloidosis is of paramount importance and is vital for disease prognostication and possibly treatment selection. This poses a serious diagnostic challenge. Our study includes a large cohort of patients with biopsy-proven cardiac amyloidosis and highlights several important findings. First, we confirm that NT-proBNP is a highly sensitive cardiac biomarker in patients with systemic AL amyloidosis and is always elevated in those with cardiac involvement (cutoff ≥300). This is consistent with a previous report by Pallidini et al,11 which demonstrated that NT-proBNP was a sensitive marker for myocardial toxicity. However, in that study, cardiac involvement was based on clinical signs, electrocardiography, and echocardiography. Of note, the NT-proBNP level should be interpreted with caution in patients with significant renal impairment as the peptide is mainly cleared via glomerular filtration.12 Second, it appears that the added benefit of TTE might be limited in patients with negative NT-proBNP in diagnosing cardiac involvement. In our study, the small cohort of patients with a negative NT-proBNP that had changes on echocardiogram mainly had a minor increase in LV strain rate. Very few of these patients (19% [5/27]) were clinically thought to have early cardiac amyloidosis. This may obviate the need for an echocardiogram in patients with a normal NT-proBNP value and low clinical suspicion of cardiac involvement. Third, among the 3 echocardiographic features that we have investigated, LV strain rate is the most sensitive. Knight et al13 reported in a large study of patients with different types of cardiac amyloidosis that LV strain rate and mitral peak velocity of early filling to early diastolic mitral annular velocity ratio by echocardiography have high probabilities of being abnormal with a low cardiac amyloid burden in patients with cardiac amyloid. This, in addition to previous reports indicating the prognostic value in patients with AL amyloidosis,14 supports the routine assessment of LV strain rate by echocardiography in patients suspected of having cardiac amyloidosis. Finally, in our study, the CMR findings indicative of cardiac involvement had a sensitivity of ∼90%, which is similar to previous reports.15,16 Direct comparison and assessment of the added benefit of CMR to echocardiography is warranted but is beyond the scope of this paper. Although noninvasive testing is highly sensitive for detection of cardiac amyloid, there remains a select group of patients that may require invasive investigation with endomyocardial biopsy. It is important to remember that a diagnosis of amyloid requires a tissue specimen with amyloid present that can be typed using techniques such as mass spectrometry. The majority of AL patients may be diagnosed with amyloidosis using a combination of bone marrow and fat aspirate biopsy. However, with the increasing recognition of wild-type transthyretin amyloidosis (primarily cardiac involvement) and, given its shared demographic of peak incidence with monoclonal gammopathy of undetermined significance, there remains a group of patients in whom endomyocardial biopsy is required for a definitive diagnosis.
The limitations of our study include the long period over which it was conducted and inclusion of patients diagnosed with AL amyloidosis before routine use of mass spectrometry–based amyloid typing in our clinical practice (instituted after 2007). In addition, although the imaging studies were all reported at our institution, this was not standardized, and significant interreporter variability could not be accounted for.
Despite these limitations, our study shows that the combination of NT-proBNP and comprehensive echocardiographic evaluation provides substantial information in diagnosing cardiac amyloidosis in the majority of patients, negating the need for endomyocardial biopsy in all patients. A negative NT-proBNP rules out clinically meaningful cardiac involvement and may obviate the routine use of TTE in patients with a low clinical suspicion of cardiac amyloidosis.
Authorship
Contribution: M.A.A. and M.H.S. designed the study, analyzed the data, wrote the first draft, and approved the final version of the manuscript; M.A.G., A.D., E.M., F.K.B., R.M.W., M.Q.L., A.L.F., M.A.H., Y.L.H., D.D., S.R.H., N.L., W.I.G., P.K., T.K., R.A.K., R.S.G., and S.V.R. performed patient management, revised the manuscript critically, and approved the final version of the manuscript; and S.K.K. designed the study, analyzed the data, wrote the first draft, approved the final version of the manuscript, and performed patient management.
Conflict-of-interest disclosure: M.A.G. received consultancy from Millenium and honoraria from Celgene, Millennium, Onyx, Novartis, SmithKline, Prothena, Ionis, and Amgen. S.K.K. received consultancy from Celgene, Millennium, Onyx, Janssen, and BMS, and research funding from Celgene, Millennium, Novartis, Onyx AbbVie, Janssen, and BMS. M.Q.L. received research funding from Celgene. D.D. received research funding from Karyopharm Therapeutics, Amgen, and Millennium Pharmaceuticals. P.K. received research funding from Takeda, Celgene, and Amgen. A.D. received research funding from Celgene, Millennium, Pfizer, and Janssen, and travel grant from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Shaji K. Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.
References
Author notes
M.A.A. and M.H.S. contributed equally to this paper.