Key Points
FLT3ITD TK inhibition impairs glycolysis and glucose utilization without equally affecting glutamine metabolism.
Combined targeting of FLT3 TK activity and glutamine metabolism decreases FLT3ITD mutant cells leukemogenic potential in vitro and in vivo.
Abstract
FLT3 internal tandem duplication (FLT3ITD) mutations are common in acute myeloid leukemia (AML) associated with poor patient prognosis. Although new-generation FLT3 tyrosine kinase inhibitors (TKI) have shown promising results, the outcome of FLT3ITD AML patients remains poor and demands the identification of novel, specific, and validated therapeutic targets for this highly aggressive AML subtype. Utilizing an unbiased genome-wide clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 screen, we identify GLS, the first enzyme in glutamine metabolism, as synthetically lethal with FLT3-TKI treatment. Using complementary metabolomic and gene-expression analysis, we demonstrate that glutamine metabolism, through its ability to support both mitochondrial function and cellular redox metabolism, becomes a metabolic dependency of FLT3ITD AML, specifically unmasked by FLT3-TKI treatment. We extend these findings to AML subtypes driven by other tyrosine kinase (TK) activating mutations and validate the role of GLS as a clinically actionable therapeutic target in both primary AML and in vivo models. Our work highlights the role of metabolic adaptations as a resistance mechanism to several TKI and suggests glutaminolysis as a therapeutically targetable vulnerability when combined with specific TKI in FLT3ITD and other TK activating mutation–driven leukemias.
Introduction
Acute myeloid leukemia (AML) is a highly heterogeneous disease at both the molecular and clinical levels. Recent sequencing efforts have helped to categorize different subtypes based on their mutation profile and its putative effect on AML pathogenesis. Common subgroups include those carrying mutations in transcription factors and epigenetic regulators, cases carrying mutations in genes encoding for components of the spliceosome machinery and cohesin complexes, and those carrying mutations in signaling genes.1,2 Within the last group, activating mutations of tyrosine kinases (TKs) are the most frequent and generally predict for a poor outcome.3 In particular, mutations in the type-III receptor TK FLT3 are present in ∼30% of AML patients, are mostly secondary to an internal tandem duplication (FLT3ITD) of the juxtamembrane domain, and predict for an increased relapse rate following standard therapies and a poor prognosis.4 Although FLT3ITD mutations are acquired relatively late in leukemia evolution1,5 and are unable to produce an AML phenotype in animal models without collaborating mutations,6 they are capable of conferring a state of oncogene addiction by activating survival pathways.7 Their importance for the maintenance of the leukemic phenotype and as a relevant therapeutic target has also been confirmed by the results of a recent phase 3 randomized study (RATIFY), where a survival benefit for patients treated with FLT3 TK inhibitor (TKI) was demonstrated for the first time,8 leading to recent US Food and Drug Administration approval of the FLT3 inhibitor midostaurin. However, despite our understanding of the role played by FLT3ITD mutations in AML and the rational design of targeted inhibitors of their TK activity, the overall outcome of AML patients carrying FLT3ITD mutations remains poor, suggesting that resistance mechanisms to targeted inhibitors might hinder the efficacy of these therapies.9 Indeed mutations in the FLT3 TK domain have already been described as a frequent mechanism of resistance.7 However, more recently, mutational analysis of patient samples obtained following relapse after FLT3-TKI treatment and a handful of preclinical studies have suggested that cellular adaptive mechanism might also play a role in FLT3-TKI resistance,10-13 although these remain overall poorly defined.
FLT3ITD mutations are known to activate survival/proliferation signaling pathways, including the phosphatidylinositol 3-kinase/AKT, Ras/MAPK, and JAK/STAT pathways14-17 that are also known to directly or indirectly alter cell metabolism.18-20 As a result, leukemias harboring FLT3ITD mutations are often associated with a very proliferative and aggressive phenotype and high tumor bulk and are accompanied by alterations in cellular metabolism to sustain this proliferative phenotype.4,21
Metabolic reprogramming has emerged as a hallmark of transformed cells,22 and several reports have recently highlighted the role of specific metabolic enzymes and metabolites in normal hematopoietic stem cell homeostasis and leukemogenesis through both direct effects on energy production and macromolecule biosynthesis and their ability to modulate redox balance, epigenetic regulation, and signaling pathways.23-29 Moreover, metabolism is able to rapidly respond to changing conditions within a cell, and it has already been shown, in both solid cancers and hematological malignancies, that metabolic adaptations, under therapeutic selective pressure, can act as key resistance mechanisms to standard therapeutics.30,31
In this work, we aimed to identify novel cellular adaptive resistance mechanisms to FLT3-TKI treatment in FLT3ITD AML. Using several unbiased complementary approaches, we identify glutamine metabolism as a protective and adaptive response to FLT3-TKI and describe the mechanisms underlying this phenotype. Finally, we validate glutaminolysis as a clinically actionable therapeutic vulnerability in both FLT3ITD and other AML subtypes carrying TK activating mutations following TKI treatment.
Methods
For more information, see the supplemental Methods (available on the Blood Web site).
Cell culture
MV411, MOLM13, THP1, and K562 were cultured in RPMI 1640 (Sigma-Aldrich) supplemented with 10% dialyzed fetal bovine serum (Sigma-Aldrich) and 1% penicillin/streptomycin/glutamine. Lineage-depleted bone marrow cells from Rosa26Cas9/+, Flt3ITD/+ mice were transduced with retrovirus constructs pMSCV-MLL-AF9-IRES-YFP, pMSCV-MLL-AF4-PGK-puro, and pMSCV-MLL-ENL-IRES-Neo and cultured in X-VIVO 20 (Lonza) supplemented with 10 ng/mL interleukin-3 (IL-3), 10 ng/mL IL-6, and 50 ng/mL stem cell factor (Peprotech).
Generation of genome-wide mutant libraries, CRISPR screening, and gRNA competition assays
Clustered regularly interspaced short palindromic repeats (CRISPR) screens were performed using the previously reported wild-type Sanger genome-wide CRISPR library.32 Guide RNA (gRNA) competition assays were performed using single and dual gRNA vectors as described previously.32 The gRNA sequences are listed in the supplemental Methods.
Liquid chromatography coupled to mass spectrometry (LC-MS) for metabolomics analysis
MV411 cells were plated at 0.5 × 106 cells/mL in media supplemented with uniformly labeled 13C (U-13C6) glucose (11 mM) or uniformly labeled 13C, 15nitrogen (U-13C5,15N2) glutamine (2 mM) (Cambridge Isotope Laboratories) for 48 hours before sampling. Details of metabolite extraction and LC-MS analysis are provided in the supplemental Methods.
Adult primary leukemia and cord blood sample drug and proliferation assays
Human AML mononuclear cells were obtained from bone marrow or peripheral blood of patients. Normal CD34 samples were obtained from leukapheresis products of myeloma/lymphoma patients in bone marrow remission. Informed consent was obtained in accordance with the Declaration of Helsinki, and the study was conducted under local ethical approval (REC 07-MRE05-44). Culture conditions for methylcellulose and liquid culture assay of primary samples were as previously described.33
In vivo experiments
MV411 cells transduced with control “scramble” short hairpin RNA (shRNA) or GLS shRNA were transplanted (3 × 106) into sublethally irradiated (2 Gy) 8- to 12-week-old NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) male mice via tail vein injection. Three days after transplant, mice were fed a doxycycline diet (1 g/kg) to induce the shRNA, and after disease dissemination, treatment was started. Mice were treated by gavage either with vehicle (22% hydroxypropyl-β-cyclodextrin/0.3% dimethyl sulfoxide) or AC220 at 1 mg/kg daily for 8 days and then 0.1 mg/kg till they succumbed to disease. Survival was measured as the time from transplantation until the point at which mice had to be humanely culled due to overt clinical symptoms typical of the MV411 xenotransplant model.34
Results
A genome-wide CRISPR/Cas9 screen identifies GLS as a synthetic lethal gene in TKI-treated FLT3ITD cells
In order to identify genes and pathways that would sensitize FLT3ITD AML to FLT3-TKI treatment in an unbiased manner, we performed a genome-wide CRISPR/Cas9 synthetic lethality screen in the FLT3ITD cell line MOLM13 during treatment with the highly potent and specific FLT3ITD inhibitor AC220 (quizartinib), which is currently being assessed in phase 3 clinical trials34 or vehicle control (Figure 1A). A total of 304 genes dropped out following AC220 treatment (defined as genes showing a drop out of ≤0.5 log2 fold change in at least 80% of gRNA at false discovery rate < 0.01) (supplemental Table 1). KEGG gene set enrichment analysis, using Enrichr software,35,36 demonstrated significant enrichment for genes involved in several pathways, including some obviously relevant to AML biology (highlighted in Figure 1B). Among these, metabolic pathways, including mostly genes involved in oxidative phosphorylation and the tricarboxylic acid (TCA) cycle, were significantly enriched (Figure 1B). Among the top metabolic genes depleted following AC220 treatment, glutaminase (GLS) demonstrated the highest number of significantly depleted gRNA (all 5 guides out of 5 targeting the gene), indicating a strong synthetic lethal interaction with AC220 (Figure 1C-D). GLS is the first enzyme in glutamine catabolism, a metabolic pathway with well-established anaplerotic and biosynthetic roles in cancer cells37 that also regulates the availability of substrates for both the TCA cycle and oxidative phosphorylation, 2 of the most affected pathways in our screen. Importantly, a potent and selective GLS inhibitor, CB839, is currently being investigated in clinical trials.38 Given the strong synthetic lethal interaction in the screen, its central role in regulating metabolic pathways shown to be affected in our screen, and the availability of a clinical grade inhibitor, we decided to further investigate the role of GLS as a clinically relevant synthetic lethal pair in AC220-treated FLT3ITD cells.
GLS gene deletion and chemical inhibition are synthetically lethal with FLT3 TKIs. (A) Schematic of the genome-wide CRISPR/Cas9 synthetic lethality screen in the FLT3ITD mutant cell line MOLM13. (B) KEGG pathways enrichment analysis of dropout genes from CRISPR/Cas9 screen sorted by combined score calculated using Enrichr software.35,36 Pathways relevant to AML biology are highlighted while the remaining are grayed out. (C) List of top 10 genes from the “metabolic pathways” gene list sorted according to average fold depletion from gRNAs. FDR, false discovery rate. (D) Bar graph depicting individual fold depletion for each gRNA targeting GLS. (E-G) Growth inhibition curves to AC220 of MOLM13 (E) and murine bone marrow cells expressing MLL/AF9-FLT3ITD (F) and MLL/AF9 (G) transduced respectively with “empty” gRNA control or 2 different gRNA targeting GLS (mean ± standard error of the mean [SEM], n = 3, P < .001 for treatment effect comparing control and both Gls knockout for panels E-F; ns, not significant for panel G; 2-way analysis of variance [ANOVA]). (H-I) Apoptosis in the FLT3ITD mutant cell lines MV411 (H) and MOLM13 (I) transduced with control scramble shRNA and GLS shRNA following treatment with AC220 0.5 nM for 48 hours (for MV411: mean ± SEM, n = 7, ****P < .0001, **P = .0086; for MOLM13: mean ± SEM, n = 3, **P = .0014; for AC220 treatments [comparison between scramble and GLS shRNA]: **P = .0050; for dimethyl sulfoxide vs AC220 comparison in the scramble shRNA; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Apoptosis in MV411 (J) and MOLM13 (K) following treatment with AC220 1 nM, CB839 100 nM or their combination (for MV411: mean ± SEM, n = 14, **P = .0033, ****P < .0001; for MOLM13: mean ± SEM, n = 16, *P = .0126, ***P = .0003; ANOVA with Tukey’s multiple comparisons). (L-M) Apoptosis in MV411 (L) and MOLM13 (M) grown in the presence (full media) or absence of glutamine following treatment with AC220 1 nM for 48 hours (for MV411 mean ± SEM, n = 24, for MOLM13 mean ± SEM, n = 11; ****P < .0001, ***P = .0007; 2-way ANOVA with Bonferroni’s multiple comparisons).
GLS gene deletion and chemical inhibition are synthetically lethal with FLT3 TKIs. (A) Schematic of the genome-wide CRISPR/Cas9 synthetic lethality screen in the FLT3ITD mutant cell line MOLM13. (B) KEGG pathways enrichment analysis of dropout genes from CRISPR/Cas9 screen sorted by combined score calculated using Enrichr software.35,36 Pathways relevant to AML biology are highlighted while the remaining are grayed out. (C) List of top 10 genes from the “metabolic pathways” gene list sorted according to average fold depletion from gRNAs. FDR, false discovery rate. (D) Bar graph depicting individual fold depletion for each gRNA targeting GLS. (E-G) Growth inhibition curves to AC220 of MOLM13 (E) and murine bone marrow cells expressing MLL/AF9-FLT3ITD (F) and MLL/AF9 (G) transduced respectively with “empty” gRNA control or 2 different gRNA targeting GLS (mean ± standard error of the mean [SEM], n = 3, P < .001 for treatment effect comparing control and both Gls knockout for panels E-F; ns, not significant for panel G; 2-way analysis of variance [ANOVA]). (H-I) Apoptosis in the FLT3ITD mutant cell lines MV411 (H) and MOLM13 (I) transduced with control scramble shRNA and GLS shRNA following treatment with AC220 0.5 nM for 48 hours (for MV411: mean ± SEM, n = 7, ****P < .0001, **P = .0086; for MOLM13: mean ± SEM, n = 3, **P = .0014; for AC220 treatments [comparison between scramble and GLS shRNA]: **P = .0050; for dimethyl sulfoxide vs AC220 comparison in the scramble shRNA; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Apoptosis in MV411 (J) and MOLM13 (K) following treatment with AC220 1 nM, CB839 100 nM or their combination (for MV411: mean ± SEM, n = 14, **P = .0033, ****P < .0001; for MOLM13: mean ± SEM, n = 16, *P = .0126, ***P = .0003; ANOVA with Tukey’s multiple comparisons). (L-M) Apoptosis in MV411 (L) and MOLM13 (M) grown in the presence (full media) or absence of glutamine following treatment with AC220 1 nM for 48 hours (for MV411 mean ± SEM, n = 24, for MOLM13 mean ± SEM, n = 11; ****P < .0001, ***P = .0007; 2-way ANOVA with Bonferroni’s multiple comparisons).
In single targeting experiments, GLS was validated as synthetically lethal with AC220 in human and murine FLT3ITD mutant, but not in wild-type FLT3 (FLT3wt) cells (Figure 1E-G; supplemental Figure 1A-C). Moreover, the genetic ablation of GLS was nontoxic in untreated FLT3ITD cells (supplemental Figure 1D-F). We then confirmed that the silencing of GLS by shRNA and its chemical inhibition, using the specific clinical grade inhibitor CB839 at concentrations shown to be inhibiting GLS enzymatic activity in a specific fashion,38 produced similar effects on cell proliferation when combined with AC220 in FLT3ITD-mutant cells, and the combination treatment induced higher levels of apoptosis than AC220 treatment alone in FLT3ITD, but not FLT3wt cells (Figure 1H-K; supplemental Figure 1G-K). In line with these findings, glutamine starvation sensitized FLT3ITD cells to AC220 while having negligible effects in untreated cells (Figure 1L-M). Taken together, these data demonstrate that glutamine metabolism represents a metabolic dependency in FLT3ITD cells that is only unmasked by FLT3-TKI, making genetic and chemical inhibition of GLS a feasible strategy to sensitize these cells to AC220.
FLT3 TK inhibition markedly reduces glycolysis without affecting glutamine uptake in FLT3ITD cells
Previous studies have demonstrated that cells carrying FLT3ITD display a highly glycolytic phenotype and enhanced central carbon metabolism.21 Indeed, gene set enrichment analysis39,40 of published gene expression datasets of untreated AML patients at diagnosis,1,41,42 demonstrate that signatures involving glucose metabolism, TCA cycle, and electron transport chain (ETC) are consistently upregulated in FLT3ITD compared with FLT3wt samples across all datasets analyzed (supplemental Figure 2A-D). Furthermore, murine bone marrow cells carrying FLT3ITD demonstrate both increased glycolytic activity/capacity and oxygen consumption compared with their FLT3wt counterpart (supplemental Figure 2E-F). Considering that glucose and glutamine are the main fuels for central carbon metabolism in cultured cells,37,43 we investigated the effects of FLT3-TKI on the utilization of these nutrients and on central carbon metabolism. Dynamic measurement of the concentration of glucose and glutamine in FLT3ITD cell-conditioned medium confirmed that while glucose uptake was almost completely blocked during treatment with AC220, glutamine uptake was only modestly reduced, and by 48 hours, it was not significantly different between treated and untreated cells (Figure 2A-D). LC-MS analysis using U-13C6-glucose confirmed a marked reduction in glucose labeling of glycolytic intermediates/products upon FLT3 TK inhibition in FLT3ITD mutant cells (Figure 2E; supplemental Table 2). The effects of AC220 on glycolysis were further confirmed by time-resolved metabolic profiling (Figure 2F-G). As might be expected based on the profound antiproliferative effects of AC220 in the same cells (supplemental Figure 3A-B), a reduction in total levels of most TCA cycle intermediates was also observed, but this was less pronounced and the presence of 13C2 citrate and 13C3 aspartate isotopologues (products, of the activity of the anaplerotic enzymes pyruvate dehydrogenase and pyruvate carboxylase, respectively) suggests that anaplerotic oxidative metabolism is still active in these cells (Figure 2H-I; supplemental Table 2). Moreover, the preservation of the unlabeled (13C0) and partially labeled (13C2) fractions of TCA cycle intermediates also suggests that alternative carbon sources, such as glutamine, were used by the cells to support the production of TCA cycle metabolites following AC220 treatment (Figure 2H; supplemental Table 2). Gene expression studies, performed prior to the induction of significant levels of apoptosis by AC220, confirmed the more pronounced effects of FLT3 inhibition on glycolytic enzymes compared with TCA cycle and anaplerotic genes, including glutaminolytic enzymes, GLS, and glutamate dehydrogenase 1 (GLUD1) (Figure 2J-M; supplemental Figure 3C-D; supplemental Table 3). Overall, these data highlight that FLT3 inhibition significantly impairs the utilization of glucose as a carbon source and particularly glycolysis in FLT3ITD cells, whereas glutamine utilization and anaplerotic oxidative metabolism via the TCA cycle were not equally affected.
FLT3 TK inhibition reduces glucose uptake and central carbon metabolism without affecting glutamine uptake. (A-D) Time-course analysis of glucose and glutamine uptake from media by MV411 (A-B) and MOLM13 (C-D) cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 3; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (E) Total and isotopologue abundance of selected glycolytic intermediates and products measured by LC-MS analysis in MV411 cell extracts treated with AC220 1 nM or vehicle control and grown in media containing uniformly labeled 13C [U-13C6] glucose (GLC) (mean ± SEM, n = 5; ***P = .0004 for total 3-phosphoglycerate and P = .0007 for total lactate; 2-tailed paired t test). (F-G) Extracellular acidification rate (ECAR) of MV411 (F) and MOLM13 (G) cells treated with AC220 1nM or vehicle control (mean ± SEM, n = 3, ****P < .0001, ***P = .0003, 2-way ANOVA with Bonferroni’s multiple comparisons). (H) Total and isotopologue levels of selected TCA cycle intermediates in MV411 cells treated as in panel E (mean ± SEM, n = 5; *** P = .0009,**P = .0011; ns, not significant; 2-tailed, paired Student t test). A.U., arbitrary units. (I) Percentage of total levels of citrate and aspartate provided respectively by the 13C2 and 13C3 fraction following AC220 treatment as in panel E (mean ± SEM, n = 5; ****P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Volcano plot for gene expression changes by RNA sequencing (n = 2 for each cell line) of MV411 and MOLM13 cells treated with AC220 1 nM compared with vehicle control, highlighting reduced expression of glycolysis genes (top) and the minimal effects on TCA cycle genes (bottom). (L-M) Quantitative PCR validation in MV411 (L) and MOLM13 (M) for the reduced expression of lactate dehydrogenase (LDHA) and glucose transporter (GLUT3) after treatment with AC220 1 nM (top) (mean ± SEM, MV411 n = 4, MOLM13 n = 5; for LDHA: **P = .0053, *** P = .0005; for GLUT3, MV411: *P = .0150; MOLM13: *P = .0387; 2-tailed, paired Student t test) and for the lack of changes in expression in glutamine metabolism genes (GLS) and glutamate dehydrogenase (GLUD1) (bottom) (mean ± SEM, n = 4; ns, not significant by 2-tailed, paired Student t test).
FLT3 TK inhibition reduces glucose uptake and central carbon metabolism without affecting glutamine uptake. (A-D) Time-course analysis of glucose and glutamine uptake from media by MV411 (A-B) and MOLM13 (C-D) cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 3; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (E) Total and isotopologue abundance of selected glycolytic intermediates and products measured by LC-MS analysis in MV411 cell extracts treated with AC220 1 nM or vehicle control and grown in media containing uniformly labeled 13C [U-13C6] glucose (GLC) (mean ± SEM, n = 5; ***P = .0004 for total 3-phosphoglycerate and P = .0007 for total lactate; 2-tailed paired t test). (F-G) Extracellular acidification rate (ECAR) of MV411 (F) and MOLM13 (G) cells treated with AC220 1nM or vehicle control (mean ± SEM, n = 3, ****P < .0001, ***P = .0003, 2-way ANOVA with Bonferroni’s multiple comparisons). (H) Total and isotopologue levels of selected TCA cycle intermediates in MV411 cells treated as in panel E (mean ± SEM, n = 5; *** P = .0009,**P = .0011; ns, not significant; 2-tailed, paired Student t test). A.U., arbitrary units. (I) Percentage of total levels of citrate and aspartate provided respectively by the 13C2 and 13C3 fraction following AC220 treatment as in panel E (mean ± SEM, n = 5; ****P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Volcano plot for gene expression changes by RNA sequencing (n = 2 for each cell line) of MV411 and MOLM13 cells treated with AC220 1 nM compared with vehicle control, highlighting reduced expression of glycolysis genes (top) and the minimal effects on TCA cycle genes (bottom). (L-M) Quantitative PCR validation in MV411 (L) and MOLM13 (M) for the reduced expression of lactate dehydrogenase (LDHA) and glucose transporter (GLUT3) after treatment with AC220 1 nM (top) (mean ± SEM, MV411 n = 4, MOLM13 n = 5; for LDHA: **P = .0053, *** P = .0005; for GLUT3, MV411: *P = .0150; MOLM13: *P = .0387; 2-tailed, paired Student t test) and for the lack of changes in expression in glutamine metabolism genes (GLS) and glutamate dehydrogenase (GLUD1) (bottom) (mean ± SEM, n = 4; ns, not significant by 2-tailed, paired Student t test).
Glutamine supports the TCA cycle and glutathione production following FLT3 inhibition
In order to understand the fate of glutamine metabolism in FLT3ITD cells following AC220 treatment, we performed LC-MS analysis upon incubation with stable isotope labeled glutamine (U-13C5,15N2-glutamine). Intracellular levels of labeled glutamine were increased in treated cells, confirming that glutamine uptake was not impaired in these cells but, as expected, given the antiproliferative effects of AC220, incorporation of labeled glutamine in TCA cycle intermediates was reduced and overall the level of most TCA cycle intermediates was decreased compared with vehicle treated cells. However, between 20% to 40% of the total pool of TCA cycle intermediates was still labeled from glutamine oxidative metabolism in AC220-treated cells compared with 30% to 60% in vehicle-treated cells, suggesting that despite a significant reduction in overall TCA cycle activity, glutamine is still a major anaplerotic substrate in FLT3-TKI–treated cells (Figure 3A-B; supplemental Table 2). Of note, AC220-treated cells were still able to produce aspartate (Figure 3A), a readout of ETC activity,44,45 indicating that their respiratory function was not compromised by FLT3-TKI treatment (total levels of aspartate were actually increased, possibly reflecting lack of utilization due to FLT3-TKI antiproliferative effects). Consistent with this hypothesis, AC220-treated cells increased their mitochondrial membrane potential and showed a trend toward increased mitochondrial mass (Figure 3C-D; supplemental Figure 4A). We did not observe any significant contribution from glutamine reductive metabolism in FLT3ITD mutant cells, and this did not change following AC220 treatment (supplemental Figure 4B).
Glutamine supports both mitochondrial function and glutathione production following FLT3 TK inhibition. (A) Total and isotopologue levels of TCA cycle intermediates, glutamate and reduced glutathione (GSH), measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control and grown in media containing U-13C5 and 15nitrogen [15N2] glutamine (GLN) (mean ± SEM, n = 5; ***P = .0005, **P = .0017, *P = .0195 for citrate, P = .0228 for malate, P = .0216 for glutamate; ns, not significant; 2-tailed, paired Student t test). A schematic representation of glutamine metabolism and labeling pattern of metabolites is also provided. (B) Percentage of total levels of TCA cycle metabolites labeled by U-13C5,15N2-GLN through its oxidative metabolism measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 5; ****P < .0001, ***P = .0008, **P = .0067; 2-way ANOVA with Bonferroni’s multiple comparisons). (C) Relative mitochondrial membrane potential of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right) (mean ± SEM, n = 3; * P = .0145; 2-tailed, paired Student t test). (D) Relative mitochondrial mass of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right panel) (mean ± SEM, n = 3; ns, not significant; P = .13; 2-tailed paired t test). (E) Relative GSH/GSSG ratio, GSH, and GSSG of MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 6; * P = .0173; ns, not significant; 2-tailed, paired Student t test). (F) Percentage of total levels of glutamine, glutamate, GSH, and GSSG labeled by U-13C5,15N2-GLN measured by LC-MS analysis in MV411 cells treated as in panel A (mean ± SEM, n = 5; **** P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons. (G) Relative GSH levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 5; **P = .0017, *P = .0471, q = 4.248; ANOVA with Tukey’s multiple comparisons). (H) Relative cytoplasmic ROS levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 14; ****P < .0001; ANOVA with Tukey’s multiple comparisons).
Glutamine supports both mitochondrial function and glutathione production following FLT3 TK inhibition. (A) Total and isotopologue levels of TCA cycle intermediates, glutamate and reduced glutathione (GSH), measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control and grown in media containing U-13C5 and 15nitrogen [15N2] glutamine (GLN) (mean ± SEM, n = 5; ***P = .0005, **P = .0017, *P = .0195 for citrate, P = .0228 for malate, P = .0216 for glutamate; ns, not significant; 2-tailed, paired Student t test). A schematic representation of glutamine metabolism and labeling pattern of metabolites is also provided. (B) Percentage of total levels of TCA cycle metabolites labeled by U-13C5,15N2-GLN through its oxidative metabolism measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 5; ****P < .0001, ***P = .0008, **P = .0067; 2-way ANOVA with Bonferroni’s multiple comparisons). (C) Relative mitochondrial membrane potential of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right) (mean ± SEM, n = 3; * P = .0145; 2-tailed, paired Student t test). (D) Relative mitochondrial mass of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right panel) (mean ± SEM, n = 3; ns, not significant; P = .13; 2-tailed paired t test). (E) Relative GSH/GSSG ratio, GSH, and GSSG of MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 6; * P = .0173; ns, not significant; 2-tailed, paired Student t test). (F) Percentage of total levels of glutamine, glutamate, GSH, and GSSG labeled by U-13C5,15N2-GLN measured by LC-MS analysis in MV411 cells treated as in panel A (mean ± SEM, n = 5; **** P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons. (G) Relative GSH levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 5; **P = .0017, *P = .0471, q = 4.248; ANOVA with Tukey’s multiple comparisons). (H) Relative cytoplasmic ROS levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 14; ****P < .0001; ANOVA with Tukey’s multiple comparisons).
In addition to supporting TCA cycle activity, glutamine, via glutamate, is also a precursor of glutathione, the major cellular antioxidant.46 Of note, the reduced/oxidized glutathione ratio (GSH/GSSG) was generally preserved in AC220-treated cells (Figure 3E), and glutathione metabolism genes, including the master regulator of antioxidant response NFE2L2, were not affected by AC220 treatment in FLT3ITD mutant cells (supplemental Figure 4C-D; supplemental Table 3). As our labeling experiments showed that glutamine largely contributes to glutamate and GSH generation (Figure 3A,F), we hypothesized that glutamine metabolism might play a role in maintaining redox homeostasis in AC220-treated cells. Consistent with this hypothesis, glutamine starvation markedly reduced GSH levels in AC220-treated cells, and these effects correlated with a significant increase in intracellular reactive oxygen species (ROS) levels (Figure 3G-H; supplemental Figure 4E). Overall, these data suggest a role for glutamine metabolism in supporting both mitochondrial function and redox homeostasis in FLT3ITD cells under the cellular stress of TK inhibition.
Effects of combined FLT3-TKI and GLS inhibitor treatment can be rescued by the glutamine downstream product α-ketoglutarate (αKG)
In order to clarify the relative importance of the metabolic pathways supported by glutamine in the survival of FLT3ITD cells upon TKI treatment, we specifically targeted both glutathione metabolism and respiratory function using respectively buthionine sulfoximine, an inhibitor of GSH synthesis,47 or phenformin, an ETC (complex I) inhibitor44 in addition to AC220. However, neither of the 2 combinations was able to fully phenocopy the effects of glutamine starvation or GLS inhibition (supplemental Figure 5A-B). We also failed to completely rescue the effects of glutamine starvation or GLS inhibition using the antioxidant N-acetylcysteine or anaplerotic substrates such as pyruvate or aspartate (supplemental Figure 5C-H). These data support a model whereby both branches of glutamine metabolism, supporting TCA cycle/mitochondrial function and GSH synthesis, are important for continued cell survival, and blocking only one of these branches is insufficient to recapitulate the effects of glutamine starvation or GLS inhibition.
To confirm this hypothesis, we used a cell-permeable form of αKG, a downstream metabolic product of glutamine metabolism, to rescue the effects of combined AC220 and CB839 treatment in FLT3ITD cells. Among other functions,48 αKG supports both the TCA cycle and glutamate production and can therefore rescue both branches of glutamine metabolism. Moreover, αKG is known to regulate redox homeostasis in cancer cells.49 Treatment of FLT3ITD cells with combined AC220 and CB839 resulted in reduced oxygen consumption, during both basal and maximal respiration, and increased intracellular ROS production compared with single agent alone. However, these effects were rescued by concomitant treatment with αKG, in keeping with its anaplerotic and antioxidant properties (Figure 4A-D). The salvage of the metabolic phenotype correlated with a complete rescue of the additional cell death related to the combination treatment by αKG (Figure 4E-F). Overall, these data further confirm the importance of glutamine metabolism in supporting both TCA cycle and redox metabolism in FLT3ITD cells treated with AC220.
Rescue of combined effects of AC220 and CB839 by αKG. (A-B) Oxygen consumption rate (OCR) in MV411 (A) and MOLM13 (B) cells treated with vehicle control, AC220 1 nM, CB839 100 nM, AC220 and CB839 combination, and AC220/CB839 combination + 4 mM dimethyl αKG measured using a Seahorse analyzer (for MV411 mean ± SEM, n = 3; for basal respiration: ** P = .0019 between AC220 and AC220+CB839, P = .018 between AC220 + CB839 and AC220 + CB839 + αKG; for maximal respiration: ***P = .0002 between AC220 and AC220 + CB839, P = .0224 between AC220 + CB839 and AC220 + CB839 + αKG, 2-way ANOVA with Bonferroni’s multiple comparisons; for MOLM13 cells: mean ± SEM, n = 3; for basal respiration: *P = .0298 between AC220 and AC220 + CB839, P = .0182 between AC220 + CB839 and AC220 + CB839 + αKG; for maximal respiration: *P = .02 between AC220 and AC220 + CB839, P = .0148 between AC220 + CB839 and AC220 + CB839 + αKG; 2-way ANOVA with Bonferroni’s multiple comparisons). (C-D) Relative cytoplasmic ROS levels of MV411 (C) and MOLM13 (D) cells treated as in panels A-B (for MV411: mean ± SEM, n = 5, * P = .0195, *** P = .0008; for MOLM13: mean ± SEM, n = 6, *P = .0345, ANOVA with Tukey’s multiple comparisons). (E-F) Apoptosis in MV411 (E) and MOLM13 (F) cells treated as in panels A-B (for MV411: mean ± SEM, n = 10, *P = .0158 between AC220 and AC220 + CB839 and *P = .0102 between AC220 + CB839 and AC220 + CB839 + αKG; for MOLM13: mean ± SEM, n = 9, *P = .0105 between AC220 and AC220+CB839, *P = .0120 between AC220 + CB839 and AC220 + CB839 + αKG, ANOVA with Tukey’s multiple comparisons).
Rescue of combined effects of AC220 and CB839 by αKG. (A-B) Oxygen consumption rate (OCR) in MV411 (A) and MOLM13 (B) cells treated with vehicle control, AC220 1 nM, CB839 100 nM, AC220 and CB839 combination, and AC220/CB839 combination + 4 mM dimethyl αKG measured using a Seahorse analyzer (for MV411 mean ± SEM, n = 3; for basal respiration: ** P = .0019 between AC220 and AC220+CB839, P = .018 between AC220 + CB839 and AC220 + CB839 + αKG; for maximal respiration: ***P = .0002 between AC220 and AC220 + CB839, P = .0224 between AC220 + CB839 and AC220 + CB839 + αKG, 2-way ANOVA with Bonferroni’s multiple comparisons; for MOLM13 cells: mean ± SEM, n = 3; for basal respiration: *P = .0298 between AC220 and AC220 + CB839, P = .0182 between AC220 + CB839 and AC220 + CB839 + αKG; for maximal respiration: *P = .02 between AC220 and AC220 + CB839, P = .0148 between AC220 + CB839 and AC220 + CB839 + αKG; 2-way ANOVA with Bonferroni’s multiple comparisons). (C-D) Relative cytoplasmic ROS levels of MV411 (C) and MOLM13 (D) cells treated as in panels A-B (for MV411: mean ± SEM, n = 5, * P = .0195, *** P = .0008; for MOLM13: mean ± SEM, n = 6, *P = .0345, ANOVA with Tukey’s multiple comparisons). (E-F) Apoptosis in MV411 (E) and MOLM13 (F) cells treated as in panels A-B (for MV411: mean ± SEM, n = 10, *P = .0158 between AC220 and AC220 + CB839 and *P = .0102 between AC220 + CB839 and AC220 + CB839 + αKG; for MOLM13: mean ± SEM, n = 9, *P = .0105 between AC220 and AC220+CB839, *P = .0120 between AC220 + CB839 and AC220 + CB839 + αKG, ANOVA with Tukey’s multiple comparisons).
Effects of combined GLS and TKI extend to other TK activating mutations, primary AML samples, and in vivo models
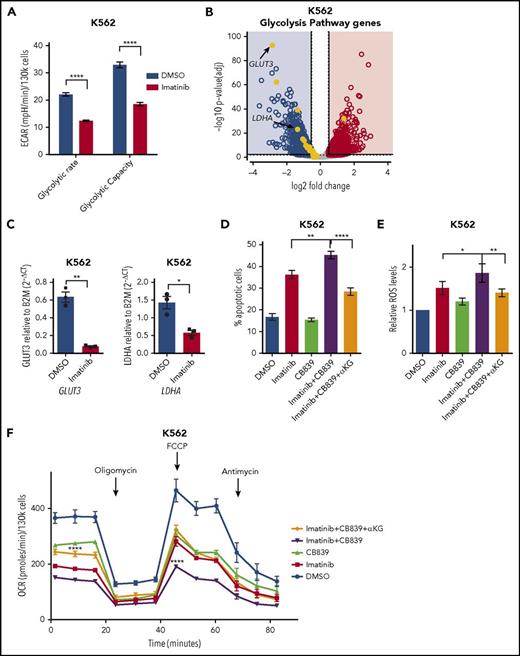
To determine whether a similar rewiring of metabolism occurs in leukemia driven by other activated TK that are amenable to targeted inhibition, we analyzed the metabolic consequences of inhibiting the chimeric BCR-ABL tyrosine kinase, which is central to the pathogenesis of chronic myeloid leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia,50 using its specific inhibitor imatinib.51 Indeed, in a BCR-ABL–positive cell line, imatinib treatment resulted in a reduction of glycolytic activity that also correlated with a decrease in gene expression levels of glycolytic enzymes (Figure 5A-C). Conversely, the effects on TCA cycle and glutathione metabolism genes were much less pronounced, and although a significant reduction in both GLS and GLUD1 gene expression levels were noted after imatinib treatment, this was <50% and much smaller than those observed on glycolytic genes (supplemental Figure 6A-C; supplemental Table 3). As observed in FLT3ITD cells, combining imatinib with CB839 led to increased apoptosis of BCR-ABL–positive cells, which correlated with enhanced intracellular ROS production and reduced oxygen consumption. Moreover, as with FLT3ITD AML, these effects could also be fully rescued by αKG (Figure 5D-F).
