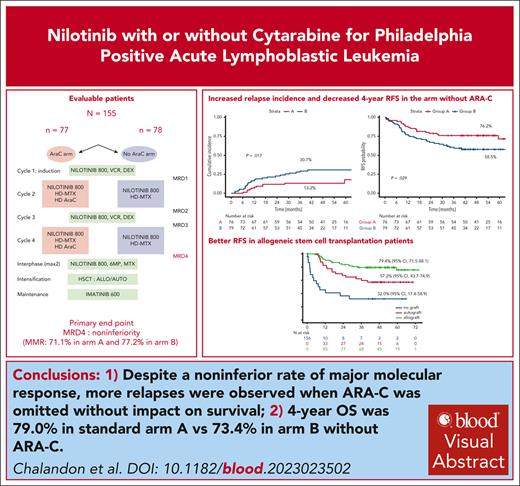
Alternating reduced-intensity and conventional chemotherapy with nilotinib followed by SCT resulted in 4-year OS of 79.4% in Ph+ ALL.
The omission of high-dose Ara-C during consolidation resulted in a significantly higher rate of relapses without affecting overall survival.
Visual Abstract
We previously demonstrated that a reduced-intensity chemotherapy schedule can safely replace hyper-CVAD (cyclophosphamide-vincristine-doxorubicin [Adriamycin]-dexamethasone) cycle 1 when combined with imatinib in adults with Philadelphia-positive acute lymphoblastic leukemia. In the present randomized GRAAPH-2014 trial, we used nilotinib and addressed the omission of cytarabine (Ara-C) in consolidation. The primary objective was the major molecular response (MMR) rate measured by BCR::ABL1 quantification after cycle 4 (end of consolidation). All patients were eligible for allogeneic stem cell transplant (SCT), whereas those in MMR could receive autologous SCT, followed by 2-year imatinib maintenance in both cases. After the enrollment of 156 of 265 planed patients, the data and safety monitoring board decided to hold the randomization because of an excess of relapse in the investigational arm. Among the 155 evaluable patients, 76 received Ara-C during consolidation (arm A) and 79 did not (arm B). Overall, 133 patients (85%) underwent SCT, 93 allogeneic and 40 autologous. The noninferiority end point regarding MMR was reached with 71.1% (arm A) and 77.2% (arm B) of patients reaching MMR. However, the 4-year cumulative incidence of relapse was higher in arm B compared with arm A (31.3% [95% confidence interval {CI}, 21.1%-41.9%] vs 13.2% [95% CI, 6.7%-21.9%]; P = .017), which translated to a lower relapse-free survival. With a median follow-up of 3.8 years, 4-year overall survival was 79.0% (95% CI, 70.6%-89.3%) in arm A vs 73.4% (95% CI, 63.9%-84.4%) in arm B (P = .35). Despite a noninferior rate of MMR, more relapses were observed when ARA-C was omitted without impact on survival. ClinicalTrials.gov ID, NCT02611492.
Introduction
During the pre–tyrosine kinase inhibitor (TKI) era, the outcome of adult patients with Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL) was rather dismal, with a 5-year overall survival in the range of 10% to 20%; and the only therapy that could increase survival was allogeneic stem cell transplantation (allo-SCT), with a 5-year overall survival (OS) in the range of 35% to 45% for those who could undergo transplantation.1,2 The advent of TKIs in the 2000s has dramatically changed this paradigm, with a 90% to 100% complete remission (CR) rate irrespective of the TKI used and a 5-year OS of ≈50% for adult patients with or without transplantation.3,4 In our prior GRAAPH-2005 trial, we conducted the first randomized trial demonstrating that a reduced-intensity induction chemotherapy combined with imatinib can be safely substituted for a standard regimen based on hyper-CVAD (cyclophosphamide-vincristine-doxorubicin [Adriamycin]-dexamethasone) plus imatinib. In this setting, patients continued to benefit from allo-SCT.5
The US intergroup study reported that combining the second-generation dasatinib with hyper-CVAD, followed by allo-SCT, in adult Ph+ ALL resulted in a relapse-free survival (RFS) of 76% (95% confidence interval [CI], 63%-91%) at 3 years.6 More recently, the MD Anderson Cancer Center (MDACC) group combined the third-generation TKI ponatinib with hyper-CVAD, showing encouraging results, with a CR rate of 100%, a complete molecular response (CMR) rate of 83%, and a 3-year event-free survival (EFS) of 70% (95% CI, 56%-80%) without the use of allo-SCT in most patients.7 The US intergroup was a national multicenter study, and the MDACC study was from a single center.
Many questions remain, however, on how to optimize the combination of potent TKIs and chemotherapy, on the opportunity to further deescalate chemotherapy, and on the need to allocate to allo-SCT in first CR. On the basis of the results of our previous trial, we initiated a randomized study using a reduced-intensity schedule for cycles 1 and 3 in combination with continuous nilotinib administration, and randomly addressed the role of high-dose cytarabine (HD-Ara-C) during cycles 2 and 4. Nilotinib was given continuously in both arms until SCT. This was followed by a systematic post-SCT imatinib maintenance for 2 years. Our aim was to decrease toxicity without impairing the depth of the BCR::ABL1 measurable residual disease (MRD) response, a potential surrogate marker for RFS. The primary end point of this noninferiority study was the achievement of a major molecular response (MMR) at the end of consolidation.
Materials and methods
Study design and participants
The prospective, open-label, noninferiority, multicenter, randomized GRAAPH-2014 trial was conducted in 79 centers in France, Belgium, and Switzerland. Patients, aged 18 to 59 years, with newly diagnosed Ph+ and/or BCR::ABL1+ B-cell precursor ALL were eligible. Patients with known chronic myeloid leukemia in blast phase, cardiac disease, renal, hepatic, or pancreatic dysfunction (serum creatinine level >1.5 upper limit of normal [ULN], bilirubin level >1.5 ULN, aspartate aminotransferase or alanine aminotransferase level >2.5 ULN, and amylase or lipase >1.5 ULN), HIV, human T-lymphotropic virus, hepatitis B virus or hepatitis C virus infection, contraindication to intensive chemotherapy, and pregnancy were not eligible. Written informed consent was obtained from all patients. The study was approved by the Institutional Ethics Committee Ile-de-France VI, France, and conducted in accordance with the Declaration of Helsinki.
In February 2019, the data safety monitoring board recommended that randomization be placed on hold, based on the detection of a higher relapse rate in the experimental over the control arm. At that time, 156 of the anticipated 265 patients were randomized. We present here the results based on the patients enrolled and randomized before February 2019 (156 patients).
Diagnosis of Ph+ ALL and MRD monitoring
Ph positivity was determined during the prephase by karyotype and/or BCR::ABL1 fluorescence in situ hybridization analysis and/or BCR::ABL1 fusion transcript detection with qualitative reverse transcription–polymerase chain reaction (RT-PCR) using standard procedures. MRD monitoring was performed by quantification of BCR::ABL1 fusion transcripts with quantitative RT–PCR centralized in 2 laboratories using standardized methods as per EuroMRD.8 BCR::ABL1 transcript levels were used to monitor MRD. MMR was defined as a BCR::ABL1/ABL1 ratio of ≤0.1% in the bone marrow and CMR by the absence of detectable MRD with a sensitivity of at least 0.01%. The primary end point was the MMR rate after cycle 4 (MRD4), at time point 4 (TP4).
Detection of mutations in the ABL kinase domain of BCR::ABL1
Mutations were centrally detected by next-generation sequencing on a MiSeq sequencer (Illumina, France) after RT-PCR amplification of BCR::ABL1 kinase domain, as recommended in the European Leukemia Net recommendations.9 Alignments and variant interpretation were done with G-ROUTE and Leaves applications, respectively, available on the MOABI platform (Assistance Publique–Hôpitaux de Paris, Paris, France). Sensitivity of detection was 3%.
Randomization and intervention
All patients received nilotinib and were randomly allocated to receive methotrexate and high-dose cytarabine (HD-Ara-C) (arm A) or methotrexate only (arm B) at inclusion. The randomization was centralized, used a 1:1 allocation ratio, performed using an interactive web-response system (CleanWeb/CTMS [Clinical Trial Master System]), and stratified according to age (≤40 vs >40 years) and transcript (m-bcr, M-bcr, or not assessable), with prespecified lists based on permutation blocks the size of which remained blinded to investigators.
Outcomes
The primary end point was the MMR rate after cycle 4 (TP4). The secondary efficacy end points were hematologic CR after cycle 1, cumulative incidence of nonrelapse mortality (NRM), cumulative incidence of relapse (CIR), RFS, that is a composite end point accounting relapse, progression and death free of relapse/progression, whichever occurred first, and OS. Response was assessed at day 29 of each cycle 1 to 4 (TP1 to TP4) and evaluated by conventional morphologic criteria together with bone marrow MRD evaluation. Hematologic CR was defined as <5% marrow blasts with adequate blood count recovery (absolute neutrophil count ≥1 × 109/L; platelets ≥100 × 109/L). For safety end points, Common Terminology Criteria for Adverse Events (CTCAE) v4.0 grade 3 and more adverse events were assessed.
Treatments and procedures
Treatments are detailed in Table 1 and the supplemental Appendix (available on the Blood website). Patients were randomized after the steroid prephase and were allocated to 4 cycles of induction/consolidations before TP4 MRD assessment. All patients received nilotinib, 400 mg twice daily (bid), on days 1 to 28, 40 mg dexamethasone on days 1 to 2, 8 to 9, 15 to 16, and 22 to 23, and 2 mg vincristine on days 1, 8, 15, and 22 for cycles 1 and 3. Patients randomized to arm A received 400 mg nilotinib bid on days 1 to 28, methotrexate, 1 g/m2 by continuous intravenous administration, on day 1, and HD-Ara-C, 3 g/m2 (decreased to 1.5 g/m2 in patients aged ≥45 years) bid on days 2 and 3 for cycles 2 and 4. Patients randomized to arm B received the same cycles 2 and 4 without Ara-C. There was no interruption of nilotinib between cycles.
GRAAPH-2014 treatments
| Treatment phases . | Drugs . | Doses . | Schedules . |
|---|---|---|---|
| Initial treatments | |||
| Prephase | PDN | 60 mg/m2 per day PO | Day −7 to −1 |
| MTX | 15 mg IT | Between day −7 and −4 | |
| Cycle 1 and 3 (both arms)∗ | VCR | 2 mg/day IV | Days 1, 8, 15, and 22 |
| DXM | 40 mg/day PO | Days 1-2, 8-9, 15-16, and 22-23 | |
| Nilotinib | 400 mg bid PO | Days 1 to 28 | |
| G-CSF | 5 μg/kg per day SC/IV | From day 15 to PMN recovery | |
| Cycle 2 and 4 arm A∗ | MTX (leucovorin rescue) | 1000 mg/m2 per 24 h CIV | Day 1 |
| Ara-C | 3000 mg/m2 per 12 h IV (1500 mg/m2 per 12 h IV if age ≥45 y) | Days 2-3 | |
| Nilotinib | 400 mg bid PO | Days 1 to 28 | |
| Peg-filgrastim | 6 mg SC | Day 6 | |
| Cycle 2 and 4 arm B∗ | MTX | 1000 mg/m2 per 24 h CIV | Day 1 |
| Nilotinib | 400 mg bid PO | Days 1 to 28 | |
| Peg-filgrastim | 6 mg SC | Day 6 | |
| Pre-SCT interphase (N = 2)∗ | Nilotinib | 300 mg bid PO | Days 1 to 14 |
| MTX | 25 mg/m2 per day PO | Days 1 and 8 | |
| 6-MP | 60 mg/m2 per day PO | Days 1 to 14 | |
| Postautologous and allogeneic SCT maintenance | Imatinib | 300 mg bid PO | For 24 months |
| CNS treatments | |||
| CNS prophylaxis | Triple IT† | N = 1 | Days 1, 8, and 15 of cycle 1 and day 1 of cycle 3 |
| Triple IT† | N = 1 | Day 9 of cycles 2 and 4 | |
| Triple IT† | N = 1 | Day 1 of the 2 interphase cycles | |
| If initial CNS involvement | Triple IT† | N = 12 | Between day −7 and until day 22 of cycle 2 |
| Triple IT† | N = 1 | As per prophylaxis for cycles 3 and 4 |
| Treatment phases . | Drugs . | Doses . | Schedules . |
|---|---|---|---|
| Initial treatments | |||
| Prephase | PDN | 60 mg/m2 per day PO | Day −7 to −1 |
| MTX | 15 mg IT | Between day −7 and −4 | |
| Cycle 1 and 3 (both arms)∗ | VCR | 2 mg/day IV | Days 1, 8, 15, and 22 |
| DXM | 40 mg/day PO | Days 1-2, 8-9, 15-16, and 22-23 | |
| Nilotinib | 400 mg bid PO | Days 1 to 28 | |
| G-CSF | 5 μg/kg per day SC/IV | From day 15 to PMN recovery | |
| Cycle 2 and 4 arm A∗ | MTX (leucovorin rescue) | 1000 mg/m2 per 24 h CIV | Day 1 |
| Ara-C | 3000 mg/m2 per 12 h IV (1500 mg/m2 per 12 h IV if age ≥45 y) | Days 2-3 | |
| Nilotinib | 400 mg bid PO | Days 1 to 28 | |
| Peg-filgrastim | 6 mg SC | Day 6 | |
| Cycle 2 and 4 arm B∗ | MTX | 1000 mg/m2 per 24 h CIV | Day 1 |
| Nilotinib | 400 mg bid PO | Days 1 to 28 | |
| Peg-filgrastim | 6 mg SC | Day 6 | |
| Pre-SCT interphase (N = 2)∗ | Nilotinib | 300 mg bid PO | Days 1 to 14 |
| MTX | 25 mg/m2 per day PO | Days 1 and 8 | |
| 6-MP | 60 mg/m2 per day PO | Days 1 to 14 | |
| Postautologous and allogeneic SCT maintenance | Imatinib | 300 mg bid PO | For 24 months |
| CNS treatments | |||
| CNS prophylaxis | Triple IT† | N = 1 | Days 1, 8, and 15 of cycle 1 and day 1 of cycle 3 |
| Triple IT† | N = 1 | Day 9 of cycles 2 and 4 | |
| Triple IT† | N = 1 | Day 1 of the 2 interphase cycles | |
| If initial CNS involvement | Triple IT† | N = 12 | Between day −7 and until day 22 of cycle 2 |
| Triple IT† | N = 1 | As per prophylaxis for cycles 3 and 4 |
Ara-C, cytarabine; CIV, continuous IV; CNS, central nervous system; DXM, dexamethasone; G-CSF, granulocyte colony-stimulating factor; IT, intrathecal; IV, intravenous; 6-MP, 6-mercaptopurine; MTX, methotrexate; PDN, prednisone; PMN, neutrophils; PO, per os (by mouth); SC, subcutaneously; VCR, vincristine.
Cycles 2, 3, and 4 and interphase start at day 29 of prior cycle.
Triple IT consisted of 15 mg MTX, 40 mg Ara-C, and 40 mg PDN IT.
Patients with MMR at TP4 were eligible to receive either an allo-SCT (from a genoidentical or unrelated 9/10 or 10/10 human leukocyte antigen–compatible donor) or an autologous SCT (auto-SCT) at the investigator’s choice. Patients with a bone marrow MRD4 less than an MMR were recommended to receive an allo-SCT from a genoidentical or unrelated 9/10 or 10/10 human leukocyte antigen–compatible donor or even from an alternate source of stem cells (cord blood, haploidentical SCT) or were discontinued from the study to enter another experimental trial. Transplant procedures are further detailed in the supplemental Appendix.
Statistical analysis
The study was designed as a noninferiority trial. The sample size computation was based on the MMR rate at TP4. We calculated that the enrollment of 250 patients (125 in each arm) would provide the trial with 90% power to show noninferiority, assuming a primary outcome of 80% in both arms, with an absolute noninferiority margin of 15%, applying a 1-sided 2-sample Z-test, and a type I error level of 5%. A sample size of 265 patients was fixed to obtain a randomized sample of 250 evaluable patients.
The primary efficacy analyses were based on the intention to treat, which included all randomized patients, who all received at least 1 dose of treatment (full analysis set). Patients who dropped out of the study before TP4 were considered to have a nonresponse with respect to the primary end point. Exact binomial 95% CI of the MMR at TP4 was computed.
The statistical methods for secondary and post hoc analyses are detailed in the supplemental Appendix. The study was approved by the Institutional Ethics Committee Ile-de-France VI, France.
Results
Participants
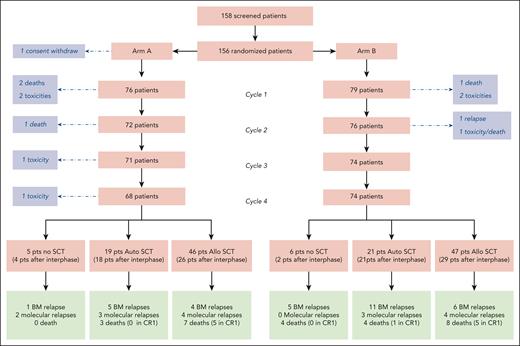
Between March 2016 and February 2019, 156 patients with Ph-positive ALL were enrolled and randomized in the study, with a median follow-up of 3.8 years. Of these patients, 77 were randomly assigned to receive the high-dose methotrexate plus HD-Ara-C regimen (arm A) and 79 the regimen without HD-Ara-C (arm B) as cycles 2 and 4. As 1 patient withdrew consent, the full analysis set included 155 patients (Figure 1).
Patient characteristics were well balanced between both randomization arms, except for more men in arm B (Table 2). Median age was 47.1 years (interquartile range, 38.8-53.8 years). Karyotype analysis was performed in all patients but failed in 4 patients (Table 2). The t(9;22)(q34;q11)/Ph chromosome was evidenced in 142 patients by karyotype. BCR::ABL1 fluorescence in situ hybridization analysis was performed and found positive in 95 patients, including the 9 who were lacking the Ph chromosome in the karyotype analysis. BCR::ABL1 detection was available in 155 patients, with 107 m-bcr and 46 M-bcr fusions and 2 variants (Table 2). Additional cytogenetic abnormalities (ACAs) were observed in 101 patients and more frequently in m-bcr than in M-bcr cases (75.5% vs 56.8%; P = .031). No marked differences in age and white blood cell count were noted in patients with M-bcr or ACA. The ACAs are described in the supplemental Table 1. TKI doses and reasons to switch from nilotinib to another TKI are also given in the supplemental Appendix.
Patient characteristics
| Characteristic . | Randomized patients . | Arm A . | Arm B . |
|---|---|---|---|
| Patients, N | 155 | 76 | 79 |
| Sex ratio, N (M/F) | 80:75 | 38:38 | 42:37 |
| Age, median (IQR), y | 47.1 (38.8-53.8) | 48.8 (38.8-54.0) | 47.0 (39.1-53.5) |
| Aged ≥40 y, N (%) | 113 (72.9) | 55 (72.4) | 58 (73.4) |
| BMI, median (IQR), kg/m2 | 24.9 (22.1-28.4) | 24.9 (22.0-29.1) | 24.7 (22.5-22.8) |
| ECOG PS 0/1/2/3/unknown | 53/73/24/2/3 | 22/39/11/2/2 | 31/34/13/0/1 |
| CNS 3 disease, N (%) | 12 (7.74) | 6 (7.89) | 6 (7.59) |
| WBC, median (IQR), 109/L | 19.4 (6.4-65.2) | 24.3 (7.2-109.0) | 18.0 (6.2-49.0) |
| Karyotype∗ | |||
| Failure, N (yes/no) | 4/151 | 2/74 | 2/77 |
| t(9;22), N (yes/no) | 142/9 | 70/4 | 72/5 |
| ACA, N (yes/no/unknown) | 101/43/11 | 50/21/5 | 51/22/6 |
| Monosomal karyotype, N (yes/no/unknown) | 36/106/11 | 16/55/5 | 22/51/6 |
| bcr subtype, m/M/variant | 107/46/2 | 50/25/1 | 57/21/1 |
| Characteristic . | Randomized patients . | Arm A . | Arm B . |
|---|---|---|---|
| Patients, N | 155 | 76 | 79 |
| Sex ratio, N (M/F) | 80:75 | 38:38 | 42:37 |
| Age, median (IQR), y | 47.1 (38.8-53.8) | 48.8 (38.8-54.0) | 47.0 (39.1-53.5) |
| Aged ≥40 y, N (%) | 113 (72.9) | 55 (72.4) | 58 (73.4) |
| BMI, median (IQR), kg/m2 | 24.9 (22.1-28.4) | 24.9 (22.0-29.1) | 24.7 (22.5-22.8) |
| ECOG PS 0/1/2/3/unknown | 53/73/24/2/3 | 22/39/11/2/2 | 31/34/13/0/1 |
| CNS 3 disease, N (%) | 12 (7.74) | 6 (7.89) | 6 (7.59) |
| WBC, median (IQR), 109/L | 19.4 (6.4-65.2) | 24.3 (7.2-109.0) | 18.0 (6.2-49.0) |
| Karyotype∗ | |||
| Failure, N (yes/no) | 4/151 | 2/74 | 2/77 |
| t(9;22), N (yes/no) | 142/9 | 70/4 | 72/5 |
| ACA, N (yes/no/unknown) | 101/43/11 | 50/21/5 | 51/22/6 |
| Monosomal karyotype, N (yes/no/unknown) | 36/106/11 | 16/55/5 | 22/51/6 |
| bcr subtype, m/M/variant | 107/46/2 | 50/25/1 | 57/21/1 |
ACA, additional chromosomal abnormality; BMI, body mass index; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; PS, performance status; F, female; IQR, interquartile range; M, male; WBC, white blood cell count.
All the 155 patients had bone marrow karyotypes, but 4 karyotypes failed; among the 151 patients with an evaluable karyotype, 142 had the t(9;22) chromosomal translocation; the remaining 9 patients (including 6 normal karyotypes) were BCR::ABL1 fluorescence in situ hybridization positive; among 144 patients, 101 had ACA and 43 did not.
Primary end point
All 4 scheduled cycles were received in 142 of 155 patients (91.6%), and 135 of those had an MRD evaluation after cycle 4. There were no differences in the characteristics of the patients who had an MRD evaluation or not after cycle 4 (supplemental Table 2). At TP4, 115 of the 155 patients (74.2%) reached MMR, including 54 (71.1%) in arm A and 61 (77.2%) in arm B. Both primary and sensitivity analyses met the primary end point of noninferiority of the reduced-intensity arm, as observed by the lower bound of the 95% CIs that did not cross the noninferiority margin of −0.15. In the primary analysis (ITT population), the difference was 6.2 percentage points (2-sided 95% CI, −7.7% to 20%) (Table 3). In the sensitivity analysis based on complete cases, the difference in MMR rates was 2.8% (95% CI, −9.6% to 15.6%).
Primary and secondary efficacy outcomes
| Variable . | Arm A, n (%) . | Arm B, n (%) . | Absolute difference (95% CI) . | P value . |
|---|---|---|---|---|
| Patients, N | 76 | 79 | ||
| Primary outcome | ||||
| MMR rate at TP4 | 54 (71.1) | 61 (77.2) | 6.1 (−7.7 to 19.96) | .0016 |
| Secondary outcomes | ||||
| CR after 1 cycle | 71 (93.4) | 75 (94.9) | 1.5 (−6.69 to 10.15) | .74 |
| Arm A, n (%) | Arm B, n (%) | Hazard ratio (95% CI) | Pvalue | |
| Relapse | 11 | 24 | 2.38 (1.17 to 4.86) | .017 |
| Treatment-related death | 8 | 9 | 1.05 (0.39 to 2.79) | .93 |
| Event (either relapse or death free of relapse) | 19 | 32 | 1.81 (1.03 to 3.20) | .040 |
| Death | 15 | 20 | 1.37 (0.70 to 2.69) | .35 |
| Variable . | Arm A, n (%) . | Arm B, n (%) . | Absolute difference (95% CI) . | P value . |
|---|---|---|---|---|
| Patients, N | 76 | 79 | ||
| Primary outcome | ||||
| MMR rate at TP4 | 54 (71.1) | 61 (77.2) | 6.1 (−7.7 to 19.96) | .0016 |
| Secondary outcomes | ||||
| CR after 1 cycle | 71 (93.4) | 75 (94.9) | 1.5 (−6.69 to 10.15) | .74 |
| Arm A, n (%) | Arm B, n (%) | Hazard ratio (95% CI) | Pvalue | |
| Relapse | 11 | 24 | 2.38 (1.17 to 4.86) | .017 |
| Treatment-related death | 8 | 9 | 1.05 (0.39 to 2.79) | .93 |
| Event (either relapse or death free of relapse) | 19 | 32 | 1.81 (1.03 to 3.20) | .040 |
| Death | 15 | 20 | 1.37 (0.70 to 2.69) | .35 |
CR, complete remission; MMR, major molecular response rate; TP, time point.
Secondary end points
Overall, hematologic CR was reached in all but 3 patients who died early, including 2 in arm A (at days 10 and 18) and 1 in arm B (at day 22). Overall hematologic CR rate was 97.4% in arm A vs 98.7% in arm B (P = .61). Besides the 3 patients who died early in cycle 1, hematologic CR was reached after cycle 1 for all patients except for 6, 3 in each arm, who achieved CR after cycle 2. Resulting CR rate after cycle 1 was 93.4% in arm A vs 94.9% in arm B (P = .74). The responses to the different cycles are described in supplemental Table 3. There was no difference in the rate of MMR response between both arms after cycles 1 through 4. This was also the case for early death at day 60 or day 120 after randomization. The only significant difference was a lower rate of patients in CMR after cycle 2 in patients in arm A (P = .031).
At the time of data cutoff for analysis in the ITT population, median follow-up was 46 months, and 52 patients had experienced an event, including 35 relapses (11 in arm A, 24 in arm B, 1 central nervous system relapse in each arm) and 17 nonrelapse deaths (8 in arm A, 9 in arm B) (Figure 2 and Table 3). The CIR was significantly higher in arm B, with a 4-year cumulative incidence of 30.7% (95% CI, 20.8%-41.2%) vs 13.2% (95% CI, 6.7%-21.9%) in arm A (cause-specific hazard ratio [HR], 2.38 [95% CI, 1.17-4.86]; P = .017; Figure 2A). There was no difference across arms in the cumulative incidence of NRM (cause-specific HR, 1.05 [95% CI, 0.39-2.79]; P = .88; Figure 2B).
Outcome by randomization arm. (A) CIR by randomization arm: at 4 years, CIR was estimated at 13.2% (95% CI, 6.7%-21.9%) in arm A vs 30.7% (95% CI, 20.8%-41.2%) in arm B (P = .017). (B) NRM by randomization arm: at 4 years, NRM was estimated at 10.6% (95% CI, 4.9%-18.8%) in arm A vs 11.4% (95% CI, 5.6%-19.6%) in arm B (P = .88). (C) OS by randomization arm: at 4 years, OS was estimated at 79.4% (95% CI, 70.6%-89.3%) in arm A vs 73.4% (95% CI, 63.9%-84.4%) in arm B (P = .27). (D) RFS by randomization arm: at 4 years, RFS was estimated at 76.2% (95% CI, 67.2%-86.4%) in arm A vs 58.5% (95% CI, 48.4%-70.7%) in arm A (P = .029).
Outcome by randomization arm. (A) CIR by randomization arm: at 4 years, CIR was estimated at 13.2% (95% CI, 6.7%-21.9%) in arm A vs 30.7% (95% CI, 20.8%-41.2%) in arm B (P = .017). (B) NRM by randomization arm: at 4 years, NRM was estimated at 10.6% (95% CI, 4.9%-18.8%) in arm A vs 11.4% (95% CI, 5.6%-19.6%) in arm B (P = .88). (C) OS by randomization arm: at 4 years, OS was estimated at 79.4% (95% CI, 70.6%-89.3%) in arm A vs 73.4% (95% CI, 63.9%-84.4%) in arm B (P = .27). (D) RFS by randomization arm: at 4 years, RFS was estimated at 76.2% (95% CI, 67.2%-86.4%) in arm A vs 58.5% (95% CI, 48.4%-70.7%) in arm A (P = .029).
Of 35 relapsed patients, 29 were tested for the acquisition of BCR::ABL1 tyrosine kinase domain mutations, 20 of 29 (69%) had at least 1 mutation and 10 of 20 (50%) of them had a T315I mutation. The details of the tyrosine kinase domain mutations are described in supplemental Figure 1.
The 4-year OS estimate was 79.4% (95% CI, 70.6%-89.3%) in arm A and 73.4% (95% CI, 63.9%-84.4%) in arm B (HR, 1.37 [95% CI, 0.70-2.69]), with no significant difference in OS between the 2 arms (P = .27 by the log-rank test; Figure 2C). Relapse-free survival was significantly shorter in arm B compared with arm A, with a 4-year RFS rate of 76.2% (95% CI, 67.1%-86.4%) in arm A and 58.5% (95% CI, 48.4%-70.7%) in arm B (HR, 1.81 [95% CI, 1.03-3.20]; P = .029) (Figure 2D).
Hematologic and other grade 3 to 4 toxicities observed during the first 4 cycles are shown in the supplemental Appendix (supplemental Table 4). As anticipated, hematologic toxicity was lower in arm B during the second and fourth cycles, with lower number of patients having absolute neutrophil count <0.5 × 109/L and platelets <20 × 109/L. This was associated with a lower incidence of infections during cycle 2 but not in cycle 4 (supplemental Table 4).
A total of 133 patients underwent an SCT, including 93 allogeneic transplants (46 in arm A and 47 in arm B) and 40 autologous transplants (19 in arm A and 21 in arm B). The transplant modalities by randomization arm are described in the supplemental Appendix (supplemental Table 5). The only difference between the arms regarding those modalities was a shorter time from CR to SCT in arm B compared with arm A (P = .007).
After allo-SCT, there was no difference across randomization arms in outcomes, in terms of CIR (HR, 1.46 [95% CI, 0.41-5.17]), NRM (HR, 1.00 [95% CI, 0.29-3.45]), RFS (HR, 1.20 [95% CI, 0.50-2.91]), and OS (HR, 1.15 [95% CI, 0.42-3.18]) (supplemental Table 5). On the other hand, after auto-SCT, there was a nonsignificant increase in CIR in arm B, with 53.2% (95% CI, 29.1%-72.5%) vs 26.3% (95% CI, 9.2%-47.4%) at 4 years (P = .11), which translated into a shorter RFS of 41.5% (95% CI, 24.8%-69.7.5%) vs 73.7% (95% CI, 56.3%-96.4%) in arm A (P = .066; supplemental Table 5).
Overall, in a time-dependent analysis, allo-SCT was associated with longer RFS compared with auto-SCT (HR, 0.46 [95% CI, 0.24 to 0.87]; P = .018) and the nongrafted cohort (HR, 0.17 [95% CI, 0.08-0.32]; P < .0001) and with a 4-year RFS of 79.4% (95% CI, 71.5%-88.1%) for allo-SCT, of 57.2% (95% CI, 43.7%-74.9%) for auto-SCT, and of 32.0% (95% CI, 17.4%-58.9% for nontransplant patients; Figure 3A). Similar differences were observed in arm B (HR, 0.35 [95% CI, 0.15-0.78]; P = .011 and HR, 0.08 [95% CI, 0.03-0.18]; P = .0001, respectively), with a 4-year RFS of 76.5% (95% CI, 65.3%-89.7%) for allo-SCT, 41.6% (95% CI, 24.8%-69.8%) for auto-SCT, and 9.1% (95% CI, 1.4%-58.9%) for nontransplant patients (Figure 3C). In arm A, improved RFS after allo-SCT was only significant vs the nongrafted cohort (HR, 0.33 [95% CI, 0.11-0.98]; P = .045) but not vs the auto-SCT (HR, 0.73 [95% CI, 0.24-2.18]; P = .57) with a 4-year RFS of 82.4% (95% CI, 72.0%-94.3%) for allo-SCT, 73.7% (95% CI, 56.3%-96.4%) for auto-SCT, and 55.7% (95% CI, 32.9%-94.3%) for nontransplant patients (Figure 3B). After autograft, there was only 1 reported transplant-related death, whereas 7 deaths occurred after relapse.
Relapse-free survival outcome by transplantation type and study arm. (A) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS. t0 was the time of hematologic CR achievement. RFS was significantly prolonged in the allo-SCT cohort vs the nongrafted cohort (hazard ratio [HR], 0.17 [95% CI, 0.08-0.32]; P < .0001) and vs the auto-SCT cohort (HR, 0.46 [95% CI, 0.24-0.87]; P = .018), and in the auto-SCT cohort vs the nongrafted cohort (HR, 0.36 [95% CI, 0.18-0.73]; P = .004). (B) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS in arm A. t0 was the time of hematologic CR achievement. RFS was statistically different between the allo-SCT cohort and the nongrafted one (HR, 0.33 [95% CI, 0.11-0.98]; P = .045) but not between the auto-SCT cohort and the nongrafted one (HR, 0.45 [95% CI, 0.13-1.55]; P = .20) nor between the allo-SCT cohort and the auto-SCT cohort (HR, 0.73 [95% CI, 0.24-2.18]; P = .57). (C) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS in arm B. t0 was the time of hematologic CR achievement. RFS was significantly prolonged in the allo-SCT cohort vs the nongrafted cohort (HR, 0.08 [95% CI, 0.03-0.18]; P = .0001) and the auto-SCT cohort (HR, 0.35 [95% CI, 0.15-0.78]; P = .011) as well as in the auto-SCT cohort (HR, 0.22 [95% CI, 0.09-0.52]; P = .0006).
Relapse-free survival outcome by transplantation type and study arm. (A) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS. t0 was the time of hematologic CR achievement. RFS was significantly prolonged in the allo-SCT cohort vs the nongrafted cohort (hazard ratio [HR], 0.17 [95% CI, 0.08-0.32]; P < .0001) and vs the auto-SCT cohort (HR, 0.46 [95% CI, 0.24-0.87]; P = .018), and in the auto-SCT cohort vs the nongrafted cohort (HR, 0.36 [95% CI, 0.18-0.73]; P = .004). (B) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS in arm A. t0 was the time of hematologic CR achievement. RFS was statistically different between the allo-SCT cohort and the nongrafted one (HR, 0.33 [95% CI, 0.11-0.98]; P = .045) but not between the auto-SCT cohort and the nongrafted one (HR, 0.45 [95% CI, 0.13-1.55]; P = .20) nor between the allo-SCT cohort and the auto-SCT cohort (HR, 0.73 [95% CI, 0.24-2.18]; P = .57). (C) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS in arm B. t0 was the time of hematologic CR achievement. RFS was significantly prolonged in the allo-SCT cohort vs the nongrafted cohort (HR, 0.08 [95% CI, 0.03-0.18]; P = .0001) and the auto-SCT cohort (HR, 0.35 [95% CI, 0.15-0.78]; P = .011) as well as in the auto-SCT cohort (HR, 0.22 [95% CI, 0.09-0.52]; P = .0006).
Post hoc analyses
We also compared the outcome of patients who had Ph+ ALL with p190 (m-bcr) or p210 transcript (M-bcr). There was no difference either in OS (P = .68) (supplemental Figure 2A) or RFS (P = .81) (supplemental Figure 2B).
In patients who had allo-SCT, there was no impact of MMR at TP4 on RFS, with patients having a 4-year RFS of 82.1% (95% CI, 73.3%-91.8%) for those in MMR vs 72.0% (95% CI, 56.4%-91.9%) for the ones who were not (P = .30; supplemental Figure 3A). The same was observed for CMR, with a 4-year RFS of 87.3% (95% CI, 74.8%-100%) vs 76.7% (95% CI, 67.4%-87.4%) for those in CMR vs not (P = .90; supplemental Figure 3B).
We looked at the impact of myeloablative conditioning (MAC) vs reduced-toxicity conditioning (RTC) regimen in allo-SCT patients, and no differences in outcomes were observed, particularly no difference in NRM, with a 4-year cumulative incidence of 12% (95% CI, 4.8%-22.6%) after MAC vs 9.5% (95% CI, 3.0%-20.7%) after RTC (P = .74), nor in CIR, which was 9.9% (95% CI, 3.6%-20.0%) and 9.6% (95% CI, 3.0%-20.9%) at 4 years after MAC and RTC, respectively (P = .86) (supplemental Appendix, supplemental Table 6).
Discussion
This study showed that more relapses were observed when Ara-C was omitted without impact on survival. Despite the decreased statistical power at 75% due to the early trial stopping after 59% of the required sample size, the primary noninferiority end point based on MRD was reached,. The study was built on our initial randomized trial in Ph+ ALL, the GRAAPH-2005, which demonstrated that reduced-intensity induction chemotherapy combined with imatinib did not increase the risk of relapse while reducing treatment-related mortality.5 Here, we used the second-generation TKI nilotinib and investigated whether the intensity of chemotherapy could be further reduced following the first induction cycle. We prolonged exposure to nilotinib before transplant with the aim of achieving deeper molecular response at transplant. As a result, we observed an important improvement in OS and RFS compared with our previous study (4-year OS of 79.4% [95% CI, 70.6%-89.3%] and RFS of 76.2% [95% CI, 67.1%-86.4%] in arm A vs in the GRAAPH-2005 4-year OS of 47.5% [95% CI, 41.2%-53.6%] and RFS of 40.4% [95% CI, 34.2%-46.6%]). The present results compare well with other studies combining nilotinib and chemotherapy, 1 with a shorter median follow-up, which reported 2-year OS and RFS of 72% among patients who were in hematologic CR,10 and another, which reported 45% OS.11 Studies with dasatinib reported lower rates of transplants and lower OS and EFS/RFS estimations, ranging from 46% to 56% and 44% to 47%, respectively.12,13 Last, in the recently updated nonrandomized MDACC study combining dose-adapted ponatinib with hyper-CVAD,14 with an 80-month follow-up, the 6-year OS and EFS were 75% (95% CI, 64%-83%) and 65% (95% CI, 54%-74%), respectively. The results of the randomized multicenter GRAAPH-2014 trial are comparable to this single-center nonrandomized study, albeit there were more transplantations in the GRAAPH-2014 trial.
Blinatumomab, a bispecific T-cell engager targeting CD19 and CD3, is likely to rapidly become a common frontline therapy in Ph+ ALL, as suggested by a recent published Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) study.15 This nonrandomized trial involved 63 patients who received a chemotherapy-free regimen, including steroids and dasatinib, until CR, followed by dasatinib and blinatumomab. The updated results of the study have just been published, with a 53-month OS and EFS of 80.7% and 74.6%, respectively.16 The MDACC approach is in line with these promising results, using the third-generation TKI ponatinib in combination with blinatumomab in a phase 2 study. With a median follow-up of 16 months, the study on 40 newly diagnosed patients with Ph+ ALL showed a CMR rate of 87% with a 1-year OS and EFS of 95% (95% CI, 80%-99%).17 The recently reported phase 3 study PhALLCON comparing ponatinib to imatinib both combined to reduced-intensity chemotherapy established a superiority of ponatinib in terms of CMR rate.18 The CMR rate in the present study was relatively low, with little increase over consecutive cycles, ranging from 5.2% after cycle 1 to 26.4% after cycle 4, but was in the range of those found in the PhALLCON study, with 16.7% with imatinib and 34.4% with ponatinib.18 On the other hand, the combination of TKI with blinatumomab gave higher rate, with 55% of CMR with dasatinib,15 and 87% with ponatinib.17
Despite similar MMR rates after 4 cycles, more relapses were observed when Ara-C was omitted, which may indicate that the limits of the chemotherapy deescalation strategy have been reached when a second-generation TKI is used. Other studies have shown that CMR, rather than MMR, might be a more accurate surrogate marker.12,19,20 In the present study, after 4 cycles of combined therapy, we observed no significant difference in CMR rates between both arms (28.9% in standard arm vs 24.1% in the experimental arm). Recent comparisons between BCR::ABL1 and IG/TR in patients with Ph+ ALL suggest that BCR::ABL1 levels may not always reflect the residual leukemic burden.21-24 Overall, we observed a lower relapse rate in the present nilotinib GRAAPH-2014 study compared with the previous imatinib GRAAPH-2005 study (22.6% vs 36.2%),5 which was even lower in the standard arm A including Ara-C (14.5%). This low relapse rate is comparable to the 9.5% reported in the dasatinib/blinatumomab GIMEMA study.15 One important point is that chemotherapy-free regimens are associated with an increased risk of central nervous system relapses not seen in the present study, emphasizing the importance of high-dose methotrexate blocks in the context of TKI-based therapy.
How to translate our results into practice? The treatment paradigm for Ph+ ALL recently shifted from chemotherapy plus TKI to immunotherapy plus TKI, and the need for allo-SCT is now challenged. However, immunotherapy with T-cell engagers is not yet available outside the United States in frontline treatment of B-cell ALL, including Ph+ ALL. This situation may change in the near future, but affordability will remain a significant barrier. Our results show that a high survival rate can be achieved outside the setting of T-cell engagers with a low TRM rate (day 120 mortality, 3.2%; 4-year NRM, 11%) when induction is based on reduced-intensity chemotherapy plus second-generation TKI, followed by consolidations with chemotherapy at conventional doses and intensification with allo-SCT. This observation is related to the design of the study with the intention to bring to allo-SCT as many patients as possible, which represented 60% of the study population. Furthermore, if chemotherapy (Ara-C) needs to be adapted in selected patients to reduce toxicity, our results suggest that overall survival may not be compromised in patients who received allo-SCT.
As discussed above, emerging strategies combining TKI with blinatumomab are associated with promising results, potentially resulting in fewer patients requiring transplant.15,17 In an attempt to answer the SCT question in this new context, we will randomize allo-SCT vs no SCT after a combined treatment strategy with ponatinib, blinatumomab, and chemotherapy in the next GRAAPH phase 3 trial.
To conclude, this study showed that a conventional approach based on reduced-intensity induction with second-generation TKI followed by conventional consolidations with TKI and intensification with allo-SCT produced a high CR rate and a 4-year overall survival in the range of 80%, independently of the BCR::ABL1 MRD status. Whether this result will be challenged using “chemotherapy-free” regimen without allo-SCT will depend on long-term results of ongoing trials and T-cell engager availability in first-line therapy.
Acknowledgments
The authors thank the patients and their families for participating in the trial and the GRAALL investigators and clinical research assistant for submitting clinical data and samples.
This study (ClinicalTrials.gov ID, NCT02611492) was sponsored by the Paris Ile-de-France Regional Clinical Research Office and supported by grants from the Programme Hospitalier de Recherche Clinique (PHRC 12-129) in France and the Swiss State Secretariat for Education, Research and Innovation, Switzerland. Novartis Pharma provided drug supply for nilotinib, and Amgen provided a research grant.
Authorship
Contribution: V.L. provided administrative support; Y.C., P.R., F.H., P.C., C.G., A.T.-B., S.C., X.T., L.V., C.B., Y.H., E.R., M.E.-B., I.P., M.J., P.T., F.P., A.B., G.R.G., S.B., M.G., N.B., and H.D. provided study materials or patients; Y.C., P.R., J.-M.C., R.K., E.C., V.L., N.B., and H.D. performed collection and assembly of data; R.K., E.C., J.-M.C., J.Q., and M.L.-P. performed central review of molecular and cytogenetic data; Y.C., P.R., S.C., N.B., and H.D. performed data analysis and interpretation; Y.C., P.R., S.C., V.L., N.B., and H.D. wrote the manuscript; and all authors performed conception and design and provided final approval of the manuscript.
Conflict-of-interest disclosure: Y.C. has received honoraria for participation in symposia and advisory boards from Merck Sharp & Dohme, Novartis, Incyte, Bristol Myers Squibb, Pfizer, AbbVie, Roche, Jazz, Gilead, Amgen, AstraZeneca, and Servier; and has received travel support from Merck Sharp & Dohme, Roche, Gilead, Amgen, Incyte, AbbVie, Janssen, AstraZeneca, Jazz, and Sanofi, all via the institution. F.H. has received honoraria for participation on symposia, advisory boards, and travel support from Amgen, Clinigen, Gilead, Incyte, Novartis, Pfizer, and Servier. J.-M.C. has received honoraria from Novartis, Incyte, Qiagen, and Cepheid. M.G. has received honoraria for participation in symposia and advisory boards from AbbVie, Amgen, AstraZeneca, Beigene, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Novartis, Incyte, Janssen-Cilag, Jazz, Roche, Pfizer, Sanofi, and Servier; and has received travel support from AbbVie, Beigene, Pfizer, and Roche. All fees went to the institution. N.B. has received honoraria from Novartis. The remaining authors declare no competing financial interests.
A complete list of the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) investigators and clinical research assistant appears in the supplemental Appendix. The GRAALL includes the France-Belgium Group for Acute Lymphoblastic Leukemia in adults and the Swiss Group for Clinical Cancer Research (SAKK). The GRAALL is an entity of the French Carnot Institute Organization for Partnerships in Leukemia.
Correspondence: Yves Chalandon, Hematology Division, Oncology Department, University Hospital of Geneva, 4 rue Gabrielle Perret-Gentil, 1211 Geneva, Switzerland; email: yves.chalandon@hug.ch; and Philippe Rousselot, Division of Hematology Centre Hospitalier de Versailles, University Versailles Saint-Quentin en Yvelines Paris Saclay UMR1184, 177 rue de Versailles, 78157 Le Chesnay cedex, Versailles, France; email: phrousselot@ght78sud.fr.
References
Author notes
Y.C. and P.R. contributed equally to this study.
For original data, please contact one of the corresponding authors, Yves Chalandon (yves.chalandon@hug.ch) or Philippe Rousselot (phrousselot@ght78sud.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Relapse-free survival outcome by transplantation type and study arm. (A) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS. t0 was the time of hematologic CR achievement. RFS was significantly prolonged in the allo-SCT cohort vs the nongrafted cohort (hazard ratio [HR], 0.17 [95% CI, 0.08-0.32]; P < .0001) and vs the auto-SCT cohort (HR, 0.46 [95% CI, 0.24-0.87]; P = .018), and in the auto-SCT cohort vs the nongrafted cohort (HR, 0.36 [95% CI, 0.18-0.73]; P = .004). (B) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS in arm A. t0 was the time of hematologic CR achievement. RFS was statistically different between the allo-SCT cohort and the nongrafted one (HR, 0.33 [95% CI, 0.11-0.98]; P = .045) but not between the auto-SCT cohort and the nongrafted one (HR, 0.45 [95% CI, 0.13-1.55]; P = .20) nor between the allo-SCT cohort and the auto-SCT cohort (HR, 0.73 [95% CI, 0.24-2.18]; P = .57). (C) Simon-Makuch plots for evaluating the impact of allo-SCT and auto-SCT on RFS in arm B. t0 was the time of hematologic CR achievement. RFS was significantly prolonged in the allo-SCT cohort vs the nongrafted cohort (HR, 0.08 [95% CI, 0.03-0.18]; P = .0001) and the auto-SCT cohort (HR, 0.35 [95% CI, 0.15-0.78]; P = .011) as well as in the auto-SCT cohort (HR, 0.22 [95% CI, 0.09-0.52]; P = .0006).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/23/10.1182_blood.2023023502/2/m_blood_bld-2023-023502-gr3.jpeg?Expires=1764954398&Signature=sSht3-KolC6EAoGFez4T9Z52tikeZOvZOfwG6Vy6QTx2hE6aH8OufTcyjRZzRhubC8o8fmTmpc8pSFENEXQQKeTvaw8~Iem-TAjY~cztWiYLoGiPLbcgjxe67R2TW7h5yd4~OGmgK6Wwrl8EPKAzlxsWCx0CE0dbiYI2FYrumlUuqrSgWBpmqjRSvKIYAbufJDLIKcgvnpCRnzY2wTK9fPMfiXXOk2dGbRPu3a7C3SZebjKjdddjKic8r2Dh6zyvq0QxBY~8Fd10ONe0h1~UqsCLC9QFfsnMKXXWbkplagq2GIcJkvX6dg8Q7uWSDKyRQayr1YjLYValrAMxu6qfdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal