In this issue of Blood, Wang et al shed light on a new role for the cytosolic iron chaperone protein poly rC binding protein 1 (PCBP1) in regulating intestinal absorption and systemic iron homestatsis.1 Iron is an essential micronutrient required by all cells for diverse cellular functions, including mitochondrial respiration, DNA replication, and protein synthesis. Beyond cellular metabolism, iron plays a critical role in systemic oxygen delivery through hemoglobin-mediated erythrocyte function.2 Previous studies have explored manipulating regulatory pathways, such as dietary changes or iron chelator administration, to modulate iron uptake.
Systemic iron balance involves intricate communication among various cell types, regulating intestinal iron absorption, erythropoiesis, and recycling of iron from senescent red blood cells. Dietary iron is absorbed in the duodenum through specific transporters on intestinal epithelial cells (IECs). Nonheme iron is first converted from ferric (Fe3+) to ferrous (Fe2+) via duodenal cytochrome b before being transported into enterocytes via the divalent metal transporter 1 (DMT1; gene name Slc11a2).3,4 Within enterocytes, absorbed iron can be stored as ferritin or exported into the bloodstream via the sole mammalian iron transporter, ferroportin (FPN; gene name Slc40a1).5
Iron absorption in the intestine is synchronized with systemic iron levels and erythropoietic demands via hepatic regulation. Hepcidin (gene name HAMP), a hepatic hormone, suppresses intestinal iron absorption by inducing degradation or blocking ferroportin function.6 However, during iron deficiency or increased erythropoietic demand, hepcidin expression is suppressed, allowing for increased iron absorption.7 Mutations affecting hepatic hepcidin and intestinal ferroportin are associated with hereditary hemochromatosis.8
PCBP1, part of a family of adaptor proteins binding cytosine-rich RNA, DNA, and ferrous complexes, plays a role in maintaining cytosolic iron in yeast and mammalian cells.9 The authors examined PCBP1's impact on iron absorption by inducing IEC-specific PCBP1 deletion in mice and observed an increase in proliferative cells in the intestinal epithelium. Although young mice showed no significant growth defects, older mice exhibited reduced body weight and lower nonheme iron levels, suggesting IEC iron imbalance and impaired absorption on PCBP1 disruption. To understand the implications of PCBP1 disruption on IEC iron homeostasis, the authors subjected mice to various iron diets. Regardless of diet, PCBP1-deficient mice maintained lower body weight, but those on high-iron diets (50 and 1000 ppm) exhibited low IEC iron and ferritin levels, indicating potential consequences of PCBP1 disruption on intestinal iron absorption and systemic homeostasis.
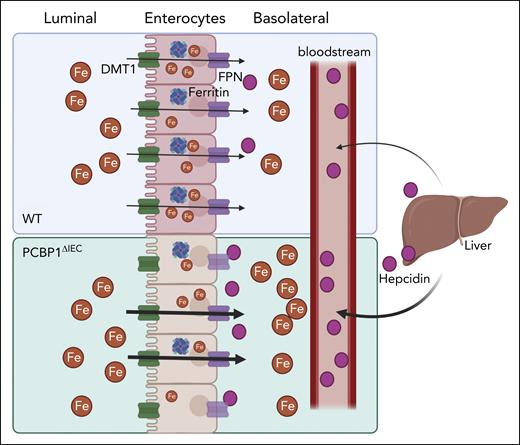
Reduced iron and ferritin levels in IEC could stem from impaired uptake via luminal iron transporter or increased efflux via ferroportin. Further investigation revealed elevated serum and tissue iron in PCBP1-deficient mice compared with wild-type mice, suggesting heightened iron absorption and efflux via IEC ferroportin (see figure). PCBP1-deficient mice exhibited heightened susceptibility to dietary iron deficiency and anemia, along with impaired erythropoiesis. This was supported by direct measurement of iron absorption using 57Fe isotope, showing decreased IEC 57Fe but increase in the plasma and liver, indicating an increase in intestinal absorption.
PCBP1 regulates intestinal iron absorption and iron efflux. Disruption of PCBP1 in the intestinal epithelium leads to an increase in intestinal iron absorption, despite a decrease in the iron exporter FPN and heightened serum hepcidin levels. WT, wild type.
PCBP1 regulates intestinal iron absorption and iron efflux. Disruption of PCBP1 in the intestinal epithelium leads to an increase in intestinal iron absorption, despite a decrease in the iron exporter FPN and heightened serum hepcidin levels. WT, wild type.
FPN, the only known mammalian iron exporter, is pivotal in regulating intestinal iron absorption; and its expression on the basolateral membrane of the IEC is directly proportional to intestinal iron uptake. Surprisingly, the authors found reduced FPN expression in IEC despite elevated iron absorption on PCBP1 deletion, challenging existing literature. Absence of changes in luminal transporter DMT1 expression and intestinal barrier integrity suggested a novel iron efflux regulation in the IEC mediated by PCBP1. Blocking FPN activity with synthetic mini hepcidin increased iron levels within IECs of PCBP1-deficient mice, suggesting the PCBP1 deletion does not alter the FPN-hepcidin axis. The authors further hypothesize that increased intracellular ferrous iron, rather than FPN activity, causes the heightened iron absorption on PCBP1 disruption.
The in vitro 3-dimensional (3D) enteroid models established from PCBP1-deficient mice confirmed that PCBP1 deletion does not affect FPN expression at the basolateral membrane of the IEC. Hepcidin treatment reduced basolateral expression of FPN in 3D enteroids, indicating intact hepcidin-ferroportin axis on PCBP1 deletion. This reduced FPN expression in PCBP1-deficient IECs could be attributed to elevated serum hepcidin levels in these mice.
PCBP1 deletion led to decreased IEC iron and ferritin levels, accompanied by reduced basolateral FPN expression and increased iron absorption. The authors hypothesized that this could stem from elevated ferrous iron within enterocytes, readily absorbed via FPN, contributing to overall increased iron absorption. However, PCBP1-deficient mice exhibited a lower labile iron pool, which increased on hepcidin treatment, suggesting increased iron efflux caused the lower IEC iron levels. Further research is needed to elucidate the molecular mechanism by which PCBP1 affects iron efflux, enhancing our comprehension of cellular signaling and systemic iron homeostasis.
The seemingly contradictory situation of increased intestinal iron absorption accompanied by reduced FPN expression in PCBP1-deficient IECs underscores the intricate intracellular signaling that shapes iron flux. This study uncovers a novel regulatory mechanism independent of posttranslational stability of FPN, suggesting potential therapeutic targets for hereditary hemochromatosis, where therapies targeting the FPN-hepcidin axis are limited because of genetic mutations.
Numerous questions remain unclear: Is PCBP1 linked to any iron-related disorders? Can disrupting PCBP1 alleviate hemochromatosis symptoms in iron overload models? How does PCBP1 influence iron absorption without directly altering the iron exporter? Follow-up studies investigating the role of PCBP1 in various iron-related disorder models will significantly enhance our understanding of systemic iron homeostasis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal