Key Points
CC-122 is a novel agent for DLBCL with antitumor and immunomodulatory activity.
CC-122 binds CRBN and degrades Aiolos and Ikaros resulting in a mimicry of IFN signaling and apoptosis in DLBCL.
Abstract
Cereblon (CRBN), a substrate receptor of the Cullin 4 RING E3 ubiquitin ligase complex, is the target of the immunomodulatory drugs lenalidomide and pomalidomide. Recently, it was demonstrated that binding of these drugs to CRBN promotes the ubiquitination and subsequent degradation of 2 common substrates, transcription factors Aiolos and Ikaros. Here we report that CC-122, a new chemical entity termed pleiotropic pathway modifier, binds CRBN and promotes degradation of Aiolos and Ikaros in diffuse large B-cell lymphoma (DLBCL) and T cells in vitro, in vivo, and in patients, resulting in both cell autonomous as well as immunostimulatory effects. In DLBCL cell lines, CC-122-induced degradation or short hairpin RNA–mediated knockdown of Aiolos and Ikaros correlates with increased transcription of interferon (IFN)–stimulated genes independent of IFN-α, -β, and -γ production and/or secretion and results in apoptosis in both activated B-cell (ABC) and germinal center B-cell DLBCL cell lines. Our results provide mechanistic insight into the cell-of-origin independent antilymphoma activity of CC-122, in contrast to the ABC subtype selective activity of lenalidomide.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for ∼40% of all non-Hodgkin lymphoma and exhibits distinct epidemiological, genetic, and clinical features.1,2 Although the addition of anti-CD20 antibodies to standard chemotherapy has improved the 5-year overall survival to 58%, a significant number of patients will relapse.3 To date, the outcomes for these relapsed and/or refractory (R/R) and high-risk patients remain particularly poor. Gene expression profiling has identified at least 2 large subgroups within DLBCL based on cell of origin (COO): a germinal center B-cell (GCB) subtype and an activated B-cell (ABC) subtype.4,5 Each subtype exhibits different pathogenic mechanisms and has different clinical outcomes, with ABC DLBCL having an inferior prognosis to chemotherapy-containing regimens in both frontline and salvage settings.4-7 Given the increased understanding of DLBCL disease biology, novel targeted agents including immunomodulatory drugs are being developed to treat high-risk and R/R DLBCL patients.8
Recent studies on the mechanism of the immunomodulatory agents, including lenalidomide and pomalidomide, reveal a new paradigm of drug action on protein homeostatic mechanisms. Crystallographic studies show that these drugs bind to a tritryptophan pocket on the surface of cereblon (CRBN), a substrate receptor protein of the Cullin 4 RING E3 ubiquitin ligase complex (CRL4CRBN).9,10 Binding of immunomodulatory drugs (IMiD) compounds to CRBN results in recruitment of 2 critical transcription factors for lymphogenesis, Aiolos and Ikaros, to CRBN leading to their ubiquitination and subsequent proteasomal degradation. In multiple myeloma cell lines, the degradation of Aiolos or Ikaros by either lenalidomide treatment or by short hairpin RNA (shRNA)–mediated knockdown reduced interferon regulatory factor 4 (IRF4) transcription and protein levels, resulting in decreased proliferative capacity.11,12 Additionally, the costimulatory effects of IMiD compounds on T-cell activation were determined to be through degradation of Aiolos and Ikaros, negative regulators of interleukin-2 (IL-2) expression.12,13 The role of Aiolos and Ikaros in DLBCL has not been defined, nor have the consequences of targeting these transcription factors in DLBCL. A novel pleiotropic pathway modifier compound originally developed for broad DLBCL activity, CC-122, has provided unique insight into this area of DLBCL biology. The chemical structure of CC-122 includes the glutarimide moiety that is known to interact with CRBN as recently described by Chamberlain et al.9 CC-122 is currently in phase 1 trials and has demonstrated single-agent clinical activity in DLBCL.14,15
Here, we report that CC-122 inhibits proliferation and induces apoptosis in a broad panel of DLBCL cell lines, reduces tumor growth in xenograft models established from ABC- and GCB-DLBCL cell lines, and stimulates IL-2 production in primary T cells. These activities are dependent on the binding of CC-122 to CRBN and subsequent ubiquitination and proteasomal degradation of Aiolos and Ikaros, resulting in direct derepression of interferon (IFN)–stimulated gene (ISG) transcription and induction of IFN-inducible proteins, ultimately leading to apoptosis. Mechanistically, we demonstrate for the first time by using inducible gene silencing that Aiolos negatively regulates transcription of ISGs in both ABC- and GCB-DLBCL cell lines. Furthermore, our findings were confirmed in a clinical setting, where administration of CC-122 to R/R-DLBCL patients resulted in decreased levels of Aiolos and Ikaros and increased IRF7 staining in lymph node biopsies and increased T-cell costimulation. Finally, our results provide a mechanistic rationale for the future clinical development of CC-122 in DLBCL and define a pathway that is not targeted by current therapeutic agents.
Materials and methods
See supplemental Materials and Methods (available on the Blood Web site) for details.
Cell culture, shRNA lentivirus production, generation of stable cell lines
Results
CC-122 inhibits proliferation and induces apoptosis in ABC and GCB DLBCL
To explore the antiproliferative activity of CC-122 in DLBCL cell lines, thymidine incorporation assays were performed in a panel of DLBCL cell lines after 5 days of treatment with CC-122. Exposure of 4 ABC-DLBCL lines (TMD8, U2932, Riva, and OCI-LY10) and 5 GCB-DLBCL lines (Karpas 422, WSU-DLCL2, SUDHL-4, OCI-LY19, and Pfeiffer) cell lines with 0.01 to 10 000 nM CC-122 for 5 days led to a marked decrease in proliferation (Figure 1A). The ABC cell lines were more sensitive than the GCB cell lines (ABC 50% inhibition concentration range, 8 nM to 6 μM; GCB 50% inhibition concentration range, 1 μM to >10 μM).
CC-122 induces growth arrest and apoptosis in ABC- and GCB-DLBCL cell lines. (A) Multiple DLBCL cell lines were treated with dimethylsulfoxide (DMSO) and CC-122 (0.01-10 000 nM) for 5 days. Proliferation for all cell lines was determined using the 3H-thymidine incorporation method. Results of 3 independent experiments are shown (mean ± standard error of the mean [SEM]). (B) DLBCL cell lines were treated with DMSO, and CC-122 (0.1-10 μM) for 7 days, after which apoptosis was measured by Annexin V and To-Pro 3 flow cytometric analysis. Graphical representation of 2 independent experiments (mean ± SEM).
CC-122 induces growth arrest and apoptosis in ABC- and GCB-DLBCL cell lines. (A) Multiple DLBCL cell lines were treated with dimethylsulfoxide (DMSO) and CC-122 (0.01-10 000 nM) for 5 days. Proliferation for all cell lines was determined using the 3H-thymidine incorporation method. Results of 3 independent experiments are shown (mean ± standard error of the mean [SEM]). (B) DLBCL cell lines were treated with DMSO, and CC-122 (0.1-10 μM) for 7 days, after which apoptosis was measured by Annexin V and To-Pro 3 flow cytometric analysis. Graphical representation of 2 independent experiments (mean ± SEM).
We next explored if this decreased proliferation eventually led to apoptosis in ABC- and GCB-DLBCL cell lines. Treatment of 0.1 to 10 μM CC-122 for 7 days resulted in a dose-dependent induction of apoptosis in GCB (WSU-DLCL2 and Karpas 422) and ABC cell lines (TMD8 and OCI-LY10) between 4.5- and 6.5-fold compared with DMSO, as measured by Annexin V and To-Pro 3 flow cytometry (Figure 1B, left: flow cytometry scatter plot; Figure 1B, right: graphical representation of flow cytometry data). Importantly, apoptosis in these cell lines was observed at clinically relevant concentrations of 0.1 to 1 μM CC-122.15
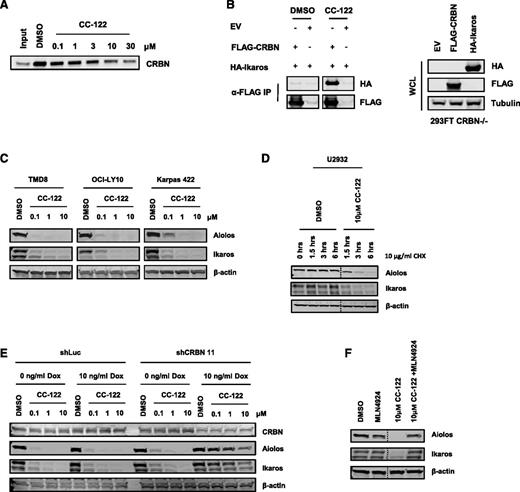
CC-122 promotes CRBN-Ikaros interaction and subsequent proteasomal degradation of Aiolos and Ikaros in vitro
The thalidomide binding domain of CRBN was recently shown to contain a hydrophobic pocket in which 3 tryptophan residues govern the binding with the glutarimide moiety within thalidomide, lenalidomide, and pomalidomide.9 As the chemical structure of CC-122 contains a glutarimide ring, we explored if CC-122 binds CRBN. As shown in Figure 2A, CRBN from U266 multiple myeloma cell extracts interacted with FG affinity beads coupled to an immobilized thalidomide analog (DMSO control lane). Furthermore, incubation of the complex with increasing concentration of free CC-122 resulted in the displacement of CRBN from the thalidomide analog–immobilized beads, consistent with CC-122 competing with thalidomide for binding CRBN. Additionally, fluorescence quenching studies on a purified C terminus fragment of CRBN (amino acids 321-440) confirmed direct binding of CC-122 with CRBN (supplemental Figure 1).
Aiolos and Ikaros are CRL4CRBN-dependent substrates of CC-122. (A) U266 cell lysates incubated with thalidomide analog bound FG affinity beads were treated with either DMSO or multiple concentrations of CC-122. Two hours later, complexes were washed, and bound protein was detected by immunoblot analysis for CRBN. (B) 293FT CRBN−/− cells were transiently transfected with pcDNA3.1, pcDNA3.1-FLAG-CRBN, or pcDNA3.1 HA-IKZF1, respectively (left panel). Whole cell extracts were then mixed with FLAG M2 beads (Sigma) for 24 hours at 4°C. Where indicated, CC-122 (20 μM) or DMSO were added into the binding reaction 24 hours before the complexes were washed, and bound protein was detected by immunoblot analysis. Cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and levels of hemagglutinin (HA), FLAG, and tubulin were assessed (right panel). (C) DLBCL cells were treated with DMSO or CC-122 (0.1-10 μM) for 12 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed by immunoblot analysis and quantification. (D) U2932 cells were pulsed with 10 μg/mL cycloheximide (CHX), then treated with DMSO or CC-122 (10 μM) for indicated time points. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (E) OCI-LY10 cells expressing shRNA targeting luciferase or CRBN were treated with either 0 or 10 ng/mL Dox for 48 hours. After 48 hours, cells were treated with DMSO or indicated doses of CC-122 for an additional 12 hours. Cell lysates were separated by SDS-PAGE, and levels of CRBN, Aiolos, and β-actin were assessed. (F) TMD8 cells were pretreated with MLN4924 (10 μM) for 1 hour, then treated with DMSO or CC-122 (10 μM) for 6 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed as before.
Aiolos and Ikaros are CRL4CRBN-dependent substrates of CC-122. (A) U266 cell lysates incubated with thalidomide analog bound FG affinity beads were treated with either DMSO or multiple concentrations of CC-122. Two hours later, complexes were washed, and bound protein was detected by immunoblot analysis for CRBN. (B) 293FT CRBN−/− cells were transiently transfected with pcDNA3.1, pcDNA3.1-FLAG-CRBN, or pcDNA3.1 HA-IKZF1, respectively (left panel). Whole cell extracts were then mixed with FLAG M2 beads (Sigma) for 24 hours at 4°C. Where indicated, CC-122 (20 μM) or DMSO were added into the binding reaction 24 hours before the complexes were washed, and bound protein was detected by immunoblot analysis. Cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and levels of hemagglutinin (HA), FLAG, and tubulin were assessed (right panel). (C) DLBCL cells were treated with DMSO or CC-122 (0.1-10 μM) for 12 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed by immunoblot analysis and quantification. (D) U2932 cells were pulsed with 10 μg/mL cycloheximide (CHX), then treated with DMSO or CC-122 (10 μM) for indicated time points. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (E) OCI-LY10 cells expressing shRNA targeting luciferase or CRBN were treated with either 0 or 10 ng/mL Dox for 48 hours. After 48 hours, cells were treated with DMSO or indicated doses of CC-122 for an additional 12 hours. Cell lysates were separated by SDS-PAGE, and levels of CRBN, Aiolos, and β-actin were assessed. (F) TMD8 cells were pretreated with MLN4924 (10 μM) for 1 hour, then treated with DMSO or CC-122 (10 μM) for 6 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed as before.
Immunomodulatory drugs in multiple myeloma cells have been shown to recruit Aiolos and Ikaros and induce their ubiquitination by the CRL4CRBN E3 ubiquitin ligase complex and subsequent proteasomal degradation.11,12 In order to assess the ability of CC-122 to recruit Ikaros to the CRL4CRBN complex, we generated HEK293FT CRBN −/− utilizing the CRISPR gene editing system. Cell lysates from HEK293FT CRBN−/− cells that were transfected with pcDNA3.1 empty vector, pcDNA3.1-FLAG-CRBN, or pcDNA3.1-HA-IKZF1 were immunoprecipitated with anti-FLAG antibody in the presence of either DMSO or 20 µM CC-122. As shown in Figure 2B, CC-122 promoted the association of Ikaros to CRBN, whereas DMSO did not. We next assessed whether the increased association of Ikaros to CRBN upon addition of CC-122 resulted in degradation of Aiolos and Ikaros in cells. In 3 DLBCL cell lines (TMD8, OCI-LY10, and Karpas 422) treated with 0.1, 1, or 10 μM CC-122 for 12 hours, there was decreased protein abundance of Aiolos and Ikaros in a concentration-dependent manner (Figure 2C). In contrast, gene expression analysis of Ikaros or Aiolos demonstrated no change in the messenger RNA (mRNA) levels (data not shown), suggesting the effect of CC-122 on Aiolos and Ikaros was primarily at the protein level and not secondary to reduced transcription. To demonstrate that the decreased protein degradation occurred independent of protein synthesis, we assessed the effects of CC-122 on Aiolos and Ikaros protein half-life by treating U2932 cells with cycloheximide to inhibit production of nascent polypeptides, followed by treatment with DMSO or 10 μM CC-122 for 1.5, 3, and 6 hours. Aiolos and Ikaros protein levels were stable in the DMSO-treated cells, indicating a normal half-life >6 hours, whereas a 36% to 72% decrease for Aiolos and a 39% to 73% decrease in Ikaros protein abundance was observed at 1.5 and 3 hours after CC-122 treatment, indicating a significantly shorter protein half-life in the presence of the drug (Figure 2D). These data underscore that degradation of Aiolos and Ikaros occurs at the protein level and does not involve changes in transcription or translation.
To test the functional requirement of CRBN for CC-122-mediated degradation of Aiolos and Ikaros, we generated stably transduced OCI-LY10 cells expressing a doxycycline (Dox)–inducible shRNA targeting CRBN or luciferase mRNA, respectively. Expression of the CRBN shRNA resulted in a considerable decrease in CRBN protein levels, with a concomitant reduction in CC-122-dependent degradation of Aiolos and Ikaros (Figure 2E). In contrast, the control luciferase shRNA affected neither CRBN levels nor degradation of the 2 substrates. We next investigated the dependence of CC-122-mediated degradation of Aiolos and Ikaros on the enzymatic activity of the E3 ligase complex. For this, we pretreated a separate ABC-DLBCL cell line, TMD8, for 1 hour with MLN4924, a small-molecule inhibitor of Cullin RING E3 ligase activity (NEDD8 activating enzyme inhibitor), prior to a 6-hour exposure of CC-122. Inhibition of the enzymatic activity of Cullin RING E3 ligase resulted in a blockade of Aiolos and Ikaros degradation (Figure 2F). Cumulatively, these results demonstrate that CRBN is the molecular target of CC-122, CC-122 binding to CRBN recruits Aiolos/Ikaros to CRL4CRBN, and E3 ligase enzymatic activity is necessary for ubiquitination of Aiolos and Ikaros and thus their proteasomal degradation induced by CC-122.
CC-122 promotes degradation of Aiolos and Ikaros in ABC-DLBCL and GCB-DLBCL xenografts and DLBCL patients
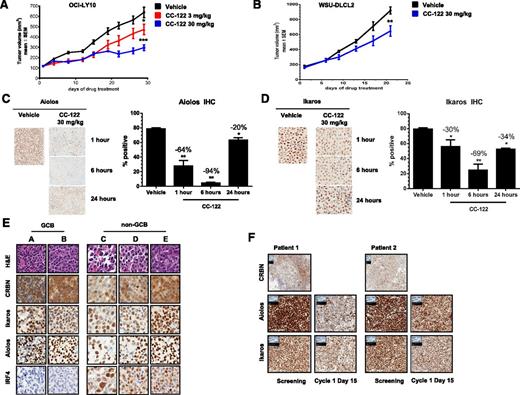
We next examined the ability of CC-122 to inhibit DLBCL xenograft growth in vivo. Treatment of female CB-17 SCID mice with CC-122 at 3 or 30 mg/kg once daily significantly decreased tumor growth in OCI-LY10 ABC-DLBCL (P = .028 and P < .001, respectively) (Figure 3A) and WSU-DLCL2 GCB-DLBCL derived xenograft models (P < .01) (Figure 3B) compared with the vehicle control. In a separate study, we assessed the ability of CC-122 to promote degradation of Ikaros and Aiolos in vivo. In the 21-day efficacy study of WSU-DLCL2 xenograft transplanted mice, tumors were excised 1, 6, or 24 hours post final dosing. Aiolos and Ikaros expression was interrogated through immunohistochemistry (IHC) and was found to be decreased 64% and 30%, respectively, compared with vehicle within 1 hour of treatment, with a maximal reduction of 94% and 69%, respectively, observed at 6 hours (Figure 3C-D). Aiolos and Ikaros levels partially recovered 24 hours postdosing with protein level within 20% and 34% of vehicle, respectively. The 24-hour postdose Aiolos and Ikaros expression represents the trough compound level following multiple doses of CC-122. When the 1-hour time point is compared with the 24-hour postdose time point, there is a significant reduction in Aiolos but not Ikaros expression; however, at the 6-hour time point, both transcription factors are significantly different from the 24-hour time point. Taken together, these data reveal that CC-122 inhibited DLBCL tumor growth in vivo and that this activity was associated with the degradation of Aiolos and Ikaros in both ABC- and GCB-DLBCL xenograft models.
CC-122 reduces tumor growth and promotes degradation of Aiolos and Ikaros in vivo and in DLBCL patients. (A) CB-17 SCID mice with OCI-LY10 xenograft tumors were treated with either vehicle or 3 or 30 mg/kg CC-122 daily. The average tumor volume of each group (n = 10) ± standard deviation (SD) is shown as a function of time. *P = .028; ***P < .001 unpaired Student t test. (B) CB-17 SCID mice with WSU-DLCL2 xenograft tumors were treated with either vehicle or 30 mg/kg CC-122 daily. The average tumor volume of each group (n = 10) ± SD is shown as a function of time. **P < .01 unpaired Student t test. (C and D) WSU-DLCL2 xenograft tumor samples were harvested at indicated time points after final dosing with either vehicle or CC-122 (30 mg/kg). Tissues were then subjected to FFPE IHC for Aiolos and Ikaros. Graphical representation of Aiolos and Ikaros degradation (mean ± SEM). *P < .01; **P < .001 1-way analysis of variance followed by Dunnett’s multiple comparison test. (E) Representative fields of DLBCL formalin fixed paraffin embedded (FFPE) samples stained with hematoxylin and eosin (H&E), CRBN, Aiolos, Ikaros, and IRF4. (F) Representative fields of FFPE samples from GCB R/R-DLBCL patients administered with 3 mg of CC-122 daily. Screening and cycle 1 day 15 biopsies were immunohistochemically stained with Aiolos, Ikaros, and CRBN antibodies as indicated.
CC-122 reduces tumor growth and promotes degradation of Aiolos and Ikaros in vivo and in DLBCL patients. (A) CB-17 SCID mice with OCI-LY10 xenograft tumors were treated with either vehicle or 3 or 30 mg/kg CC-122 daily. The average tumor volume of each group (n = 10) ± standard deviation (SD) is shown as a function of time. *P = .028; ***P < .001 unpaired Student t test. (B) CB-17 SCID mice with WSU-DLCL2 xenograft tumors were treated with either vehicle or 30 mg/kg CC-122 daily. The average tumor volume of each group (n = 10) ± SD is shown as a function of time. **P < .01 unpaired Student t test. (C and D) WSU-DLCL2 xenograft tumor samples were harvested at indicated time points after final dosing with either vehicle or CC-122 (30 mg/kg). Tissues were then subjected to FFPE IHC for Aiolos and Ikaros. Graphical representation of Aiolos and Ikaros degradation (mean ± SEM). *P < .01; **P < .001 1-way analysis of variance followed by Dunnett’s multiple comparison test. (E) Representative fields of DLBCL formalin fixed paraffin embedded (FFPE) samples stained with hematoxylin and eosin (H&E), CRBN, Aiolos, Ikaros, and IRF4. (F) Representative fields of FFPE samples from GCB R/R-DLBCL patients administered with 3 mg of CC-122 daily. Screening and cycle 1 day 15 biopsies were immunohistochemically stained with Aiolos, Ikaros, and CRBN antibodies as indicated.
We next compared expression of CRBN, Aiolos, or Ikaros protein levels by IHC on FFPE samples from 20 DLBCLs, 5 tonsils, and 10 normal lymph node biopsies. Interestingly, normal GCBs exhibited modestly higher levels of CRBN expression compared with DLBCL cells (mean H score of 256 and 208; P = .024). However, there were no significant differences between lymphoma cells and normal GCBs with respect to the expression levels of Aiolos (mean H score of 198 and 211, respectively) and Ikaros (mean H score of 175 and 179, respectively) (supplemental Figure 2A-B). To explore the association of COO molecular subtypes to CRBN, Aiolos, and Ikaros expression, we used the Hans classification scheme together with IHC staining for these 3 proteins on tissue microarrays containing 90 FFPE samples from DLBCL patients. As shown in representative images in Figure 3E, CRBN, Aiolos, and Ikaros were expressed in both GCB and non-GCB DLBCL. IRF4 protein expression, as expected, was observed at much higher levels in the non-GCB subtype.
Finally, we analyzed lymph node biopsies from R/R-DLBCL patients enrolled in a single-arm CC-122 clinical trial (www.clinicaltrials.gov, #NCT01421524) and administered the drug at a dose of 3 mg once daily. Biopsies taken from 2 GCB patients at baseline and after ∼15 days of daily dosing were subjected to IHC staining for CRBN, Aiolos, and Ikaros. IHC staining was positive at baseline in multiple representative biopsies for all 3 markers (Figure 3F). Exposure to CC-122 reduced expression levels of Aiolos and Ikaros in each patient by 25% to 50% demonstrating the utility of these 2 proteins as pharmacodynamic markers of CC-122.
CC-122 mimics IFN signaling in DLBCL
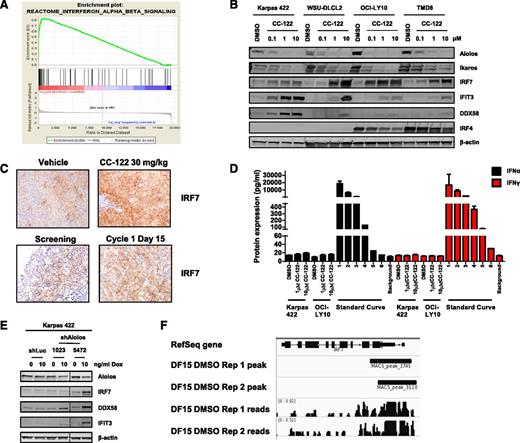
To gain insight into the molecular basis of CC-122 activity in DLBCL cells, we performed gene expression profiling on OCI-LY10 cells treated with DMSO or 1 μM CC-122 for 18 hours to identify CC-122-modulated genes. Comparison of OCI-LY10 cells exposed to 1 μM CC-122 vs the corresponding DMSO control yielded 1374 significantly differentially expressed probes (805 upregulated and 569 downregulated vs DMSO; supplemental Table 1). Gene-set enrichment analysis identified a significant association of CC-122 with 31 upregulated pathway-based gene categories (supplemental Table 2), including autoimmune signaling, cell adhesion molecules, glutathione metabolism, and negative regulators of retinoic acid inducible gene 1 (RIG1). Interestingly, a signature of type I IFN signaling (REACTOME_INTERFERON_ALPHA_BETA_SIGNALING, FDR = 0.001335) (Figure 4A) was highly represented in CC-122-treated cells compared with DMSO. To validate this observation, OCI-LY10 cells were treated with 1 or 10 μM CC-122 or positive control IFN-α for 6 or 24 hours. We observed transcriptional upregulation of IRF7 and 2 other representative ISGs with defined proapoptotic functions of caspase 3 activation and cytochrome C release from the mitochondria, interferon inducible protein 27 and XIAP associated factor 1,18,19 at the mRNA level within 6 hours (supplemental Figure 3A) or 24 hours (supplemental Figure 3B) of treatment. We next confirmed that CC-122 increased the protein levels of IRF7 and 2 additional IFN-regulated proteins, interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) and DEAD box protein 58 (DDX58), concomitant with Aiolos and Ikaros degradation in a concentration-dependent manner (Figure 4B). A modest downregulation of IRF4 was also observed in the ABC cell lines treated with CC-122. In xenograft mice implanted with the WSU-DLCL2 GCB-DLBCL cell line, we confirmed these findings as IRF7 levels assessed by IHC were found to increase when the animals were treated with 30 mg/kg CC-122 to the vehicle control (Figure 4C, top panel). Furthermore, after ∼15 days of CC-122 administration in a non-GCB R/R-DLBCL patient resulted in increased intratumoral IRF7 levels compared with the baseline lymph node biopsy (Figure 4C, bottom panel). To test if the treatment with CC-122 led to production and/or secretion of IFN, we measured secretion of IFN-α or IFN-γ by enzyme-linked immunosorbent assay (ELISA), 72 hours after CC-122 treatment in Karpas 422 and OCI-LY10 cells. Surprisingly, no increase in secretion of either IFN-α or IFN-γ (Figure 4D) and no increase in the transcription of IFN-β1 mRNA (supplemental Figure 3B) were observed. These results suggest that CC-122-mediated effects on the IFN pathway were independent of autocrine type I and II IFN secretion and signaling.
CC-122 induces IFN-regulated proteins. (A) OCI-LY10 cells were treated with CC-122 (1 μM) for 18 hours, and gene expression profiling was performed. Gene set enrichment analysis REACTOME_INTERFERON_ALPHA_BETA_SIGNALING enrichment plot is shown for a category identified as positively enriched with CC-122 treatment in OCI-LY10 cells. False discovery rate = 0.001335. (B) DLBCL cells were treated with DMSO or CC-122 (0.1-10 μM) for 72 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, IRF7, DDX58, IFIT3, IRF4, and β-actin were assessed. (C) WSU-DLCL2 xenograft tumor samples were harvested 24 hours after dosing with either vehicle or CC-122 (30 mg/kg; top panel). Representative fields of FFPE samples from a non-GCB R/R-DLBCL patients administered with 3 mg of CC-122 daily (bottom). Screening and cycle 1 day 15 biopsies were obtained. Tissues were then subjected to FFPE IHC for IRF7. (D) OCI-LY10 and Karpas 422 cells were treated with DMSO or CC-122 (1-10 μM) for 72 hours, supernatants were harvested, and IFN-α and IFN-γ expression was measured by ELISA. A control standard curve of 10 000 pg/mL IFN-α or IFN-γ with fivefold serial dilutions was also determined. Data shown are the mean of 2 independent experiments tested in triplicate (±SD). (E) Inducible luciferase or Aiolos shRNA Karpas 422 cell lines were treated with 0 or 10 ng/mL of Dox for 3 days. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, IRF7, DDX58, IFIT3, and β-actin were assessed. (F) Depiction of chromatin immunoprecipitation sequencing data from DF15 multiple myeloma cells showing Aiolos binding at the IRF7 promoter.
CC-122 induces IFN-regulated proteins. (A) OCI-LY10 cells were treated with CC-122 (1 μM) for 18 hours, and gene expression profiling was performed. Gene set enrichment analysis REACTOME_INTERFERON_ALPHA_BETA_SIGNALING enrichment plot is shown for a category identified as positively enriched with CC-122 treatment in OCI-LY10 cells. False discovery rate = 0.001335. (B) DLBCL cells were treated with DMSO or CC-122 (0.1-10 μM) for 72 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, IRF7, DDX58, IFIT3, IRF4, and β-actin were assessed. (C) WSU-DLCL2 xenograft tumor samples were harvested 24 hours after dosing with either vehicle or CC-122 (30 mg/kg; top panel). Representative fields of FFPE samples from a non-GCB R/R-DLBCL patients administered with 3 mg of CC-122 daily (bottom). Screening and cycle 1 day 15 biopsies were obtained. Tissues were then subjected to FFPE IHC for IRF7. (D) OCI-LY10 and Karpas 422 cells were treated with DMSO or CC-122 (1-10 μM) for 72 hours, supernatants were harvested, and IFN-α and IFN-γ expression was measured by ELISA. A control standard curve of 10 000 pg/mL IFN-α or IFN-γ with fivefold serial dilutions was also determined. Data shown are the mean of 2 independent experiments tested in triplicate (±SD). (E) Inducible luciferase or Aiolos shRNA Karpas 422 cell lines were treated with 0 or 10 ng/mL of Dox for 3 days. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, IRF7, DDX58, IFIT3, and β-actin were assessed. (F) Depiction of chromatin immunoprecipitation sequencing data from DF15 multiple myeloma cells showing Aiolos binding at the IRF7 promoter.
Ikaros family transcription factors are known to negatively regulate transcription of target genes through direct interaction with the DNA near the transcriptional start site affecting chromatin remodeling. Ikaros and Aiolos repress transcription through 2 different mechanisms: by the recruitment of the Mi-2β containing nucleosome remodeling deacetylase (NuRD) complex resulting in histone deacetylation,20,21 and by modulating the basal transcriptional machinery in a non-histone deacetylase (HDAC) mechanism through interactions with C-terminal binding protein (CtBP) and CtBP-interacting protein (CtIP).22 The Karpas 422 cells (GCB DLBCL) do not express IRF4 and enable elucidation of the effects of Aiolos on ISG stimulation independent of B-cell differentiation. To further understand the specific role of Aiolos in CC-122-mediated upregulation of the IFN-regulated proteins, we generated Karpas 422 cell lines stably transduced with inducible shRNA constructs targeting either luciferase (shLuc) as a control or Aiolos mRNA via 2 unique shRNA constructs (shAiolos 1023 and shAiolos 5472). As shown in Figure 4E, induction of shRNAs targeting Aiolos but not luciferase in Karpas 422 cells with Dox led to a twofold decrease in Aiolos protein levels at 3 days. Additionally, in shRNA cell lines where Aiolos was reduced, a concomitant increase in IRF7, DDX58, and IFIT3 protein was observed (Figure 4E). Finally, analysis of Aiolos chromatin immunoprecipitation sequencing23 performed in DF15 multiple myeloma cells demonstrated positive binding of Aiolos to the IRF7 transcriptional start site (Figure 4F). Taken together, these results show that CC-122 activates genes in the IFN pathway by promoting Aiolos degradation in both ABC- and GCB-DLBCL cell lines, likely by removing their repressive activity on the ISGs.
Differential activities of CC-122 and lenalidomide in DLBCL
Lenalidomide has demonstrated potent activity in clinical trials with R/R-DLBCL subjects, especially in the ABC subtype.24-26 Our previous preclinical studies also demonstrated that lenalidomide had preferential antiproliferative activity in ABC-DLBCL cell lines vs GCB-DLCBL cell lines.16 In contrast, CC-122 exhibited antiproliferative activity in both ABC- and GCB-DLBCL cell lines; thus, we wanted to explore the potential difference in the underlying mechanisms for the differential effects exhibited by the 2 drugs.
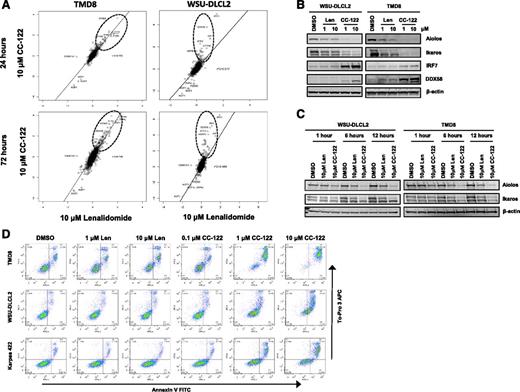
We next investigated the relative abundance of various proteins in WSU-DLCL2 GCB-DLBCL cells after treatment with 10 µM CC-122 or 10 µM lenalidomide. In this experiment, total proteins were isolated from drug-treated cells and analyzed by tandem mass tag mass spectrometry, and log2 ratios of relative protein abundance from the 2 treatments were compared. Scatter plots of the mass spectrometric data from ABC TMD8 cells demonstrated that those proteins that increased in relative abundance at 24 and 72 hours, including the IFN-regulated proteins, were commonly regulated by lenalidomide and CC-122 with r2 values of 0.752 and 0.746 (Figure 5A). Additionally, proteomics were performed on WSU-DLCL2 cells treated with equivalent concentrations of CC-122 and lenalidomide for 24 and 72 hours. Strikingly, although CC-122 treatment increased the relative abundance of IFN-stimulated proteins such as IFIT3 and DDX58 by 5- to 15-fold, exposure to lenalidomide did not result in increased levels of these proteins. Moreover, the r2 values comparing CC-122 and lenalidomide treatments in WSU-DLCL2 were 0.517 and 0.488 indicating a greater difference in the effects of the 2 drugs in GCB cells compared with ABC cells. To confirm the findings, validation experiments were performed in WSU-DLCL2 and TMD8 cells treated with DMSO, lenalidomide (1-10 μM), and CC-122 (1-10 μM) for 72 hours. As shown in Figure 5B, lenalidomide treatment did not result in increased IRF7 and DDX58 protein levels in these cells, whereas treatment with CC-122 dramatically increased their abundance. TMD8 cells treated with lenalidomide slightly increased levels of IRF7 and DDX58 compared with DMSO, whereas CC-122 greatly increased the abundance of these proteins. Additionally, in both cells lines CC-122 promoted a much greater reduction in the levels of Aiolos and Ikaros compared with lenalidomide.
Differential activities of CC-122 in GCB-DLBCL compared with lenalidomide. (A) Effect of 10 μM lenalidomide exposure (log2 fold-change vs DMSO) plotted against 10 μM CC-122 exposure at 24 and 72 hours in TMD8 and WSU-DLCL2 cells. Key proteins (IKZF1, IKZF3) and proteins manifestly affected more by one compound than the other are displayed, along with r2 for linear regression. (B) DLBCL cells were treated with DMSO, lenalidomide (Len), or CC-122 for 72 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, IRF7, DDX58, and β-actin were assessed. (C) DLBCL cells were treated with DMSO, lenalidomide (10 μM), or CC-122 (10 μM) for 1, 6, or 12 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (D) DLBCL cell lines were treated with DMSO, lenalidomide (1-10 μM), or CC-122 (0.1-10 μM) for 7 days, after which apoptosis was measured by Annexin V and To-Pro 3 flow cytometric analysis.
Differential activities of CC-122 in GCB-DLBCL compared with lenalidomide. (A) Effect of 10 μM lenalidomide exposure (log2 fold-change vs DMSO) plotted against 10 μM CC-122 exposure at 24 and 72 hours in TMD8 and WSU-DLCL2 cells. Key proteins (IKZF1, IKZF3) and proteins manifestly affected more by one compound than the other are displayed, along with r2 for linear regression. (B) DLBCL cells were treated with DMSO, lenalidomide (Len), or CC-122 for 72 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, IRF7, DDX58, and β-actin were assessed. (C) DLBCL cells were treated with DMSO, lenalidomide (10 μM), or CC-122 (10 μM) for 1, 6, or 12 hours. Cell lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (D) DLBCL cell lines were treated with DMSO, lenalidomide (1-10 μM), or CC-122 (0.1-10 μM) for 7 days, after which apoptosis was measured by Annexin V and To-Pro 3 flow cytometric analysis.
Next, we explored differences in rate of degradation between CC-122 and lenalidomide. We treated WSU-DLCL2 and TMD8 with DMSO, 10 μM lenalidomide, or 10 μM CC-122 for 1, 6, and 12 hours and immunoblotted for Aiolos and Ikaros. As shown in Figure 5C, lenalidomide induced Aiolos and Ikaros degradation of 33% to 43% and 23% to 31% in WSU-DLCL2 and TMD8, respectively, whereas CC-122 mediated degradation of Aiolos and Ikaros by 78% to 80% and 52% to 70% within 12 hours in WSU-DLCL2 and TMD8, respectively. These results demonstrate a concentration- and time-dependent difference between lenalidomide and CC-122 in the rate and extent of Aiolos and Ikaros degradation in GCB cells and corresponding changes in upregulation of ISG transcription and abundance of proteins. Finally, we compared the ability of CC-122 and lenalidomide to induce apoptosis in the ABC TMD8 cells and 2 GCB cell lines, WSU-DLCL2 and Karpas 422. Lenalidomide treatment in the TMD8 cells resulted in a twofold increase in apoptosis, whereas there was no measureable increase in either the WSU-DLCL2 or Karpas 422 cells. By contrast, CC-122 induced apoptosis from 4- to 8.5-fold in each of the 3 cell lines compared with DMSO (Figure 5D). These data suggest a potential mechanism for the broader spectrum of CC-122 activity in DLBCL encompassing GCB DLBCL.
CC-122 costimulates T cells via Aiolos and Ikaros degradation
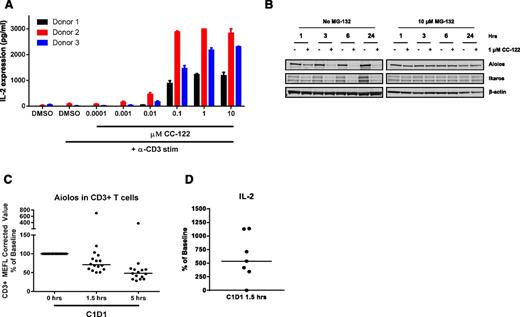
Aiolos and Ikaros have been described as repressors of IL-2 expression and secretion, a marker of activation in primary T cells.13,21,27 Given the degradation observed of Aiolos and Ikaros in DLBCL cells, we investigated the effects of CC-122 treatment on IL-2 expression in primary T cells. Exposure of CD3-stimulated purified T cells from 3 donors to various concentrations of CC-122 led to a dramatic increase in IL-2 expression and secretion (Figure 6A). We next examined the effect of CC-122 on Aiolos and Ikaros protein expression in anti-CD3-stimulated primary human T cells treated with DMSO or 1 µM CC-122 for 1, 3, 6, and 24 hours. Degradation of both Aiolos and Ikaros was observed as early as 1 hour after drug treatment and a decrease of Aiolos and Ikaros of 96% and 81%, respectively, at 6 hours (Figure 6B). Furthermore, a 30-minute pretreatment with MG-132, a proteasome inhibitor, stabilized Aiolos and Ikaros confirming that degradation was mediated through the proteasome.
CC-122 induces degradation of Aiolos and Ikaros in primary T cells and increased IL-2 secretion. (A) Primary T cells were pretreated with DMSO or CC-122 (0.001-10 µM) for 1 hour prior to anti-CD3 stimulation. On day 2, supernatants were harvested and assayed for IL-2 expression by ELISA. Data shown are from 3 independent normal donors tested in triplicate (± SEM). (B) Primary T cells were pretreated with DMSO or 1 μM CC-122 for 1 hour prior to anti-CD3 stimulation in the presence or absence of 10 μM MG-132. At indicated time points, cells were harvested, lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (C) Viably frozen peripheral blood mononuclear cells from baseline cycle 1 day 1 (C1D1) at 0 hours and C1D1 (1.5 and 5 hours postadministration of CC-122) were analyzed by flow cytometry for Aiolos expression in CD3+ T cells. MEFL, molecules of equivalent fluorescein. (D) Whole blood drawn from DLBCL patients on C1D1 at 0 hours and 1.5 hours postadministration CC-122 were incubated for 48 hours at 37°C with anti-CD3 antibody. Supernatants were harvested and IL-2 expression was measured by luminex.
CC-122 induces degradation of Aiolos and Ikaros in primary T cells and increased IL-2 secretion. (A) Primary T cells were pretreated with DMSO or CC-122 (0.001-10 µM) for 1 hour prior to anti-CD3 stimulation. On day 2, supernatants were harvested and assayed for IL-2 expression by ELISA. Data shown are from 3 independent normal donors tested in triplicate (± SEM). (B) Primary T cells were pretreated with DMSO or 1 μM CC-122 for 1 hour prior to anti-CD3 stimulation in the presence or absence of 10 μM MG-132. At indicated time points, cells were harvested, lysates were separated by SDS-PAGE, and levels of Aiolos, Ikaros, and β-actin were assessed. (C) Viably frozen peripheral blood mononuclear cells from baseline cycle 1 day 1 (C1D1) at 0 hours and C1D1 (1.5 and 5 hours postadministration of CC-122) were analyzed by flow cytometry for Aiolos expression in CD3+ T cells. MEFL, molecules of equivalent fluorescein. (D) Whole blood drawn from DLBCL patients on C1D1 at 0 hours and 1.5 hours postadministration CC-122 were incubated for 48 hours at 37°C with anti-CD3 antibody. Supernatants were harvested and IL-2 expression was measured by luminex.
We extended our studies further to investigate CC-122 effects on Aiolos degradation in peripheral T cells from R/R-DLBCL patients receiving a 3-mg once-daily dose in the phase 1 trial. Peripheral blood mononuclear cells isolated from whole blood samples collected immediately prior to CC-122 administration and 1.5 and 5 hours post a single CC-122 dose were analyzed for Aiolos expression by flow cytometric analysis. In CD3+ T cells, a median decrease of 27% and 48% in Aiolos expression was observed at 1.5 and 5 hours postdosing, respectively, compared with baseline samples (Figure 6C). Additionally, ex vivo anti-CD3 stimulation of whole blood drawn prior to or 1.5 hours after a single dose of CC-122 administration led to a median increase in IL-2 levels of 477% in the 1.5-hour samples compared with baseline pretreatment (Figure 6D). These combined results demonstrate that CC-122 is a potent costimulator of T cells both in vitro and in DLBCL patients.
Discussion
In this study, we describe the activity of a new chemical entity, CC-122, with broad cytotoxic activity in DLBCL independent of COO, as well as immunomodulatory activity. Although CC-122 binds and utilizes a common target, CRBN, shared with lenalidomide or pomalidomide, recent crystallographic data demonstrate how structurally distinct IMiD compounds evoke different cellular and molecularly defined responses, yet bind the same target. Additional recent studies on the immunomodulatory agents have elucidated, in part, the mechanism of action in multiple myeloma cells and primary T cells.11-13 Both activities involve drug binding to CRBN and subsequent degradation of substrate proteins, specifically the transcription factors Aiolos and Ikaros, mediated by the CRL4CRBN E3 ligase complex. The Ikaros family of transcription factors comprises proteins with zinc-finger domain–mediated DNA binding capabilities, and they serve as key regulators of lineage commitment in lymphoid progenitor cells and lymphoid differentiation.28,29 However, their precise role in B-cell malignancies such as multiple myeloma and lymphoma is yet to be defined.
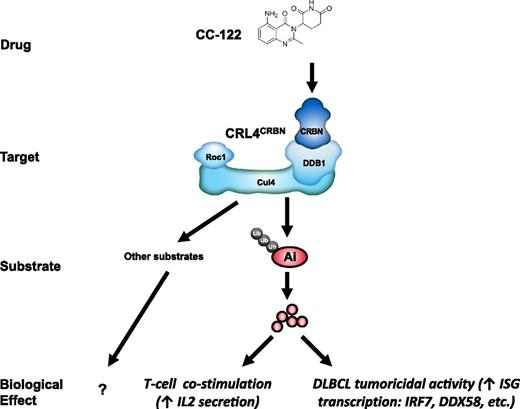
In this study based on our cumulative observations, we propose a hypothetical model for CC-122 activity (Figure 7) whereby CC-122 binding to CRBN results in the increased ability of CRL4CRBN to recruit Aiolos and Ikaros, resulting in (1) activation of T cells and (2) derepression of ISGs leading to tumoricidal activity in DLBCL (Figure 7).
Model of CC-122 costimulation of T cells and tumoricidal activity through degradation of Aiolos and Ikaros. Binding of CC-122 to CRBN promotes the interaction with Aiolos and Ikaros to CRL4CRBN leading to their ubiquitination and subsequent proteasomal destruction. Degradation of Aiolos and Ikaros results in costimulation of T cells and anti-DLBCL activity. Ai, Aiolos; Cul4, cullin 4; DDB1, DNA damage binding protein-1; Roc1, regulator of cullins 1; Ub, ubiquitin.
Model of CC-122 costimulation of T cells and tumoricidal activity through degradation of Aiolos and Ikaros. Binding of CC-122 to CRBN promotes the interaction with Aiolos and Ikaros to CRL4CRBN leading to their ubiquitination and subsequent proteasomal destruction. Degradation of Aiolos and Ikaros results in costimulation of T cells and anti-DLBCL activity. Ai, Aiolos; Cul4, cullin 4; DDB1, DNA damage binding protein-1; Roc1, regulator of cullins 1; Ub, ubiquitin.
Previous preclinical studies indicated that lenalidomide had preferential antiproliferative activity in ABC-DLBCL cell lines vs GCB-DLBCL cell lines.16,30 Interestingly, a retrospective analysis of 2 single-agent lenalidomide R/R-DLBCL clinical trials24,25 comparing COO classification by Han’s IHC algorithm to clinical response indicated a significant difference in the response of non-GCB patients vs GCB patients. Overall response rate was 52.9% (non-GCB) vs 8.7% (GCB) (P = .006), and complete response rate was 23.5% compared with 4.3%, respectively. In a separate single-agent lenalidomide clinical trial, patients were subtyped by gene expression profiling. ABC-DLBCL patients treated with lenalidomide when compared with GCB patients showed greater improvements in overall response rate (45.5% vs 21.4%), progression-free survival (82 weeks vs 13.2 weeks), and overall survival (108.4 weeks vs 30 weeks).26 In contrast to lenalidomide, CC-122 appears to possess broader cell autonomous activity than lenalidomide, spanning both the ABC- and GCB-DLBCL subtypes. Although the precise mechanism is not fully known, we suggest 2 possibilities to explain the differential activity of CC-122 and lenalidomide. First, although CC-122 and lenalidomide both mediate destruction of Aiolos and Ikaros, there may be distinct substrates for each drug as hypothesized by Chamberlain et al.9 In fact, a lenalidomide-specific substrate, casein kinase 1α, which is not affected by CC-122, was identified in our proteomics experiment (Figure 5A). It is possible that CC-122 utilizes specific and unique substrates to mediate some of its biological effects distinct from lenalidomide. Identification and characterization of new substrates may help substantiate this hypothesis. Secondly, although previous work highlighted the role of IRF4 regulation in the induction of an IFN autocrine loop in response to lenalidomide,30 our data refine this mechanism by describing a direct regulation of ISG by Aiolos independent of IRF4 and IFN secretion. CC-122’s activity in GCB DLBCL may be an indication of greater ISG promoter occupancy, and thus repression, by Aiolos in GCB compared with ABC DLBCL, as CC-122 induces a greater depth and faster kinetics of Aiolos degradation than lenalidomide. This hypothesis would require a differential dependence on Aiolos repressive activities between the 2 molecular subtypes and the ability to degrade this substrate below a threshold in GCB DLBCL that lenalidomide cannot attain, as we have not observed differences in expression of Aiolos between GCB and non-GCB patient biopsies (supplemental Figure 4).
Our results have additional implications for the clinical development of CC-122 in DLBCL. For example, we demonstrate in our studies that a classical “double hit” lymphoma cell line (Karpas 422)31 is sensitive to CC-122 and that c-myc protein levels are reduced after CC-122 treatment (supplemental Figure 5). A segment of high-risk DLBCL patients defined by gene rearrangements or elevated protein levels of c-myc in combination with BCL2 or BCL6 expression are associated with a particularly poor prognosis when receiving rituximab, cyclophosphamide, doxorubicine, vincristine, prednisone (R-CHOP).32,33 In mouse B and T cells, Aiolos and Ikaros are direct transcriptional repressors of the c-myc promoter.20,34 Although c-myc protein levels are decreased in response to CC-122-mediated degradation of these 2 substrates, the Karpas 422 cell line has a c-myc rearrangement suggesting the c-myc downregulation is partially mediated through indirect mechanism(s). These observations highlight that additional studies are needed to understand the possible role of c-myc regulation by Aiolos and Ikaros in this specific disease subset and may warrant further clinical investigation of CC-122 in this high-risk population.
In addition to the cell-autonomous effects, CC-122’s immunomodulatory properties also have clinical implication. Specifically, DLBCL patients with decreased expression of major histocompatibility complex class II at the mRNA and protein levels and fewer CD8+ tumor infiltrating lymphocytes have a poor overall survival independent of other prognostic factors.35 The potential for CC-122 to produce an antitumor effect through immunomodulation and enhanced immune surveillance is under investigation. Future studies will further elucidate the contribution of this mechanism to the therapeutic effect in the clinic as well as rationale combination strategies with other immuno-oncology agents such as checkpoint inhibitors or novel targeted agents.
Taken together, our results demonstrate that CC-122 is a broadly active antilymphoma drug that shares a common target with lenalidomide but is differentiated from lenalidomide in terms of molecular mechanisms of action, which may guide the course of its clinical development in DLBCL. CC-122 targeting of Aiolos reveals the role in the transcriptional repression of IFN signature genes in DLBCL. Although IFN-α has been employed therapeutically for decades in various types of lymphoma, it has limited clinical efficacy in high-grade lymphomas and comes with significant dose-limiting toxicity.36 CC-122 circumvents exogenous and endogenous IFN and induces DLBCL apoptosis through a unique mode of action.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kenye Sebastian and Yan Ren for the excellent immunohistochemical work, and Ryan Kunz and Steven Gygi of the Thermo Fisher Scientific Center for Multiplexed Proteomics at Harvard Medical School for their assistance in the tandem mass tag mass spectrometry experiments.
Authorship
Contribution: All authors designed and performed experiments and/or analyzed data; and P.R.H., A.K.G., A.K., A.T., R.C., and T.O.D. wrote the manuscript.
Conflict-of-interest disclosure: All authors are employed by Celgene Corporation. All authors except M.D.A. and H.C. have equity ownership in Celgene Corporation.
Correspondence: Rajesh Chopra, Celgene Corporation, 86 Morris Ave, Summit, NJ 07901; e-mail: rachopra@celgene.com.

![Figure 1. CC-122 induces growth arrest and apoptosis in ABC- and GCB-DLBCL cell lines. (A) Multiple DLBCL cell lines were treated with dimethylsulfoxide (DMSO) and CC-122 (0.01-10 000 nM) for 5 days. Proliferation for all cell lines was determined using the 3H-thymidine incorporation method. Results of 3 independent experiments are shown (mean ± standard error of the mean [SEM]). (B) DLBCL cell lines were treated with DMSO, and CC-122 (0.1-10 μM) for 7 days, after which apoptosis was measured by Annexin V and To-Pro 3 flow cytometric analysis. Graphical representation of 2 independent experiments (mean ± SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/6/10.1182_blood-2015-02-628669/4/m_779f1.jpeg?Expires=1769102590&Signature=Mh-VZ7w47id2HAolwUID3ZbbHSCd-42awm776VPMPIGaqxFNDvQJ95~TkenRNEVgPSdlOn3eMbtwDDQuYEFKdE9he5rWk4fYzUBlbTyxNX9He5N~Ygw0hvDs7A2UBUDd9BLMUtij5bzwfkZjmLxt2~R2M9nOOiA5JcUmgInHqsQWkKkac40p2HVWJepqzBnWT43fLFK25GsxAlft41AIrgJjY3uZbQX2RUJquKbMYgQzrsQ~mN9KpjqkSvRKIqZPvvbGvh-9RuMfrD4wQSdS1mk-IH-RONv4ayw59Rob6Ukxw8Byg7irQE~QOLo--Scf-Oyldm3NNo8Ns34C6i88WQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)