Issue Archive
Table of Contents
EDITORIAL
Introduction to a review series on RNA therapeutics in hematology
Small molecule inhibitors and antibody therapies have led the way for targeted therapy of a range of hematologic disorders; however, the number of amenable targets is limited. RNA-directed therapies can be a solution for targets deemed “undruggable,” with modulation of RNA expression through a variety of methods, expanding the therapeutic possibilities. In this Review Series edited by Associate Editor Jason Gotlib, 3 articles highlight areas in which RNA therapeutics are most advanced: acute hepatic porphyria, transthyretin amyloidosis, and hemophilia. This series offers insight into the promise of these new therapies.
BLOOD COMMENTARIES
REVIEW SERIES
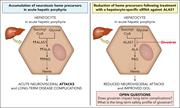
RNA interference therapy in acute hepatic porphyrias
Clinical Trials & Observations
Small molecule inhibitors and antibody therapies have led the way for targeted therapy of a range of hematologic disorders; however, the number of amenable targets is limited. RNA-directed therapies can be a solution for targets deemed “undruggable,” with modulation of RNA expression through a variety of methods, expanding the therapeutic possibilities. In this Review Series edited by Associate Editor Jason Gotlib, 3 articles highlight areas in which RNA therapeutics are most advanced: acute hepatic porphyria, transthyretin amyloidosis, and hemophilia. This series offers insight into the promise of these new therapies.
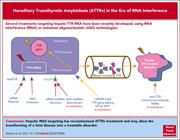
Hereditary transthyretin amyloidosis in the era of RNA interference, antisense oligonucleotide, and CRISPR-Cas9 treatments
Clinical Trials & Observations
Small molecule inhibitors and antibody therapies have led the way for targeted therapy of a range of hematologic disorders; however, the number of amenable targets is limited. RNA-directed therapies can be a solution for targets deemed “undruggable,” with modulation of RNA expression through a variety of methods, expanding the therapeutic possibilities. In this Review Series edited by Associate Editor Jason Gotlib, 3 articles highlight areas in which RNA therapeutics are most advanced: acute hepatic porphyria, transthyretin amyloidosis, and hemophilia. This series offers insight into the promise of these new therapies.
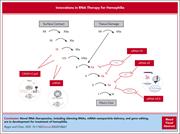
Innovations in RNA therapy for hemophilia
Small molecule inhibitors and antibody therapies have led the way for targeted therapy of a range of hematologic disorders; however, the number of amenable targets is limited. RNA-directed therapies can be a solution for targets deemed “undruggable,” with modulation of RNA expression through a variety of methods, expanding the therapeutic possibilities. In this Review Series edited by Associate Editor Jason Gotlib, 3 articles highlight areas in which RNA therapeutics are most advanced: acute hepatic porphyria, transthyretin amyloidosis, and hemophilia. This series offers insight into the promise of these new therapies.
HEMATOPOIESIS AND STEM CELLS
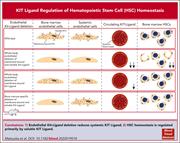
Loss of endothelial membrane KIT ligand affects systemic KIT ligand levels but not bone marrow hematopoietic stem cells
Data suggest that endothelial KIT ligand production by vascular endothelial cells plays a critical role in regulating hematopoietic stem cell numbers. However, KIT ligand is expressed in membrane-bound and soluble forms. Matsuoka and colleagues clarify the relative contribution of these 2 forms of KIT ligand in regulating hematopoietic stem cells in the bone marrow niche. Through a series of elegant experiments, the authors demonstrate that stem cell regulation is mediated by circulating soluble KIT ligand since deletion of membrane KIT ligand does not affect stem cell numbers.
LYMPHOID NEOPLASIA
Resolving therapy resistance mechanisms in multiple myeloma by multiomics subclone analysis
Poos et al used a multiomics approach to define multiple myeloma subclones leading to chemotherapy resistance. The authors performed whole genome sequencing, single cell transcriptomics, chromosomal accessibility assays and utilized mitochondrial DNA mutations to demonstrate the complex heterogeneity of myeloma relapse. They identified 3 parallel mechanisms for relapse, including preexisting subclones with survival advantage, evolution of new subclones, and changing subclone interactions with the marrow microenvironment.
MYELOID NEOPLASIA
Venetoclax abrogates the prognostic impact of splicing factor gene mutations in newly diagnosed acute myeloid leukemia
Clinical Trials & Observations
Splicing factor (SF) gene mutations are classified as adverse risk in the European LeukemiaNet risk stratification of acute myeloid leukemia (AML). Senapati et al performed a retrospective analysis of 994 newly diagnosed patients with AML, of which 27% had an SF mutation, to determine the impact of SF mutations in patients treated with low-intensity regimens. In patients treated with intensive therapy, the negative impact of SF mutations on survival was confirmed. In those treated with venetoclax, whether in a low-intensity regimen or added to an intensive regimen, survival was improved, and the negative impact of SF mutations was abrogated.
RED CELLS, IRON, AND ERYTHROPOIESIS
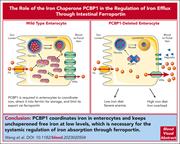
The iron chaperone poly(rC)-binding protein 1 regulates iron efflux through intestinal ferroportin in mice
Wang and colleagues describe a new layer of regulation of iron metabolism. Poly(rC)-binding protein 1 (PCBP1) is a cytosolic iron chaperone that regulates iron flux within the intestinal enterocyte. PCBP1 sequesters free iron within intestinal epithelial cells and directs it to ferritin, thereby decreasing iron absorption. The authors demonstrate that mice lacking PCBP1 have greatly reduced retention of iron in enterocytes and excess efflux through ferroportin despite upregulation of hepcidin. Whether there are defects in PCBP1 that lead to abnormal iron metabolism remains to be investigated.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Immunostaining of mouse testes for KIT+ spermatogonial precursor cells (SPCs [KIT, red]), endothelium (VE-cadherin, green), Sertoli cells (β3-tubulin, blue), and nuclei counterstained with DAPI (gray) showing KIT+ SPCs primarily located next to Sertoli niche cells. Deletion of membrane KIT ligand (mKitL) in Sertoli niche cells results in reduced SPCs and testes size, whereas there is no hematopoietic stem cell phenotype when mKitL is deleted in bone marrow endothelial niche cells. See the article by Matsuoka et al on page 1622.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals





Untangling the role of KIT ligand in HSC regulation