Combined effects of TKIs and CB839 in BCR-ABL–positive leukemia. (A) Glycolytic rate and capacity of BCR-ABL mutated K562 cells treated with vehicle control or imatinib 2 µM (mean ± SEM, n = 3, P < .0001 for both glycolytic rate and capacity, respectively; 2-way ANOVA with Bonferroni’s multiple comparison). (B) Volcano plot for gene expression changes by RNA sequencing of K562 cells treated with imatinib 2 µM or vehicle control, highlighting reduced expression of genes involved in glycolysis in treated cells (n = 2). (C) Quantitative PCR validation in K562 cells for the reduction in expression levels of GLUT3 (mean ± SEM, n = 3; **P = .0081; 2-tailed paired t test) and LDHA (mean ± SEM, n = 3; *P = .0132; 2-tailed paired t test) after treatment as in panel B. (D) Apoptosis in K562 cells treated with vehicle control, imatinib 2 µM, CB839 100 nM, imatinib and CB839 combination, and imatinib/CB839 combination + 4 mM αKG (mean ± SEM , n = 9; **P = .0019 between imatinib and imatinib + CB839; ****P < .0001 between imatinib + CB839 and imatinib + CB839 + αKG; ANOVA with Tukey’s multiple comparisons). (E) Relative cytoplasmic ROS levels in BCR-ABL mutated K562 cells treated as in panel D (mean ± SEM, n = 6; *P = .0495 between imatinib and imatinib + CB839; **P = .0089 between imatinib + CB839 and imatinib + CB839 + αKG; ANOVA with Sidak’s multiple comparisons). (F) Oxygen consumption rate in K562 cells treated as in panel D (mean ± SEM, n = 3; for basal respiration: P = .0606 between imatinib and imatinib + CB839; P < .0001 between imatinib + CB839 and imatinib + CB839 + αKG; for maximal respiration: ****P < .0001 between both imatinib vs imatinib + CB839 and imatinib + CB839 vs imatinib + CB839 + αKG; 2-way ANOVA with Bonferroni’s multiple comparisons). DMSO, dimethyl sulfoxide; ECAR, extracellular acidification rate.
Combined effects of TKIs and CB839 in BCR-ABL–positive leukemia. (A) Glycolytic rate and capacity of BCR-ABL mutated K562 cells treated with vehicle control or imatinib 2 µM (mean ± SEM, n = 3, P < .0001 for both glycolytic rate and capacity, respectively; 2-way ANOVA with Bonferroni’s multiple comparison). (B) Volcano plot for gene expression changes by RNA sequencing of K562 cells treated with imatinib 2 µM or vehicle control, highlighting reduced expression of genes involved in glycolysis in treated cells (n = 2). (C) Quantitative PCR validation in K562 cells for the reduction in expression levels of GLUT3 (mean ± SEM, n = 3; **P = .0081; 2-tailed paired t test) and LDHA (mean ± SEM, n = 3; *P = .0132; 2-tailed paired t test) after treatment as in panel B. (D) Apoptosis in K562 cells treated with vehicle control, imatinib 2 µM, CB839 100 nM, imatinib and CB839 combination, and imatinib/CB839 combination + 4 mM αKG (mean ± SEM , n = 9; **P = .0019 between imatinib and imatinib + CB839; ****P < .0001 between imatinib + CB839 and imatinib + CB839 + αKG; ANOVA with Tukey’s multiple comparisons). (E) Relative cytoplasmic ROS levels in BCR-ABL mutated K562 cells treated as in panel D (mean ± SEM, n = 6; *P = .0495 between imatinib and imatinib + CB839; **P = .0089 between imatinib + CB839 and imatinib + CB839 + αKG; ANOVA with Sidak’s multiple comparisons). (F) Oxygen consumption rate in K562 cells treated as in panel D (mean ± SEM, n = 3; for basal respiration: P = .0606 between imatinib and imatinib + CB839; P < .0001 between imatinib + CB839 and imatinib + CB839 + αKG; for maximal respiration: ****P < .0001 between both imatinib vs imatinib + CB839 and imatinib + CB839 vs imatinib + CB839 + αKG; 2-way ANOVA with Bonferroni’s multiple comparisons). DMSO, dimethyl sulfoxide; ECAR, extracellular acidification rate.
Finally, we sought to confirm our findings in more physiological and clinically relevant models. Using primary AML samples from patients carrying a FLT3ITD mutation, we found that AC220 treatment led to a reduction in glycolytic capacity, and combined treatment with AC220 and CB839 led to a reduction in basal oxygen consumption (Figure 6A-B). These effects correlated with a further reduction in the viability of FLT3ITD primary samples following combined treatment that appeared proportional to the levels of FLT3 mutation, as measured by variant allele frequency, in each sample, whereas similar effects were not observed in FLT3wt samples (Figure 6C; supplemental Figure 7A-B). The combined treatment also led to reduced colony-forming cell output in bone marrow murine cells and AML primary samples carrying FLT3ITD mutations, whereas similar effects were not observed in normal CD34+ samples or FLT3wt AML patient samples, suggesting that these effects are specific to FLT3ITD cells (supplemental Figure 7C-F). Finally, we tested the effects of combined GLS and FLT3 TK inhibition in vivo using FLT3ITD MV411 cells stably expressing a doxycycline inducible GLS or scrambled shRNA alongside a red fluorescent protein (RFP) reporter to allow tracking of shRNA expression. We transplanted shRNA GLS or scrambled cells into recipient immunocompromised mice and allowed leukemia to develop, at which point we fed mice with a doxycycline-containing diet and also initiated AC220 treatment by oral gavage. MV411-generated leukemias are extremely aggressive but also highly sensitive to FLT3 inhibitor treatment34 (data not shown). Using a low dose of AC220 in combination with a doxycycline-containing diet, all mice succumbed to disease while on treatment. However, despite the very aggressive nature of this leukemia, mice transplanted with cells carrying shRNA targeting GLS showed a modest but statistically significant increase in survival compared with mice transplanted with control cells (Figure 6D). We also observed that the mice transplanted with shRNA targeting GLS had lower levels of disease burden in bone marrow and spleen (as measured by percentage of RFP-positive cells within human CD45 cells) and a trend toward smaller spleen size (Figure 6E-F; supplemental Figure 7G). Finally, GLS depletion was measured in vivo from human cells isolated from mouse organs. Interestingly, in the shRNA GLS transduced cells, AC220 treatment resulted in a 50% reduction in GLS knockdown efficiency, suggesting preferential killing of cells with lower levels of GLS expression (supplemental Figure 7H).
Combined effects of AC220 and CB839 in FLT3ITDprimary samples and in vivo. (A) Extracellular acidification rate (ECAR) of primary FLT3ITD mutated AML samples treated with vehicle control or AC220 2.5 nM measured using a Seahorse analyzer (mean ± SEM, n = 4, maximal glycolytic capacity; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (B) Oxygen consumption rate (OCR) of primary FLT3ITD mutated AML samples treated with vehicle control, AC220 2.5 nM, CB839 100 nM, or their combination measured using a Seahorse analyzer. Real-time basal and maximal respiration are shown and in the inset a bar charts for the basal respiration in the 4 different conditions is shown (mean ± SEM, n = 4; **P = .0034 between AC220 and AC220 + CB839; ANOVA with Tukey’s multiple comparisons). (C) Relative viability in primary FLT3ITD mutated AML samples treated with vehicle control, AC220 2.5 nM, CB839 100 nM, or their combination. Far left panel shows a summary plot for all 5 patients (mean ± SEM, n = 5; **P = .0176 between AC220 and AC220 + CB839; ANOVA with Tukey’s multiple comparisons). The other panels show data for each individual patient (PT) with VAF (variant allele frequency) for FLT3ITD. Note in PT5, AC220 was used at 5 nM given the low variant allele frequency for FLT3ITD. (D) Survival curve of mice transplanted respectively with MV411 transduced with control scramble shRNA (n = 9) and GLS shRNA (n = 8) after treatment with AC220 (P = .0030 by log-rank test). (E-F) Percentage of RFP-positive cells, measured by flow cytometry, within 45 positive human cells from the bone marrow (E) and spleen (F) of mice transplanted respectively with MV411 transduced with control scramble shRNA (n = 9) and GLS shRNA (n = 8) after treatment with AC220 (box and whiskers showing minimum to maximum range for bone marrow [*P = .0201] and spleen [**** P < .0001]; unpaired Student t test). (G) Schematic model showing the action mechanism of combined GLS and FLT3 TK inhibition. FLT3ITD mutant cells use both glucose and glutamine to support their metabolism (left). FLT3-TKI treatment (AC220) blocks glucose uptake and mostly glycolysis, rendering the cells dependent on glutamine metabolism (middle). GLS gene silencing, chemical inhibition (CB839), or glutamine starvation enhances the efficacy of FLT3-TKI by blocking glutamine metabolism and its ability to support both TCA cycle/mitochondrial function and GSH synthesis/redox metabolism (right).
Combined effects of AC220 and CB839 in FLT3ITDprimary samples and in vivo. (A) Extracellular acidification rate (ECAR) of primary FLT3ITD mutated AML samples treated with vehicle control or AC220 2.5 nM measured using a Seahorse analyzer (mean ± SEM, n = 4, maximal glycolytic capacity; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (B) Oxygen consumption rate (OCR) of primary FLT3ITD mutated AML samples treated with vehicle control, AC220 2.5 nM, CB839 100 nM, or their combination measured using a Seahorse analyzer. Real-time basal and maximal respiration are shown and in the inset a bar charts for the basal respiration in the 4 different conditions is shown (mean ± SEM, n = 4; **P = .0034 between AC220 and AC220 + CB839; ANOVA with Tukey’s multiple comparisons). (C) Relative viability in primary FLT3ITD mutated AML samples treated with vehicle control, AC220 2.5 nM, CB839 100 nM, or their combination. Far left panel shows a summary plot for all 5 patients (mean ± SEM, n = 5; **P = .0176 between AC220 and AC220 + CB839; ANOVA with Tukey’s multiple comparisons). The other panels show data for each individual patient (PT) with VAF (variant allele frequency) for FLT3ITD. Note in PT5, AC220 was used at 5 nM given the low variant allele frequency for FLT3ITD. (D) Survival curve of mice transplanted respectively with MV411 transduced with control scramble shRNA (n = 9) and GLS shRNA (n = 8) after treatment with AC220 (P = .0030 by log-rank test). (E-F) Percentage of RFP-positive cells, measured by flow cytometry, within 45 positive human cells from the bone marrow (E) and spleen (F) of mice transplanted respectively with MV411 transduced with control scramble shRNA (n = 9) and GLS shRNA (n = 8) after treatment with AC220 (box and whiskers showing minimum to maximum range for bone marrow [*P = .0201] and spleen [**** P < .0001]; unpaired Student t test). (G) Schematic model showing the action mechanism of combined GLS and FLT3 TK inhibition. FLT3ITD mutant cells use both glucose and glutamine to support their metabolism (left). FLT3-TKI treatment (AC220) blocks glucose uptake and mostly glycolysis, rendering the cells dependent on glutamine metabolism (middle). GLS gene silencing, chemical inhibition (CB839), or glutamine starvation enhances the efficacy of FLT3-TKI by blocking glutamine metabolism and its ability to support both TCA cycle/mitochondrial function and GSH synthesis/redox metabolism (right).
Discussion
In this study, we used orthogonal unbiased approaches, including CRISPR/Cas9 synthetic lethality screen, metabolomics, and gene expression analysis, to reveal that FLT3ITD cells develop a metabolic dependency on glutamine metabolism after FLT3 TK inhibition. Targeted inhibition of FLT3 TK activity appears to suppress the enhanced central carbon metabolism typical of FLT3ITD cells by mostly hindering glucose uptake and utilization thus predominantly reversing the glycolytic phenotype. However, TCA cycle activity and respiratory function, although reduced, are less affected and are supported by continuous uptake of glutamine, the other main fuel for central carbon metabolism. Combined suppression of FLT3 TK activity and glutamine metabolism using both GLS chemical inhibition and gene silencing leads to an increased cell death in FLT3ITD cells, including models previously shown to be already highly sensitive to FLT3 TK inhibition. We also extend these findings to a model of leukemia carrying BCR-ABL TK activating mutations, primary AML samples, and in vivo models. We demonstrate that glutamine metabolism supports both the TCA cycle and redox metabolism upon FLT3 TK inhibition, and, through rescue experiments, we further validate the role of all these branches of glutamine metabolism in cellular survival (Figure 6G). Our data expand the findings of a recent report on the activity of combined GLS and FLT3 TK inhibition in FLT3ITD AML by providing in depth mechanistic explanation for these findings and extending their validity to primary AML samples and other TK activating mutated leukemias.52 Moreover, they also explain mechanistically previous published observations suggesting that combined targeting of FLT3 TK activity and redox metabolism or ETC might enhance toxicity in FLT3-mutated AML.53,54 However, it is entirely plausible that another consequence of glutamine metabolism might also underlie, at least in part, the effects of targeting it. For instance, branched chain amino acids, produced by the transamination of glutamine derived glutamate, have recently been shown to support the maintenance and progression of myeloid leukemias.27
GLS has recently emerged as a therapeutic target in both solid and hematological malignancies, and potent GLS inhibitors, including the one used in this study, have now entered clinical trials in several malignancies (NCT02071862 and NCT02071927). GLS is the most abundant isoform present in hematopoietic cells and has already been suggested as a potential therapeutic target in AML.55 However, our data show that specifically in FLT3ITD mutated AML, GLS inhibition, on its own, produces only mild antiproliferative effects and only becomes a metabolic vulnerability following FLT3 TK inhibition, with similar effects not observed in normal cells or leukemic cells that lack TK activating mutations. Our results therefore suggest a therapeutic window for this combination therapy and confirm its specificity and potential utility in several TK mutated leukemias.
Of note, the best effects in combination with FLT3 TK inhibition were observed when FLT3ITD cells were starved of glutamine rather than following GLS inhibition. This suggests that FLT3ITD mutated cells might also rely on ancillary pathways of glutamine metabolism releasing its γ-nitrogen and producing glutamate. Moreover, glutamine γ-nitrogen is a central substrate for the biosynthesis of nucleotides, NAD, amino acids, and glucosamine-6-phosphate,56 and given that this function is not targeted by GLS inhibition, it is also plausible that these glutamine-dependent metabolic pathways support cell survival after AC220 and combination treatment.
The ability of FLT3-TKI to predominantly revert the glycolytic phenotype, while having a less pronounced effect on TCA cycle activity and oxidative metabolism, is another important observation stemming from this work. With regards to this it is noteworthy that, consistent with our findings, 2 recent reports have suggested that both AML and chronic myeloid leukemia therapy–resistant cells display increased mitochondrial mass and a high oxidative phosphorylation status that is therapeutically actionable.57,58 However, the exact mechanisms whereby some metabolic phenotypes are particularly dependent on FLT3 TK activity and how the described metabolic adaptations are established remain unknown, and these fundamental questions merit further studies. Speculatively the ability of FLT3 TK to control glycolysis could be explained by its activation of AKT,17 which can modulate transcription factors, such as FOXO, known to regulate glycolysis,59 or directly control the activity of several glycolytic enzymes.21,60,61 However an improved understanding of the molecular mechanisms leading to this metabolic phenotype and whether other anaplerotic substrates, such as fatty acids, also contribute to oxidative phosphorylation in therapy resistant cells might help to clarify the basis of resistance and help target it more effectively.
In summary, our results highlight the importance of FLT3 mutations and downstream signaling in the control of leukemia cell metabolism, extend our understanding of the role of metabolic adaptations in the resistance to treatment with FLT3 and other TK inhibitors, and provide an example of a complementary unbiased approach to study the role of metabolism in leukemia and as a tool for the design of novel and specific therapeutic strategies targeting cell metabolism in AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Welcome Trust Sanger Institute facility for the MiSeq run.
P.G. is funded by the Wellcome Trust (109967/Z/15/Z), and was previously supported by the Academy of Medical Sciences and Lady Tata Memorial Trust. The B.J.P.H. laboratory is funded by the European Research Council, the Medical Research Council, Bloodwise, the Kay Kendall Leukaemia Fund, the Cambridge National Institute for Health Research Biomedical Research Centre, and core support grants to the Wellcome Trust Medical Research Council Cambridge Stem Cell Institute. C.F. and A.S.H.C. are funded by the Medical Research Council (core grant to the Cancer Unit). P.M.-P. is supported by a grant from Cancer Research UK (C56179/A21617). D.S. is a postdoctoral fellow of the Mildred-Scheel Organisation, German Cancer Aid. This research was supported by the Cambridge Institute for Medical Research Flow Cytometry Core Facility.
Authorship
Contribution: P.G., C.F., and B.J.P.H. conceived the study; P.G., G.G., K.T., A.S.H.C., G.V., C.F., and B.J.P.H. designed and/or conducted experiments, performed data analysis and interpretation, and informed study direction; P.M.-P., F.B., L.M., L.D.L., H.Y., D.S., S.J.H. helped with experimental work; S.V. and J.M.L.D. performed bioinformatics analyses; P.G., C.F., and B.J.P.H. drafted the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian J. P. Huntly, Cambridge Institute for Medical Research, Cambridge Biomedical Campus, Hills Rd, CB2 0XY Cambridge, United Kingdom; e-mail: bjph2@cam.ac.uk; and Paolo Gallipoli, Cambridge Institute for Medical Research, Cambridge Biomedical Campus, Hills Rd, CB2 0XY Cambridge, United Kingdom; e-mail: pg413@cam.ac.uk.
References
Author notes
G.G., K.T., and A.S.H.C. contributed equally to this study.
C.F. and B.J.P.H. contributed equally to this study.


![GLS gene deletion and chemical inhibition are synthetically lethal with FLT3 TKIs. (A) Schematic of the genome-wide CRISPR/Cas9 synthetic lethality screen in the FLT3ITD mutant cell line MOLM13. (B) KEGG pathways enrichment analysis of dropout genes from CRISPR/Cas9 screen sorted by combined score calculated using Enrichr software.35,36 Pathways relevant to AML biology are highlighted while the remaining are grayed out. (C) List of top 10 genes from the “metabolic pathways” gene list sorted according to average fold depletion from gRNAs. FDR, false discovery rate. (D) Bar graph depicting individual fold depletion for each gRNA targeting GLS. (E-G) Growth inhibition curves to AC220 of MOLM13 (E) and murine bone marrow cells expressing MLL/AF9-FLT3ITD (F) and MLL/AF9 (G) transduced respectively with “empty” gRNA control or 2 different gRNA targeting GLS (mean ± standard error of the mean [SEM], n = 3, P < .001 for treatment effect comparing control and both Gls knockout for panels E-F; ns, not significant for panel G; 2-way analysis of variance [ANOVA]). (H-I) Apoptosis in the FLT3ITD mutant cell lines MV411 (H) and MOLM13 (I) transduced with control scramble shRNA and GLS shRNA following treatment with AC220 0.5 nM for 48 hours (for MV411: mean ± SEM, n = 7, ****P < .0001, **P = .0086; for MOLM13: mean ± SEM, n = 3, **P = .0014; for AC220 treatments [comparison between scramble and GLS shRNA]: **P = .0050; for dimethyl sulfoxide vs AC220 comparison in the scramble shRNA; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Apoptosis in MV411 (J) and MOLM13 (K) following treatment with AC220 1 nM, CB839 100 nM or their combination (for MV411: mean ± SEM, n = 14, **P = .0033, ****P < .0001; for MOLM13: mean ± SEM, n = 16, *P = .0126, ***P = .0003; ANOVA with Tukey’s multiple comparisons). (L-M) Apoptosis in MV411 (L) and MOLM13 (M) grown in the presence (full media) or absence of glutamine following treatment with AC220 1 nM for 48 hours (for MV411 mean ± SEM, n = 24, for MOLM13 mean ± SEM, n = 11; ****P < .0001, ***P = .0007; 2-way ANOVA with Bonferroni’s multiple comparisons).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f1-1.jpeg?Expires=1769143714&Signature=huy6ngMowz5KQ2vV1r3evr9bR7agxC18edNEvaYaVFaRYbHRGbaSQyXUNB6SUOcsckdZE00YSki2nwJc06IhBjPQ6zpJBBqbmzNs3bzYSGE-HT8FbHuA0bs23Anl~9bJYL5NNmFNRF4OWz4MYtgwwHdAj7stVbTizpkxo0GU5g6fWxgbnDMoYLk38AxNYzNlO4XXbzKl3USGLKyVfFd2gJjoDUnqaEWoOMuPQrTHp6-8-8PueZVyKSeNMcehApG3ULR9TlOVBhkTo9qW5Mh~xe1Mahg6ao7pzbjgp5Zrg-KfInCApYjXTcM2AZyFwdcDVlBlFfDyErhMVoJREdfTJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![GLS gene deletion and chemical inhibition are synthetically lethal with FLT3 TKIs. (A) Schematic of the genome-wide CRISPR/Cas9 synthetic lethality screen in the FLT3ITD mutant cell line MOLM13. (B) KEGG pathways enrichment analysis of dropout genes from CRISPR/Cas9 screen sorted by combined score calculated using Enrichr software.35,36 Pathways relevant to AML biology are highlighted while the remaining are grayed out. (C) List of top 10 genes from the “metabolic pathways” gene list sorted according to average fold depletion from gRNAs. FDR, false discovery rate. (D) Bar graph depicting individual fold depletion for each gRNA targeting GLS. (E-G) Growth inhibition curves to AC220 of MOLM13 (E) and murine bone marrow cells expressing MLL/AF9-FLT3ITD (F) and MLL/AF9 (G) transduced respectively with “empty” gRNA control or 2 different gRNA targeting GLS (mean ± standard error of the mean [SEM], n = 3, P < .001 for treatment effect comparing control and both Gls knockout for panels E-F; ns, not significant for panel G; 2-way analysis of variance [ANOVA]). (H-I) Apoptosis in the FLT3ITD mutant cell lines MV411 (H) and MOLM13 (I) transduced with control scramble shRNA and GLS shRNA following treatment with AC220 0.5 nM for 48 hours (for MV411: mean ± SEM, n = 7, ****P < .0001, **P = .0086; for MOLM13: mean ± SEM, n = 3, **P = .0014; for AC220 treatments [comparison between scramble and GLS shRNA]: **P = .0050; for dimethyl sulfoxide vs AC220 comparison in the scramble shRNA; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Apoptosis in MV411 (J) and MOLM13 (K) following treatment with AC220 1 nM, CB839 100 nM or their combination (for MV411: mean ± SEM, n = 14, **P = .0033, ****P < .0001; for MOLM13: mean ± SEM, n = 16, *P = .0126, ***P = .0003; ANOVA with Tukey’s multiple comparisons). (L-M) Apoptosis in MV411 (L) and MOLM13 (M) grown in the presence (full media) or absence of glutamine following treatment with AC220 1 nM for 48 hours (for MV411 mean ± SEM, n = 24, for MOLM13 mean ± SEM, n = 11; ****P < .0001, ***P = .0007; 2-way ANOVA with Bonferroni’s multiple comparisons).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f1-2.jpeg?Expires=1769143714&Signature=FoMK~e4-KvrJiTqakiigOTZvM1Qnz9inR6qigGMaRXKfb4B9VokNS~bJLlA9iJWw-XoYb3zA9DnO1QgW4NFtFwvphjur9FXdcytfVKt6fjkmxyplL5VieotO-uI0PnJQkH630zv4Yd6SwTtNek-OKjkd6-GsQePTWFFTCHTrW3hMxjxq50sKdLFikpugSKNWnZf9bG44Y4EvMWhpJG1JEQd89wQa6WpfI2SSdKqTAhEMzBqRG3-hXFkV7kxNOX1vZtVO6ZUdFjjncIgwr9wAuKb9Q-KENTcpQA4aOaVQjBjDYw1O13-j4l8V-LgjBo8MDPLZAuOSHe0VpkKRX0dU2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![FLT3 TK inhibition reduces glucose uptake and central carbon metabolism without affecting glutamine uptake. (A-D) Time-course analysis of glucose and glutamine uptake from media by MV411 (A-B) and MOLM13 (C-D) cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 3; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (E) Total and isotopologue abundance of selected glycolytic intermediates and products measured by LC-MS analysis in MV411 cell extracts treated with AC220 1 nM or vehicle control and grown in media containing uniformly labeled 13C [U-13C6] glucose (GLC) (mean ± SEM, n = 5; ***P = .0004 for total 3-phosphoglycerate and P = .0007 for total lactate; 2-tailed paired t test). (F-G) Extracellular acidification rate (ECAR) of MV411 (F) and MOLM13 (G) cells treated with AC220 1nM or vehicle control (mean ± SEM, n = 3, ****P < .0001, ***P = .0003, 2-way ANOVA with Bonferroni’s multiple comparisons). (H) Total and isotopologue levels of selected TCA cycle intermediates in MV411 cells treated as in panel E (mean ± SEM, n = 5; *** P = .0009,**P = .0011; ns, not significant; 2-tailed, paired Student t test). A.U., arbitrary units. (I) Percentage of total levels of citrate and aspartate provided respectively by the 13C2 and 13C3 fraction following AC220 treatment as in panel E (mean ± SEM, n = 5; ****P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Volcano plot for gene expression changes by RNA sequencing (n = 2 for each cell line) of MV411 and MOLM13 cells treated with AC220 1 nM compared with vehicle control, highlighting reduced expression of glycolysis genes (top) and the minimal effects on TCA cycle genes (bottom). (L-M) Quantitative PCR validation in MV411 (L) and MOLM13 (M) for the reduced expression of lactate dehydrogenase (LDHA) and glucose transporter (GLUT3) after treatment with AC220 1 nM (top) (mean ± SEM, MV411 n = 4, MOLM13 n = 5; for LDHA: **P = .0053, *** P = .0005; for GLUT3, MV411: *P = .0150; MOLM13: *P = .0387; 2-tailed, paired Student t test) and for the lack of changes in expression in glutamine metabolism genes (GLS) and glutamate dehydrogenase (GLUD1) (bottom) (mean ± SEM, n = 4; ns, not significant by 2-tailed, paired Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f2-1.jpeg?Expires=1769143714&Signature=u5FvFkEAZTWus3OH77OvuZ2a0Cy1mj7IFwF1Zf9EraBRrcLmvQJQNx7y9fUxYx6-38XbwVJcsYGJZax5tz~P3DK1mscoHE8i37m1e5KbqRUNNVgBy7VdohuWdnVh-vPj732ekJAptYzekg2adjtxaSeucTzBa5r9-uEfkWMtx~8PZEJlm1lkXThaoZ6kQ2AtQYeEYBRNmsPF2EEckRd71tzRCyzFt1am8TXM~rlVhxdD0CtRB68pnUrhxSpJRfLsGu1YlCdpfYiL~yUySo5rDHQVMDOB-ZkQZPiwYmmtoVylm4lxsKWESp5PoUOWXqeC9i2J4m3Gcf8PvnO9H6gJSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![FLT3 TK inhibition reduces glucose uptake and central carbon metabolism without affecting glutamine uptake. (A-D) Time-course analysis of glucose and glutamine uptake from media by MV411 (A-B) and MOLM13 (C-D) cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 3; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (E) Total and isotopologue abundance of selected glycolytic intermediates and products measured by LC-MS analysis in MV411 cell extracts treated with AC220 1 nM or vehicle control and grown in media containing uniformly labeled 13C [U-13C6] glucose (GLC) (mean ± SEM, n = 5; ***P = .0004 for total 3-phosphoglycerate and P = .0007 for total lactate; 2-tailed paired t test). (F-G) Extracellular acidification rate (ECAR) of MV411 (F) and MOLM13 (G) cells treated with AC220 1nM or vehicle control (mean ± SEM, n = 3, ****P < .0001, ***P = .0003, 2-way ANOVA with Bonferroni’s multiple comparisons). (H) Total and isotopologue levels of selected TCA cycle intermediates in MV411 cells treated as in panel E (mean ± SEM, n = 5; *** P = .0009,**P = .0011; ns, not significant; 2-tailed, paired Student t test). A.U., arbitrary units. (I) Percentage of total levels of citrate and aspartate provided respectively by the 13C2 and 13C3 fraction following AC220 treatment as in panel E (mean ± SEM, n = 5; ****P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons). (J-K) Volcano plot for gene expression changes by RNA sequencing (n = 2 for each cell line) of MV411 and MOLM13 cells treated with AC220 1 nM compared with vehicle control, highlighting reduced expression of glycolysis genes (top) and the minimal effects on TCA cycle genes (bottom). (L-M) Quantitative PCR validation in MV411 (L) and MOLM13 (M) for the reduced expression of lactate dehydrogenase (LDHA) and glucose transporter (GLUT3) after treatment with AC220 1 nM (top) (mean ± SEM, MV411 n = 4, MOLM13 n = 5; for LDHA: **P = .0053, *** P = .0005; for GLUT3, MV411: *P = .0150; MOLM13: *P = .0387; 2-tailed, paired Student t test) and for the lack of changes in expression in glutamine metabolism genes (GLS) and glutamate dehydrogenase (GLUD1) (bottom) (mean ± SEM, n = 4; ns, not significant by 2-tailed, paired Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f2-2.jpeg?Expires=1769143714&Signature=GC2yc6YWOW0H9mQO4QVbWBNtj6Zl3JpApOuEtTz4rdiCRSOXmlDvET746P1br4Q9wc1TLg-elznKFOxuzz3hMEg6MQWAfIB8FISx8hIeZTB3df6wHuVFCP45NC7J~DaPzpHFZpgFO2n4EVOPbJA4WIY-ZVjc697XNXQdbTEyvHtewk9LsdVkC9ayb9Xr5DqTDySWSFKEZuIC1H-RgEq-weSEbT3J8oOJnh5XKu~tWSjNAt~cCYa0~EXKj8X0pVa4EMyJvDB3oHnU7E7ngFdGEPLwR9OuYLYB35DIOg0S-8gj8rsEqrZZ1mDhPB3CVEXOLQ0qkmLYgt4hchWuzDldAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Glutamine supports both mitochondrial function and glutathione production following FLT3 TK inhibition. (A) Total and isotopologue levels of TCA cycle intermediates, glutamate and reduced glutathione (GSH), measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control and grown in media containing U-13C5 and 15nitrogen [15N2] glutamine (GLN) (mean ± SEM, n = 5; ***P = .0005, **P = .0017, *P = .0195 for citrate, P = .0228 for malate, P = .0216 for glutamate; ns, not significant; 2-tailed, paired Student t test). A schematic representation of glutamine metabolism and labeling pattern of metabolites is also provided. (B) Percentage of total levels of TCA cycle metabolites labeled by U-13C5,15N2-GLN through its oxidative metabolism measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 5; ****P < .0001, ***P = .0008, **P = .0067; 2-way ANOVA with Bonferroni’s multiple comparisons). (C) Relative mitochondrial membrane potential of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right) (mean ± SEM, n = 3; * P = .0145; 2-tailed, paired Student t test). (D) Relative mitochondrial mass of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right panel) (mean ± SEM, n = 3; ns, not significant; P = .13; 2-tailed paired t test). (E) Relative GSH/GSSG ratio, GSH, and GSSG of MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 6; * P = .0173; ns, not significant; 2-tailed, paired Student t test). (F) Percentage of total levels of glutamine, glutamate, GSH, and GSSG labeled by U-13C5,15N2-GLN measured by LC-MS analysis in MV411 cells treated as in panel A (mean ± SEM, n = 5; **** P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons. (G) Relative GSH levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 5; **P = .0017, *P = .0471, q = 4.248; ANOVA with Tukey’s multiple comparisons). (H) Relative cytoplasmic ROS levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 14; ****P < .0001; ANOVA with Tukey’s multiple comparisons).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f3-1.jpeg?Expires=1769143714&Signature=AD2RfCuBOsHQlhkIn1aV0luKkhjhocuW9UfGZmXkw~E-iMehDT3GrqLRAbdA2vcX6gaU01PNCpMh6YoequhGGprWaOEmQRnFgJljfWV5NXbqHiJ4Mekm0ST3m3PnMHBaV74hu74OQMEXCO-KAgnWHXxSIlf4p1YPzHj-opMiv0HSI9lQTKlyqzV4ZURIUj8OmWNwjeGkHXcXZQXVsIKCLGQZN74O0izv8pT2nEPSjfwYD-uJhra6u-dSAzFpn4GP45XGtxlRR8GpdR3rY4CuqvqI0vkBEo4z6aTeJfMMeF5QoF5viJB27X8yxoyj1VnfiTbhtnzGX2eGBl3crtFSiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Glutamine supports both mitochondrial function and glutathione production following FLT3 TK inhibition. (A) Total and isotopologue levels of TCA cycle intermediates, glutamate and reduced glutathione (GSH), measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control and grown in media containing U-13C5 and 15nitrogen [15N2] glutamine (GLN) (mean ± SEM, n = 5; ***P = .0005, **P = .0017, *P = .0195 for citrate, P = .0228 for malate, P = .0216 for glutamate; ns, not significant; 2-tailed, paired Student t test). A schematic representation of glutamine metabolism and labeling pattern of metabolites is also provided. (B) Percentage of total levels of TCA cycle metabolites labeled by U-13C5,15N2-GLN through its oxidative metabolism measured by LC-MS analysis in MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 5; ****P < .0001, ***P = .0008, **P = .0067; 2-way ANOVA with Bonferroni’s multiple comparisons). (C) Relative mitochondrial membrane potential of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right) (mean ± SEM, n = 3; * P = .0145; 2-tailed, paired Student t test). (D) Relative mitochondrial mass of MV411 cells treated with AC220 1 nM or vehicle control (left) with representative flow cytometry histogram (right panel) (mean ± SEM, n = 3; ns, not significant; P = .13; 2-tailed paired t test). (E) Relative GSH/GSSG ratio, GSH, and GSSG of MV411 cells treated with AC220 1 nM or vehicle control (mean ± SEM, n = 6; * P = .0173; ns, not significant; 2-tailed, paired Student t test). (F) Percentage of total levels of glutamine, glutamate, GSH, and GSSG labeled by U-13C5,15N2-GLN measured by LC-MS analysis in MV411 cells treated as in panel A (mean ± SEM, n = 5; **** P < .0001; ns, not significant; 2-way ANOVA with Bonferroni’s multiple comparisons. (G) Relative GSH levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 5; **P = .0017, *P = .0471, q = 4.248; ANOVA with Tukey’s multiple comparisons). (H) Relative cytoplasmic ROS levels of MV411 cells treated with AC220 1 nM or vehicle control in the presence or absence of glutamine (mean ± SEM, n = 14; ****P < .0001; ANOVA with Tukey’s multiple comparisons).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f3-2.jpeg?Expires=1769143714&Signature=E1GMABSiN3ltHkLpvQmBvzcnECG8zwPT3cu3Xh4UvzSYn5NtFNw3Jmma-EBjO5bFAVtRnQ5FLyMf2QoxKflIdsp0etvc4YI~L4Jz63sOGgUMJI8YqhW8WqMYKZKBLngDieCruHIIs5BLap9hv5BdmNm1GakFKF~ir8h6NcyND-HMN20Ork3cqtEnTXQ~ARGw3j5h8sVlGSbBmFEuhgXPODilvZuCaIsckpVjqxEoemC5igozUqmuLL0G2Yj2UREUN1ALe~SqFzRvrYjOIBrbEnyEAchZpJUJtrL-clUY9NRL7l6qiQb5HpTph0XPegw-vYgjptl78iGStQUB-~aLHA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Combined effects of AC220 and CB839 in FLT3ITD primary samples and in vivo. (A) Extracellular acidification rate (ECAR) of primary FLT3ITD mutated AML samples treated with vehicle control or AC220 2.5 nM measured using a Seahorse analyzer (mean ± SEM, n = 4, maximal glycolytic capacity; ****P < .0001; 2-way ANOVA with Bonferroni’s multiple comparisons). (B) Oxygen consumption rate (OCR) of primary FLT3ITD mutated AML samples treated with vehicle control, AC220 2.5 nM, CB839 100 nM, or their combination measured using a Seahorse analyzer. Real-time basal and maximal respiration are shown and in the inset a bar charts for the basal respiration in the 4 different conditions is shown (mean ± SEM, n = 4; **P = .0034 between AC220 and AC220 + CB839; ANOVA with Tukey’s multiple comparisons). (C) Relative viability in primary FLT3ITD mutated AML samples treated with vehicle control, AC220 2.5 nM, CB839 100 nM, or their combination. Far left panel shows a summary plot for all 5 patients (mean ± SEM, n = 5; **P = .0176 between AC220 and AC220 + CB839; ANOVA with Tukey’s multiple comparisons). The other panels show data for each individual patient (PT) with VAF (variant allele frequency) for FLT3ITD. Note in PT5, AC220 was used at 5 nM given the low variant allele frequency for FLT3ITD. (D) Survival curve of mice transplanted respectively with MV411 transduced with control scramble shRNA (n = 9) and GLS shRNA (n = 8) after treatment with AC220 (P = .0030 by log-rank test). (E-F) Percentage of RFP-positive cells, measured by flow cytometry, within 45 positive human cells from the bone marrow (E) and spleen (F) of mice transplanted respectively with MV411 transduced with control scramble shRNA (n = 9) and GLS shRNA (n = 8) after treatment with AC220 (box and whiskers showing minimum to maximum range for bone marrow [*P = .0201] and spleen [**** P < .0001]; unpaired Student t test). (G) Schematic model showing the action mechanism of combined GLS and FLT3 TK inhibition. FLT3ITD mutant cells use both glucose and glutamine to support their metabolism (left). FLT3-TKI treatment (AC220) blocks glucose uptake and mostly glycolysis, rendering the cells dependent on glutamine metabolism (middle). GLS gene silencing, chemical inhibition (CB839), or glutamine starvation enhances the efficacy of FLT3-TKI by blocking glutamine metabolism and its ability to support both TCA cycle/mitochondrial function and GSH synthesis/redox metabolism (right).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/15/10.1182_blood-2017-12-820035/4/m_blood820035f6.jpeg?Expires=1769143714&Signature=Fsa8KnUlOHNKbEWAAxK8ItfPCYkOFYPgXui7JEktIRwVunF9yL9BZ~iry3OwdoVTIKI9DeAwLne7WM0EFkUiePKqUWjigzRlpgmj14zC9g3wPo5D-H7qOwn1i0Gw9WsWJkIETR8amuWakCZx441g-u9dhSxZXnWflYhsDnrT4wrqovpMqqCYFBXcrguxgkzlEo-O53Tkp6urfNBDO~CJpSrz8iXS20gxiWdP6OOsh1EeRVoIC5vXoJIRNZtN1GDoPJ-5z6i2dz3C~XLPbSWQnALON1zGHNnnjz-C7y-o1zsgq69P-yvwPs6NvwlrcQiI3QLpfhBkYNKPJdkf3CJJ4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal