Key Points
IL-6 from splenic stromal cells prevents CLL cells from responding strongly to TLR ligands.
IL-6–signaling inhibitors enhance TLR-mediated responses of CLL cells in vitro and in vivo.
Abstract
The regulation of toll-like receptor (TLR) signaling in a tumor microenvironment is poorly understood despite its importance in cancer biology. To address this problem, TLR7-responses of chronic lymphocytic leukemia (CLL) cells were studied in the presence and absence of a human stromal cell-line derived from a leukemic spleen. CLL cells alone produced high levels of tumor necrosis factor (TNF)-α and proliferated in response to TLR7-agonists. A signal transducer and activator of transcription 3 -activating stromal factor, identified as interleukin (IL)-6, was found to upregulate microRNA (miR)-17 and miR-19a, target TLR7 and TNFA messenger RNA, and induce a state of tolerance to TLR7-agonists in CLL cells. Overexpression of the miR-17-92 cluster tolerized CLL cells directly and miR-17 and miR-19a antagomiRs restored TLR7-signaling. Inhibition of IL-6 signaling with antibodies or small-molecule Janus kinase inhibitors reversed tolerization and increased TLR7-stimulated CLL cell numbers in vitro and in NOD-SCIDγcnull mice. These results suggest IL-6 can act as tumor suppressor in CLL by inhibiting TLR-signaling.
Introduction
Chronic lymphocytic leukemia (CLL) is incurable with conventional cytotoxic chemotherapy, but inhibitors of the signaling pathways that mediate proliferation of CLL cells are proving to be highly effective and changing treatment paradigms for this disease.1 Circulating CLL cells originate in proliferation centers (PCs) in lymphoid organs where they sometimes divide autonomously as a result of activating mutations in signaling pathways.2 However, the major drivers of CLL in vivo are thought to be external signals from the PC microenvironment, including antigens, cytokines, and chemokines provided by other CLL cells, T cells, monocyte-derived nurse cells, and stromal cells.3,4
Toll-like receptor (TLR) signaling is also important in CLL biology.5 CLL patients are immunocompromised and bacterial infections may stimulate tumor growth by activating complexes of TLR1, TLR2, and TLR6 on CLL cells.5 Viral infections and processes of cell turnover and damage that accompany tumor progression may lead to aberrant production of single- and double-stranded DNA, RNA, and microRNA (miR) that activate TLR7 and TLR9 on CLL cells.5,6 In some instances, stimulation of TLRs may have therapeutic effects. For example, the expression of TLR7 is restricted mainly to CLL cells,6 and activation of TLR7 by imidazoquinolines such as resiquimod6 can sensitize CLL cells to cytotoxic drugs and immune cells in vitro7,8 and clear cutaneous leukemic infiltrates upon topical administration.9 However, these agents have limited therapeutic activity following systemic delivery,10 suggesting TLR7-signaling is impaired in PCs in vivo. A greater knowledge of TLR-signaling processes in the CLL microenvironment would help clarify how they promote tumor progression and might be manipulated for therapeutic purposes.
PCs are not easily accessible and in vitro models are necessary to study TLR-signaling in this compartment. Such models usually involve the activation of circulating cells11,12 and do not account for important contributions of noncirculating stromal cells. Bone marrow (BM) stromal cells from CLL patients are relatively easy to obtain,13 but the roles of lymph node and splenic stromal cells have not been studied extensively despite the importance of these sites for disease progression. In this study, we have developed a stromal cell line from the spleen of a CLL patient and used it to identify a major role for interleukin (IL)-6 in the regulation of TLR7 signaling.
Methods
CLL samples
CLL cells and peripheral blood mononuclear cells (PBMCs) were isolated as before4 from consenting CLL patients, diagnosed by standard criteria14 and untreated for at least 3 months. Patient characteristics and identification numbers are listed in supplemental Table 1, available on the Blood Web site. Splenocytes were obtained from consenting patients undergoing elective splenectomies.15 Protocols were approved by the Sunnybrook Ethics Review Board.
Antibodies and reagents
Fluorescent human CD19, CD83, CD5, and tumor necrosis factor (TNF)-α antibodies were from Pharmingen (San Francisco, CA). Resiquimod (TLR-7/8 agonist) and CpG ODN2006 (TLR-9 agonist) were from Alexis Biochemicals (San Diego, CA). IL-2 (Novartis Canada Inc., Dorval, Quebec), interferon (IFN)-α2b (Schering Canada, Dorval, Quebec), vincristine sulfate (Faulding Canada Inc., Kirkland, Quebec), and actemra (Roche Canada, Mississauga, ON) were purchased from the hospital pharmacy. IL-6 for in vitro experiments was from R&D Systems (Minneapolis, MN) and IL-6 for in vivo experiments was from BioLegend (San Diego, CA). Ruxolitinib and tofacitinib were from Selleck Chemicals (Houston, TX). Anti–IL-10 antibodies were from eBioscience (San Diego, CA). Staphylococcal enterotoxin A (SEA) was from Toxin Technology Inc. (Madison, WI). Antibodies to phosphorylated and native forms of signal transducer and activator of transcription 3 (STAT3), IκB, IKKα, IRAK1, p42/44 ERK, STAT1, STAT5, AMPK, p100/p52 nuclear factor κB (NF-κB)2, and secondary antibodies were from Cell Signaling Technology (Beverly, MA). β-Actin antibodies were from Sigma (St. Louis, MO). Receptor tyrosine kinases (RTKs) activated in CLL cells by splenic stromal cell supernatant were profiled with the Proteome Profiler Human Phospho-RTK Antibody Array Kit (R&D).
Cell culture and activation
Unless otherwise specified, culture and activation of CLL cells with resiquimod and IL-2 were performed in serum-free AIM-V medium (Life Technologies, Carlsbad, CA) as before.8,16 Viable cells were counted manually in a hemocytometer using trypan-blue exclusion. Otherwise, 1 μCi of [3H]thymidine was added to the cultures after 48 hours for a subsequent 18 hours. Thymidine incorporation was then measured in a β scintillation counter as before.17
SEA-reactive T cells were made from PBMCs stimulated with SEA (1 ng/mL) in AIM-V plus 10% fetal calf serum for 10 to 14 days. Effectors were collected by density gradient centrifugation and suspended in AIM-V. SEA-redirected killing assays were then carried out with TLR7-activated CLL cells used as targets as before.7
Splenic stromal cell lines
Spleen sections were transported from the operating suite in RPMI 1640 and cut into 3 to 4 mm pieces with scissors. Splenocytes were released into transfer media with the end of a sterile 5-mL syringe plunger and centrifuged at 250 × g for 5 minutes. The cell pellet was re-suspended in media and plated on 10-cm culture dishes for 3 weeks. When some cells attached to the plastic, nonadherent cells were removed and the adherent cells were cultured for another 2 weeks, collected by scraping, and then cultured in limiting dilution to obtain stromal cell lines that were routinely cultured in Minimum Essential Media (Sigma-Aldrich, Oakville, ON) supplemented with 10% fetal bovine serum, 100 U/mL penicillin (Gibco, Grand Island, NY), and 10−4 M 2-mercaptoethanol (Sigma).
Conditioned medium
Stromal cells were grown to confluence in propagation media and replaced by AIM-V. The serum-free media was removed 48 hours later and centrifuged to remove any contaminating cells.
Immunophenotyping and membrane TNF (mTNF)-α detection
Flow cytometry was performed as before.8
Cytokine measurements
TNF-α in culture supernatants was measured with a commercial ELISA kit (R&D).18 A C-series custom antibody array for 21 STAT3-activating cytokines and growth factors was purchased from Ray Biotech (Norcross, GA).
Immunoblotting
Protein extraction and western blot analysis were performed as before.4
Lentivirus vector construction
Short hairpin RNA (shRNA)-STAT3.
An shRNA template was designed and cloned into the BamHI and BsmBI sites of plasmid green flourescent protein (pGFP)-C-shlenti (OriGene, Rockville, MD).19 Coding regions used to target the human STAT3 sequence were selected as small interfering RNA (siRNA) target sequences by using the RNAi design algorithm at http://katahdin.mssm.edu/siRNA/RNAi.cgi?type=shRNA. Correct insertions of shRNA cassettes were confirmed by restriction mapping and direct DNA sequencing (supplemental Figure 8).
miR-17-92, -17, and miR-19a.
To generate lentiviruses expressing miR-17-92, miR-17, and miR-19a, precursor overhang sequences from 5′ NotI- and 3′ XhoI-restriction sites were amplified for cloning purposes by PCR using gene-specific primers as described in supplemental Figure 9. Products were inserted into NotI and XhoI sites of pGFP-C-lenti-miR, a lentiviral expression vector, which was modified by replacing GFP from pGFP-C-lenti (OriGene) with an H1 expression cassette to provide constitutive RNA polymerase III-dependent transcription of miR transcripts and generate pGFP-C-lenti-miR-17-92, pGFP-C-lenti-miR-17, or pGFP-C-lenti-miR-19a. Correct sequences and insertions were confirmed by DNA sequencing for each plasmid (supplemental Figure 8).
AntagomiR-17 and miR-19a.
To generate miR-17 or miR-19 loss-of-function phenotypes, anti–miR-17 and anti–miR-19 were designed with the RNAi design algorithm at http://katahdin.mssm.edu/siRNA/RNAi.cgi?type=shRNA and cloned into the BamHI and BsmBI sites of a modified pGFP-C-shlenti-miR (OriGene) in which the U6 promoter from the pGFP-C-shlenti vector (OriGene) was replaced with the H1-antagomir expression cassette containing miR-shRNAs. Mature functional anti–miR-17 and anti–miR-19 sequences are shown in supplemental Figure 9.
Virus generation
Lentiviruses were generated by co-transfecting 5 μg of lentiviral vector and 6 μg of packaging plasmids (OriGene) in 293T cells using lipofectamine 2000 reagent (Invitrogen). Supernatants were collected after 48 hours, spun at 3000 rpm/min, filtered through a 0.45 μm filter, and used directly to infect cells.
Lentiviral transduction of primary CLL cells
Freshly isolated CLL cells (2 × 106) in 4 mL medium per well of a 6-well plate were treated with polyarginine (Sigma) for 20 minutes before adding 0.5 mL of lentiviral particles. The solution was swirled gently for a few seconds. Cells were collected after 12 hours and centrifuged at 1050 rpm for 15 minutes to remove smaller cells that were not transfected. Larger cells remaining in suspension were cultured with fresh AIM-V + 2-mercaptoethanol (3 mL) for 12 to 48 hours.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Quantification of specific RNA transcripts was performed as before20 with the primers listed in supplemental Figure 9.
Quantification of mature miR
Total RNA was extracted with the mirVana miRNA Isolation Kit (Life Technologies, Carlsbad, CA) and complementary DNA was synthesized using the miRNA RT Assay (TaqMan). Expression of mature miR-17, miR-18, miR-19a, miR-20, miR-19b, and miR-92 was analyzed with miR-specific primers (supplemental Figure 9) and normalized to hypoxanthine phosphoribosyltransferase (HGTP).
Luciferase assay
NIH-3T3 cells were plated into 12-well plates at 3 to 4 × 105 cells per well for 24 hours. Then, 0.2 μg pMIR-REPORT Luciferase Reporter Plasmid (Ambion, Austin, TX) expressing wild-type or mutated miR-17 or miR-19a binding sites in TNF or TLR7 3′-untranslated regions (3′-UTRs) and 0.8 μg miR-19a or miR-17 expressing pGFP-C-shLenti-miR vectors were added to each well using lipofectamine 2000 in 4 triplicates. Mutagenesis of miR binding sites was carried out with the QuickChangeXL Mutagenesis Kit (Stratagene). Primers for cloning are described in supplemental Figure 9. For the miR-17-92 promoter luciferase reporter construct, overhang sequences from 5′ Hind III- and 3′ SacI-restriction sites were amplified by RT-PCR and then inserted into the HindIII and SacI sites of pGL4.28 (pGL4.28 promoter wild type). The predicted STAT3 binding site was altered by introducing 4-point mutations (pGL4.28 promoter *STAT3), and an empty pGL4.28 vector was used as a control. HepG2 cells were used to study IL-6–mediated regulation of the miR-1-92 promoter due to weak responses of NIH-3T3 cells to IL-6. Relative luciferase units (RLU) were measured 48 hours posttransfection of NIH-3T3 cells or 6 hours after stimulation with IL-6 of HepG2 cells transfected 48 hours earlier using the Luciferase Reporter Assay System (Ambion) on a Synergy 2 Microplate Reader (BioTek Instruments, Winooski, VT).
In vivo experiments
NOD-SCIDγcnull mice were bred and maintained at the Toronto Medical Discovery Tower, MaRs Centre (Toronto, ON). Mice (8 to 12 weeks old) were irradiated (2.0 Gy), IV injected with 5 × 107 PBMCs from CLL patients, along with biweekly intraperitoneal injections of stromal cell conditioned media (1 mL), and either resiquimod (100 μg intraperitoneally) or resiquimod plus actemra (100 μg IV). Actemra and resiquimod were given immediately and 30 minutes after the conditioned media, respectively. Mice were euthanized after 1 week. For the experiments with IL-6 in place of stromal conditioned media, intramuscular injections of IL-6 (50 ng) were given daily for 20 days until the day before the mice were euthanized.
Statistics
Unpaired Student t tests were calculated with GraphPad Prism Software. P values <.05 were considered significant. For the mega-analysis of the xenograft experiments, control mice (treated with resiquimod and either stromal supernatant or IL-6) were paired with mice from the same experiment that were also treated with actemra. The average of the differences in splenic CLL cell numbers for each pair was tested for significance with a 2-sided paired Student t test for 20 df.21
Results
Derivation of a CLL splenic stromal line
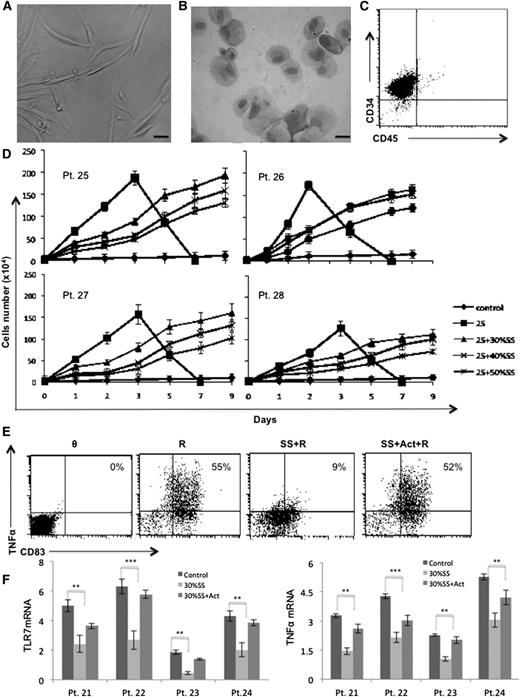
As described in “Methods,” a stromal line was derived from the spleen of patient #79 that was removed for symptom relief.15 The cells appeared spindle-shaped and epithelioid by light-microscopy (Figure 1A-B). They did not express CD45, consistent with a nonhematopoietic origin, but did express low levels of the endothelial marker CD34 (Figure 1C).22 Similar lines were made from two other spleens.
Effect of stromal factors on TLR7-signaling responses. (A) Morphology of CLL splenic stromal cells are shown by direct light microscopy or (B) following May-Grünwald/Giemsa-staining of a cytospin preparation. Scale bars, 1 mm. (C) Flow cytometry indicate the cells do not express the hematopoietic marker CD45. (D) 104 CLL cells were cultured alone or with IL-2 and resiquimod in the indicated concentrations of supernatant from confluent stromal cells cultured in AIM-V for 48 hours. Viable cells were counted daily for 9 days by trypan-blue exclusion. Averages and standard errors of 3 separate measurements are shown for 4 different patient samples. (E-F) CLL cells were cultured overnight in the presence or absence of 30% conditioned media with or without the IL-6 receptor antibody actemra (15 μg/ml) and then stimulated with resiquimod (1 μg/mL). Membrane TNF-α and CD83 expression were measured 4 hours later by flow cytometry. An example for patient #22 is shown (E) with similar results obtained for 8 other patient samples. Numbers in the top right quadrants indicate percentages of mTNF-α+CD83+ cells. (F) Before resiquimod activation, TLR7 (left panel) and TNFA (right panel) transcripts were measured by PCR and normalized to HPRT. Averages and standard errors of results from 3 independent measurements for 4 different patient samples are shown. **P < .01; ***P < .001. Act, actemra; Pt, patient; R, resiquimod; SS, stromal supernatant.
Effect of stromal factors on TLR7-signaling responses. (A) Morphology of CLL splenic stromal cells are shown by direct light microscopy or (B) following May-Grünwald/Giemsa-staining of a cytospin preparation. Scale bars, 1 mm. (C) Flow cytometry indicate the cells do not express the hematopoietic marker CD45. (D) 104 CLL cells were cultured alone or with IL-2 and resiquimod in the indicated concentrations of supernatant from confluent stromal cells cultured in AIM-V for 48 hours. Viable cells were counted daily for 9 days by trypan-blue exclusion. Averages and standard errors of 3 separate measurements are shown for 4 different patient samples. (E-F) CLL cells were cultured overnight in the presence or absence of 30% conditioned media with or without the IL-6 receptor antibody actemra (15 μg/ml) and then stimulated with resiquimod (1 μg/mL). Membrane TNF-α and CD83 expression were measured 4 hours later by flow cytometry. An example for patient #22 is shown (E) with similar results obtained for 8 other patient samples. Numbers in the top right quadrants indicate percentages of mTNF-α+CD83+ cells. (F) Before resiquimod activation, TLR7 (left panel) and TNFA (right panel) transcripts were measured by PCR and normalized to HPRT. Averages and standard errors of results from 3 independent measurements for 4 different patient samples are shown. **P < .01; ***P < .001. Act, actemra; Pt, patient; R, resiquimod; SS, stromal supernatant.
Inhibition of TLR7 signaling by splenic stromal factors
To model PCs in vitro, circulating CLL cells were cultured with IL-2 and resiquimod (called “2S” in the figures) to represent factors encountered by CLL cells in this compartment.16 Splenic stromal cells were incorporated into the model by coculturing them with CLL cells. Without stromal cells, TLR7-activated CLL cells proliferated rapidly for a few days and then died (Figure 1D). Coculture on confluent stromal cells slowed the proliferation of activated CLL cells and allowed them to grow for a longer time (Figure 1D). CLL cells did not adhere to stromal cells, suggesting that the outcome of TLR7-signaling was being influenced by stromal cell secreted factors.
Conditioned media was then prepared from stromal cells as described in “Methods” and TLR7-signaling was assessed with a previously described flow cytometric assay.8 TLR7 activates multiple signaling pathways, especially NF-κB, that culminate in the expression of genes such as TNFA and CD83.6,7 Surface membrane TNF-α and CD83 levels can then be used to indicate effective TLR7-signaling. Overnight culture of CLL cells in stromal cell conditioned media significantly inhibited TLR7-signaling in this assay (Figure 1E, middle panels), associated with decreased TLR7 and TNFA messenger RNA (mRNA) expression (Figure 1F). Inhibition of TLR7-mediated phosphorylation of a number of important intermediates in the NF-κB pathway, including IKKα, IκB, p65 NF-κB, and IRAK1, was confirmed by immunoblotting (supplemental Figure 1). The inability to make TNF-α in response to TLR-agonists coupled to decreased TLR expression is called “tolerization.”8
Stromal cell-derived IL-6 accounts for TLR7 tolerization
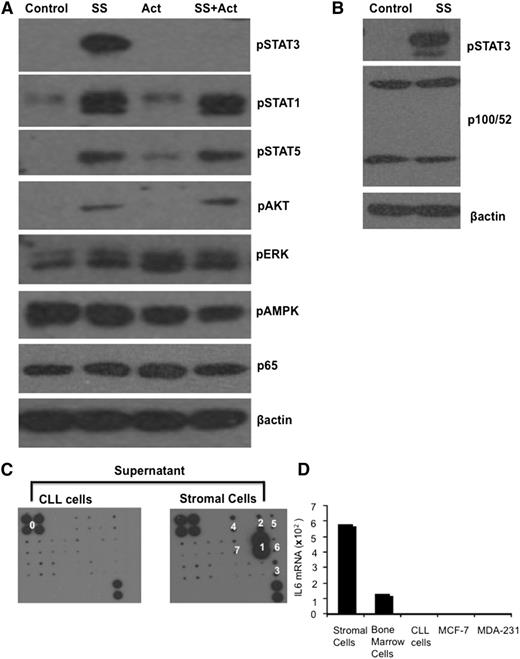
Signaling pathways activated in CLL cells by stromal cell conditioned media were studied to indicate the factor(s) affecting TLR7 responses. For example, B-cell activating factor (BAFF) is made by supporting cells in the CLL microenvironment and promotes tumor progression.23 BAFF activates the noncanonical NF-κB pathway, indicated by cleavage of p100 and the appearance of p52 in the p52/RelB active heterodimer. CLL cells treated with the stromal supernatant did not show increased expression levels of p52 (Figure 2B), excluding a role for BAFF in this system. In contrast, immunoblotting revealed robust activation of STAT3 in CLL cells by stromal factors (Figure 2A-B). Evidence for phosphorylation of STAT1, STAT5, and AKT was also obtained (Figure 2A), but the importance of STAT3 in modulating TLR responses7,16 prompted efforts to identify the STAT3-activating factor in the stromal supernatant.
IL-6 production by stromal cells. (A-B) Purified CLL cells were treated with stromal cell conditioned media (final concentration 30%) in the presence or absence of actemra for 4 hours. Levels of the indicated phosphorylated and native signaling molecules, along with p65 and p100/52 were then measured in whole cell lysates by immunoblotting with β-actin as a loading control. (C) Conditioned media from stromal cells and primary CLL cells were incubated with the custom antibody microarray described in “Methods.” Stromal cells made IL-6, granulocyte macrophage colony-stimulating factor, vascular endothelial growth factor, Axl, growth hormone, IL-9, and IL-11 (marked 1, 2, 3, 4, 5, 6, and 7, respectively) with IL-6 produced in highest amounts. The upper left four spots and lower right two spots (marked ‘0”) are positive controls. (D) IL-6 mRNA was measured by real-time PCR in the stromal line, a BM stromal cell line from another CLL patient (BS), primary CLL cells, and MCF-7 and MDA-231 breast cancer cells. Splenic stromal cells expressed high levels of IL-6 compared with these cells.
IL-6 production by stromal cells. (A-B) Purified CLL cells were treated with stromal cell conditioned media (final concentration 30%) in the presence or absence of actemra for 4 hours. Levels of the indicated phosphorylated and native signaling molecules, along with p65 and p100/52 were then measured in whole cell lysates by immunoblotting with β-actin as a loading control. (C) Conditioned media from stromal cells and primary CLL cells were incubated with the custom antibody microarray described in “Methods.” Stromal cells made IL-6, granulocyte macrophage colony-stimulating factor, vascular endothelial growth factor, Axl, growth hormone, IL-9, and IL-11 (marked 1, 2, 3, 4, 5, 6, and 7, respectively) with IL-6 produced in highest amounts. The upper left four spots and lower right two spots (marked ‘0”) are positive controls. (D) IL-6 mRNA was measured by real-time PCR in the stromal line, a BM stromal cell line from another CLL patient (BS), primary CLL cells, and MCF-7 and MDA-231 breast cancer cells. Splenic stromal cells expressed high levels of IL-6 compared with these cells.
Integrin receptors, RTKs, or Janus kinases (JAKs) can phosphorylate STAT3.24 Integrin receptor signaling was not considered due to the absence of adhesive phenomena in vitro. RTK ligands did not have a major role as indicated by the phosphoproteome array described in “Methods” (not shown). To identify responsible cytokines, a custom antibody array was designed to measure 21 different STAT3-activating cytokines and growth factors. The array revealed low granulocyte macrophage colony-stimulating factor, vascular endothelial growth factor, Axl, growth hormone, IL-9, and IL-11 but high IL-6 production by the stromal cells (Figure 2C). IL-6 mRNA levels in splenic stromal cells were higher than in a BM stromal cell line from another patient, primary CLL cells, and breast cancer cells used as controls (Figure 2D).
IL-6 tolerized CLL cells directly (Figure 3A-C and supplemental Figure 2) and inhibited proliferative responses to resiquimod (Figure 3D) in a dose-dependent manner with a concentration of 100 ng/mL used for most experiments. Importantly, IL-6 receptor blocking antibodies restored TLR7 and TNFA expression along with TLR7-mediated production of TNF-α and proliferation by CLL cells in the presence of splenic stromal cell conditioned media or IL-6 (Figure 1E-F and Figure 3A-D). The small molecule JAK inhibitors ruxolitinib and tofacitinib, which block IL-6–mediated STAT3 activation,25 gave similar results (supplemental Figure 2). Changes in proliferative responses were confirmed by tritiated thymidine incorporation, as well as manual cell counting (Figure 3E and supplemental Figure 4C). Taken together, the results indicated that TLR7-signaling responses in CLL cells were inhibited by IL-6 from stromal cells.
Effect of IL-6 on responses of CLL cells to resiquimod. (A) Purified CLL cells (2 × 106 cells/ml) were cultured overnight alone or with IL-6 (100 ng/ml) with or without actemra (15 μg/ml). Some cells were then stimulated with resiquimod and membrane TNF-α, and CD83 expression determined by flow cytometry after 4 hours. An example for patient #9 is shown with similar results obtained for 6 other samples. Numbers in the top right quadrants indicate percentages of mTNF-α+CD83+ cells. (B-C)TLR7 (B) and TNFA (C) transcript levels were determined in the remaining cells by real-time PCR and normalized to HPRT transcripts. The average and standard deviation (SD) of 4 independent experiments for 4 different patient samples are shown. (D-E) Purified CLL cells (104 cells) were cultured alone or with IL-2 and resiquimod in the presence or absence of IL-6 or stromal supernatant with or without actemra or ruxolitinib. Viable cells were counted after 8 to 12 days (D). [3H]thymidine uptake was measured after 48 hours (E). Averages and standard errors from triplicate wells or 3 separate cell counts are shown for 4 different patient samples. IL-6 tolerized CLL cells to TLR7 agonists and slowed the growth of activated CLL cells. *P < .05; **P < .01; ***P < .001. Ru, ruxolitinib.
Effect of IL-6 on responses of CLL cells to resiquimod. (A) Purified CLL cells (2 × 106 cells/ml) were cultured overnight alone or with IL-6 (100 ng/ml) with or without actemra (15 μg/ml). Some cells were then stimulated with resiquimod and membrane TNF-α, and CD83 expression determined by flow cytometry after 4 hours. An example for patient #9 is shown with similar results obtained for 6 other samples. Numbers in the top right quadrants indicate percentages of mTNF-α+CD83+ cells. (B-C)TLR7 (B) and TNFA (C) transcript levels were determined in the remaining cells by real-time PCR and normalized to HPRT transcripts. The average and standard deviation (SD) of 4 independent experiments for 4 different patient samples are shown. (D-E) Purified CLL cells (104 cells) were cultured alone or with IL-2 and resiquimod in the presence or absence of IL-6 or stromal supernatant with or without actemra or ruxolitinib. Viable cells were counted after 8 to 12 days (D). [3H]thymidine uptake was measured after 48 hours (E). Averages and standard errors from triplicate wells or 3 separate cell counts are shown for 4 different patient samples. IL-6 tolerized CLL cells to TLR7 agonists and slowed the growth of activated CLL cells. *P < .05; **P < .01; ***P < .001. Ru, ruxolitinib.
TLR9 is also expressed by CLL cells and mediates TNF-α production.5,6 To determine the effect of IL-6 on TLR9 responses, CLL cells were cultured overnight with IL-6 and then stimulated with CpG oligonucleotides (supplemental Figure 3). Like TLR7, TLR9 responses were also tolerized by IL-6.
The outcomes of cytokine- and TLR-signaling responses are related to underlying biological properties of CLL cells.4,26 However, no obvious correlation was seen between the ability of IL-6 to tolerize TLR7 responses and clinical patient characteristics (supplemental Table 1). CLL cells with 17 deletions and loss of p53 function exhibit especially aberrant responses to cytokines and TLR ligands.4,27 We specifically looked at 17p-deleted patient samples (patients #87 to #90; supplemental Table 1) and found IL-6 could tolerize TLR7 responses in this group (Figure 3E and supplemental Figure 4).
Stimulatory effect of IL-6 blockade on TLR-driven responses of CLL cells in vivo
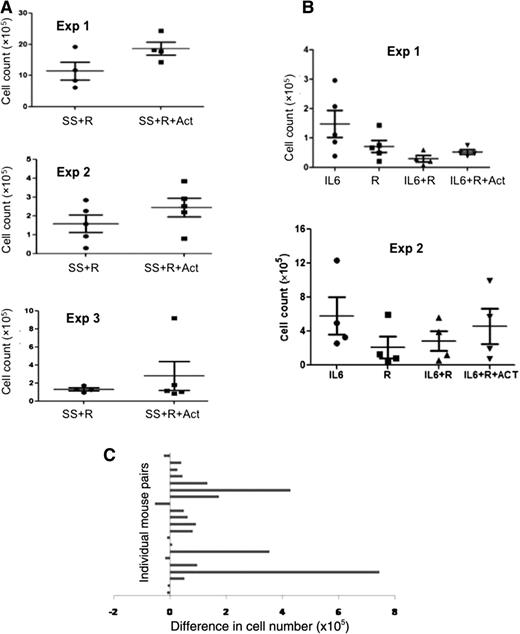
The above results suggested that IL-6 is made by stromal cells and inhibits TLR7-mediated proliferation of CLL cells in vitro, which can be reversed by blockade of IL-6 signaling. To study these effects in vivo, NOD-SCIDγcnull mice were injected with PBMCs from CLL patients followed by resiquimod. To model the stromal compartment of PCs that is missing in PBMCs, mice were also injected with splenic stromal cell conditioned media with or without actemra as described in “Methods.” Human CD5+CD19+ CLL cells in mouse spleens were counted after 7 days. The results from 3 experiments with different patient samples indicated actemra increased CLL cell numbers in response to TLR7 stimulation (Figure 4A).
Effect of IL-6–signaling inhibitors on TLR7-mediated growth of CLL cells in vivo. (A) Immunodeficient mice were engrafted with 5 × 107 PBMCs, given bi-weekly injections of splenic stromal cell conditioned media (ie, SS) (1 ml) or (B) daily injections of IL-6, treated with resiquimod (100 μg) with or without actemra (100 μg), and euthanized 1 week later. CLL cells in spleens were calculated from total cell counts in a hemocytometer and percentages of human CD5+CD19+ cells measured by flow cytometry. The results of separate experiments using PBMCs from different patients are shown. (C) Paired differences in splenic CLL cell numbers with and without actemra are shown for all experiments.
Effect of IL-6–signaling inhibitors on TLR7-mediated growth of CLL cells in vivo. (A) Immunodeficient mice were engrafted with 5 × 107 PBMCs, given bi-weekly injections of splenic stromal cell conditioned media (ie, SS) (1 ml) or (B) daily injections of IL-6, treated with resiquimod (100 μg) with or without actemra (100 μg), and euthanized 1 week later. CLL cells in spleens were calculated from total cell counts in a hemocytometer and percentages of human CD5+CD19+ cells measured by flow cytometry. The results of separate experiments using PBMCs from different patients are shown. (C) Paired differences in splenic CLL cell numbers with and without actemra are shown for all experiments.
Similar experiments were carried out with 2 other patient samples using IL-6 in place of stromal cell conditioned media (Figure 4B). Note that CLL cells alone do not engraft well in immunodeficient mice,15 so control groups without concomitant IL-6 and/or resiquimod were not included. IL-6 in the absence of TLR signals yielded the greatest engraftment of CLL cells, whereas IL-6 in combination with resiquimod produced low-level engraftment. Again, actemra increased CLL cell numbers in response to TLR7 stimulation in the presence of IL-6 (Figure 4B).
Differences between splenic CLL cell numbers in pairs of mice treated with and without actemra are detailed in Figure 4C and used to show the differences were highly significant (P < .01). Although it is unclear if the higher numbers of CLL cells are caused by increased local proliferation, altered homing, or greater survival, the data are consistent with the idea that TLR responses in vivo are inhibited by IL-6, resulting in decreased engraftment.
Effect of IL-6 on sensitivity of TLR7-activated CLL cells to cytotoxic agents
We had previously found that TLR7-tolerized CLL cells were more sensitive to killing by cytotoxic drugs and immune effector cells.7,8 Because IL-6 could prevent TLR7-mediated TNF-α production, we wondered if it might interfere with sensitization to cytotoxic agents. Accordingly, CLL cells were treated with IL-2 and resiquimod with or without IL-6 for 2 days and then exposed to vincristine or short-term SEA-reactive T-cell lines as cytotoxic effectors. IL-6 actually enhanced the in vitro sensitivity of activated CLL cells to these agents. Importantly, blockade of IL-6 signaling with actemra or ruxolitinib strongly inhibited the killing of TLR7-activated CLL cells (supplemental Figure 5).
Regulation of TLR7-signaling pathway components by miR-17 and miR-19a
A number of previously described mechanisms might account for the inhibition of TLR7 signaling by IL-6. IL-6 can induce IL-10, a potent inhibitor of TLR signaling and TNF-α production,25 but anti–IL-10 antibodies did not prevent TLR7 tolerization by the stromal cell conditioned media (not shown). IL-6 can induce suppressor of cytokine signaling proteins, which inhibit signaling through TLRs.28 However, no convincing evidence for suppressor of cytokine signaling 1 to 3 induction by stromal factors was observed by RT-PCR (not shown).
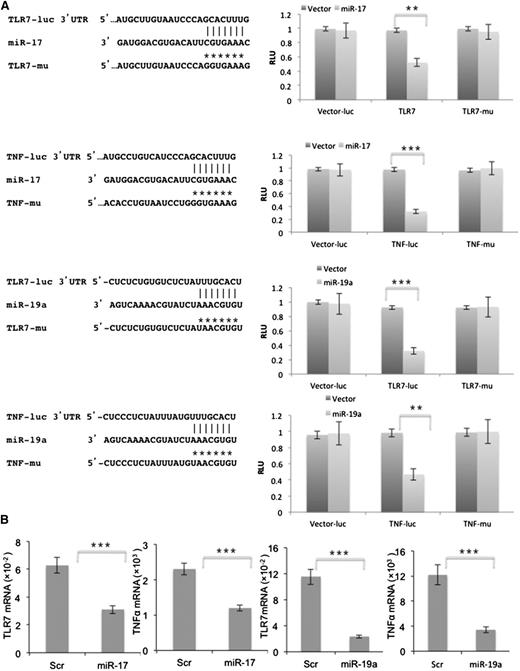
TLR7 tolerization is associated with downregulation of TLR7 and TNFA mRNA (Figure 1F)8 and miRs are important regulators of gene expression.29 IL-6 might then inhibit TLR7 signaling through miR. miRs are 22 nucleotide noncoding RNA molecules that bind to seed sequences in 3′-UTRs of mRNAs, preventing their transcription in ribosomes.30 In silico analyses indicated potential seed sequences for both miR-17 and miR-19a in the 3′-UTRs of TNFA and TLR7 (Figure 5A). These miRs are members of the miR-17-92 cluster on chromosome 13.29,30 Luciferase assays with NIH-3T3 cells transiently transfected with reporter genes containing these seed sequences, along with miR-17 (Figure 5A, top 2 rows) or miR-19a (Figure 5A, bottom 2 rows) expression vectors were consistent with regulation of TLR7 and TNFA by miR-17 and miR-19a. Mutation of the binding sites for miR-17 and miR-19a in TLR7 and TNFA restored luciferase activity (Figure 5A).
TLR7 and TNFA are miR-17 and miR-19a targets. (A) Luciferase assays were performed with reporter vectors containing native and mutated TLR7 (first and third rows) and TNFA (second and fourth rows) 3′UTRs after transient transfection into NIH-3T3 cells stably expressing miR-17 (top 2 rows) or miR-19a (bottom 2 rows). Shown on the left are schematic representations of the TLR7 and TNFA 3′UTR sequences containing potential miR-17 and miR-19a binding sites. Positions of the mutations are marked as *’s and the corresponding constructs are called TLR7-μ and TNF-μ. Luciferase activity is presented relative to that obtained with transfection of the control vector. (B) Splenic stromal cells were transfected with pGFP-C-lenti-miR-17 (left panels) or pGFP-C-lenti-miR-19a (right panels) viruses. Endogenous TLR7 and TNFA levels, measured by real-time PCR after 48 hours and normalized to HPRT, were significantly downregulated compared with cells transfected with the scrambled control. The average and SD of 3 separate experiments are shown. **P < .01; ***P < .001. RLU, relative luciferase units; Scr, scrambled control.
TLR7 and TNFA are miR-17 and miR-19a targets. (A) Luciferase assays were performed with reporter vectors containing native and mutated TLR7 (first and third rows) and TNFA (second and fourth rows) 3′UTRs after transient transfection into NIH-3T3 cells stably expressing miR-17 (top 2 rows) or miR-19a (bottom 2 rows). Shown on the left are schematic representations of the TLR7 and TNFA 3′UTR sequences containing potential miR-17 and miR-19a binding sites. Positions of the mutations are marked as *’s and the corresponding constructs are called TLR7-μ and TNF-μ. Luciferase activity is presented relative to that obtained with transfection of the control vector. (B) Splenic stromal cells were transfected with pGFP-C-lenti-miR-17 (left panels) or pGFP-C-lenti-miR-19a (right panels) viruses. Endogenous TLR7 and TNFA levels, measured by real-time PCR after 48 hours and normalized to HPRT, were significantly downregulated compared with cells transfected with the scrambled control. The average and SD of 3 separate experiments are shown. **P < .01; ***P < .001. RLU, relative luciferase units; Scr, scrambled control.
Further support for regulation of TNFA and TLR7 by miR-17 and miR-19a was provided by transient transfection of miR-17 and miR-19a expression vectors into splenic stromal cells (Figure 5B). The low levels of endogenous TLR7 mRNA in these cells were decreased further after transfection with miR-17 (Figure 5B, left graph) or miR-19a (Figure 5B, third graph from left) vectors. Baseline TNFA expression was higher than TLR7 in stromal cells and was also lowered when intracellular miR-17 and miR-19a were increased by transfection (Figure 5B, second and fourth graphs from left).
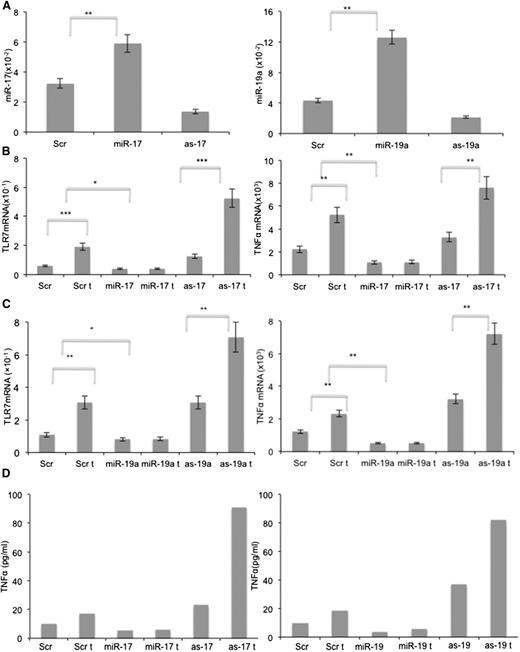
Splenic stromal cells were also transfected with expression vectors containing antisense sequences of miR-17 and miR-19 to neutralize endogenous levels of these miRs (Figure 6A, left and right panels). The low levels of endogenous TLR7 (Figure 5B) precluded direct stimulation of splenic stromal cells with resiquimod. However, IFNs increase both TLR7 and TNF-α expression.31 Therefore, splenic stromal cells engineered to overexpress miR-17 and miR-19a by transfection with the respective expression vectors, or with decreased expression of these miRs by transfection with the antisense vectors, were treated with IFN-α2B. IFN-treatment (called “t” in the x-axes) increased TLR7 and TNFA mRNA expression after 24 hours by two to threefold (Figure 6B-C). Both miR-17 and miR-19a prevented these increases, whereas antisense miR-17 and miR-19a increased baseline expression of TLR7 and TNFA mRNA as well as the magnitude of the increase from IFN (Figure 6A-B). Changes in TNFA mRNA were reflected in TNF-α protein levels in culture supernatants (Figure 6D). Taken together, these results suggested miR-17 and miR-19a can lower TLR7 and TNFA expression and inhibit TLR7-signaling responses.
Downregulation of TLR7 and TNFA by miR-17-92. Splenic stromal cells were transfected with pGFP-C-lenti-miR-17, pGFP-C-lenti-miR-19a, pGFP-C-shlenti-antimiR-17, or pGFP-C-shlenti-antimiR-19 vectors and stimulated with medium or IFN-α (1000 U/ml) for 24 hours. TLR7 and TNFA expression were then measured by RT-PCR. (A) Quantification of miR-17 and miR-19a confirmed increased expression in transfected miR-17 and miR-19a cell lines and decreased expression in transfected as-miR-17 and as-miR-19a cells compared with control cells. (B) Increased miR-17 and (C) miR-19a levels inhibited IFN-α–mediated induction of TLR7 (B-C; left panels) and TNFA (B-C; right panels) mRNA, whereas the antisense inhibitors had opposite effects. The average and SD of the results of 3 independent measurements are shown. (D) TNF-α proteins in 24-hour culture supernatants were measured by enzyme-linked immunosorbent assay. *P < .05, **P < .01, ***P < .001.
Downregulation of TLR7 and TNFA by miR-17-92. Splenic stromal cells were transfected with pGFP-C-lenti-miR-17, pGFP-C-lenti-miR-19a, pGFP-C-shlenti-antimiR-17, or pGFP-C-shlenti-antimiR-19 vectors and stimulated with medium or IFN-α (1000 U/ml) for 24 hours. TLR7 and TNFA expression were then measured by RT-PCR. (A) Quantification of miR-17 and miR-19a confirmed increased expression in transfected miR-17 and miR-19a cell lines and decreased expression in transfected as-miR-17 and as-miR-19a cells compared with control cells. (B) Increased miR-17 and (C) miR-19a levels inhibited IFN-α–mediated induction of TLR7 (B-C; left panels) and TNFA (B-C; right panels) mRNA, whereas the antisense inhibitors had opposite effects. The average and SD of the results of 3 independent measurements are shown. (D) TNF-α proteins in 24-hour culture supernatants were measured by enzyme-linked immunosorbent assay. *P < .05, **P < .01, ***P < .001.
Increased miR-17 and miR-19a expression in CLL cells by IL-6 via STAT3
IL-6 increases miR-17-92 expression in pulmonary endothelial cells and lung cancer cells through STAT3.32-34 Negative regulation of TLR7 signaling in CLL cells by IL-6 might then result from STAT3 activation and increased expression of the miR-17-92 cluster. Consistent with this idea, IL-6 phosphorylated STAT3 on tyrosine 705 in CLL cells (supplemental Figure 6A, left panel) and increased expression of each member of the cluster (supplemental Figure 6A, right panel). These changes were reversed by actemra (supplemental Figure 6A).
To demonstrate a connection between IL-6–mediated STAT3 activation and miR-17-92 cluster transcription, CLL cells were infected with a lentivirus expressing shRNA to downregulate STAT3 protein levels (supplemental Figure 6B, left panel). All members of the miR-17-92 cluster increased following stimulation with IL-6 in CLL cells transfected with a scramble control but not in STAT3–shRNA-transfected cells (supplemental Figure 6B, right panel).
A 33 bp region in the promoter of the miR-17-92 contains a predicted STAT3 binding site (supplemental Figure 6C, top panel).32 We confirmed that treatment with IL-6 of NIH-3T3 cells transfected 48 hours earlier with a luciferase construct driven by the miR-17-92 promoter containing this site resulted in increased fluorescence. Inactivation of this site by site-directed mutagenesis reversed responsiveness to IL-6 (supplemental Figure 6C). The results suggested strong control of miR-17-92 by IL-6 through STAT3.
Modulation of TLR signaling in primary CLL cells by genetic manipulation of miR-17 and miR-19a
If IL-6 acts through STAT3 to increase miR-17-92 expression and downregulate TLR7 and TNFA mRNA, then overexpression of the miR-17-92 cluster in CLL cells should inhibit TLR7-signaling responses directly, which should be restored by neutralizing miR-17 and/or miR-19a. To address this, lentiviruses expressing the entire miR-17-92 cluster, antisense miR-17, and antisense miR-19a were constructed as described in “Methods.” Overexpression of miR-17-92 in CLL cells significantly decreased TLR7 and TNFA mRNA expression within 12 hours, compared with cells infected with the scrambled control (Figure 7A, left panels). Conversely, infection with viral vectors expressing antisense miR-17 and miR-19a increased TNFA and TLR7 expression (Figure 7A, right panels).
Effect of miR-17 and miR-19a on TLR7 responses in primary CLL cells. (A) CLL cells were infected with lentiviruses expressing a scrambled vector control, the miR-17-92 cluster or antisense miR-17 and miR-19a. TNFA (upper panels) and TLR7 (lower panels) were measured by RT-PCR after 12 hours. Results for 4 patient samples are shown and indicate suppression of TLR7 and TNFA expression by miR-17 and miR-19a in primary leukemia cells. (B-C) Infected cells were treated with resiquimod (1 μg/ml) and mTNF-α and CD83 expression determined by flow cytometry after 4 hours. Numbers in the right upper quadrants indicate percentages of mTNF-α+CD83+ cells (B). Results for 4 patient samples are shown (C). Overexpression of miR-17-92 mimics the tolerizing effect of stromal factors and blockade of miR-17, miR-19a, or both, restores the responsiveness to TLR7-agonists in the presence of stromal factors. (D) Schematic diagram showing the effect of IL-6 on TLR7 signaling in a leukemia microenvironment. ***P < .05.
Effect of miR-17 and miR-19a on TLR7 responses in primary CLL cells. (A) CLL cells were infected with lentiviruses expressing a scrambled vector control, the miR-17-92 cluster or antisense miR-17 and miR-19a. TNFA (upper panels) and TLR7 (lower panels) were measured by RT-PCR after 12 hours. Results for 4 patient samples are shown and indicate suppression of TLR7 and TNFA expression by miR-17 and miR-19a in primary leukemia cells. (B-C) Infected cells were treated with resiquimod (1 μg/ml) and mTNF-α and CD83 expression determined by flow cytometry after 4 hours. Numbers in the right upper quadrants indicate percentages of mTNF-α+CD83+ cells (B). Results for 4 patient samples are shown (C). Overexpression of miR-17-92 mimics the tolerizing effect of stromal factors and blockade of miR-17, miR-19a, or both, restores the responsiveness to TLR7-agonists in the presence of stromal factors. (D) Schematic diagram showing the effect of IL-6 on TLR7 signaling in a leukemia microenvironment. ***P < .05.
Consistent with these results, CLL cells infected with miR-17-92–expressing lentiviruses exhibited decreased responses to resiquimod (Figure 7B, top row, compare first two dot-plots on the left). Overnight incubation in splenic stromal cell conditioned media impaired responsiveness to resiquimod in this assay as before (Figure 7B, compare left dot-plots in the top and bottom rows), which was exacerbated by overexpression of miR-17-92 (Figure 7B, bottom row, compare first two dot-plots on the left). However, infection of CLL cells with viruses expressing antisense mRNA to miR-17, miR-19a, or both, restored TLR-signaling responses regardless of the presence of stromal factors (Figure 7B, bottom row). Similar results were seen with 3 other patient samples (Figure 7C).
Discussion
This study lends insight into the interactions between immunoreceptor signals in a human cancer microenvironment. The results suggest (1) stromal cells in secondary lymphoid organs make IL-6, which induces a state of tolerance to TLR7 agonists in CLL cells characterized by downregulated TLR7 and TNFA mRNA expression (Figures 1, 2, and 7D); (2) seed sequences for miR-17 and miR-19a in the 3′ UTRs of TLR7 and TNFA are involved in transcriptional regulation of these genes (Figures 5 and 6); (3) IL-6 increases miR-17-92 cluster expression through a STAT3 promoter element (supplemental Figure 6); (4) increased expression of the cluster downregulates TLR7 and TNFA, which can be prevented by neutralizing miR-17 and/or miR-19a (Figure 7); and (5) inhibition of IL-6 signaling, by preventing IL-6 from binding to its receptor or blocking downstream signaling from the receptor, enhances proliferative responses of CLL cells to TLR7 agonists in vitro and in vivo (Figures 3 and 4).
Tolerization of TLR signaling in CLL cells by IL-6 appears to be a general phenomenon that is unrelated to underlying clinical properties of the leukemia cells. Immunoglobulin heavy chain variable mutational status was not available for our studies but CLL cells with 17p-deletions that are particularly sensitive to cytokine and TLR signals4,27 also exhibited IL-6–mediated TLR7-tolerization (supplemental Figure 4). A small subgroup of CLL patients have mutations in TLR signaling genes including MYD88, associated with evidence for activation of the NF-κB pathway,35 and subset #4 CLL cells with stereotyped immunoglobulin heavy chain variable 4 to 34 B-cell receptors exhibit innate tolerance to TLR7 agonists.36 Because these CLL subtypes may already have TLR-signaling pathways occupied, they might be expected to not be tolerized by IL-6. However, the numbers of patients with such phenotypes (2% to 4% and 1%, respectively)35,36 are small and would not be apparent in the sample size used for these experiments.
The conclusions of these studies followed from the finding that high levels of IL-6 were made by a stromal cell line derived from the spleen of a CLL patient. Although IL-6 was not measured directly in splenic microenvironments, high IL-6 levels, which could originate from sources other than stromal cells, are found in the serum of CLL patients and correlate with more aggressive disease.37 Immunohistochemistry also detects IL-6 in most CLL lymph nodes.38 Evidence that CLL cells encounter IL-6 in PCs is suggested by microarray studies that demonstrate increased expression of the IL-6 signature gene IL4RA by recent emigrants to the circulation.39,40 Published gene-array data also suggest TLR7 is decreased when CLL cells enter lymph nodes41 (supplemental Figure 7).
The miR-17-92 cluster on chromosome 13q31 is important in many cancers, including CLL.29,30 It is often upregulated by chromosomal amplification or Myc-driven gene transcription, and generally activates PI3K and NF-κB signaling pathways to promote tumor progression.42 However, individual members of the cluster may have distinct functions and we showed miR-17 has tumor-suppressive properties in certain contexts.29 Here, we suggest that IL-6–mediated induction of miR-17 and miR-19a may slow CLL progression by downregulating expression of TLR7 and TNFA in CLL cells and inhibiting their proliferative responses to endogenous TLR7 ligands. This mechanism adds to previous mechanisms of TLR tolerization in leukemia cells.8 Interestingly, IL-6 has been shown before to inhibit proliferation of CLL cells in response to TNF-α but a mechanism was not provided.43
IL-6 is traditionally considered a pro-inflammatory cytokine but it exhibits context-dependent immunoregulatory properties.40 Our findings suggest IL-6 may contribute to immune suppression in a tumor microenvironment by downregulating the ability of cells to respond to TLR7 agonists. Similarly, IL-6 and STAT3 are usually considered oncogenic proteins24,44 but they have tumor suppressor properties in some contexts.45 Consistent with this idea, short-term engraftment of CLL cells was enhanced by IL-6 in the absence of exogenous TLR signaling (Figure 4B), suggesting a tumor-promoting effect of IL-6 in this model. However, TLR7 and TLR9 signaling are involved in the pathogenesis of progressive CLL.5,6 Our results with exogenous TLR agonists suggest IL-6 is a tumor suppressor in this case, by slowing leukemia progression driven by endogenous TLR7 and TLR9 signaling. Strategies to counter IL-6 signaling with blocking antibodies or signal transduction inhibitors are currently being explored as cancer treatments46 but may also have context-dependent outcomes. If IL-6 has a tumor-suppressing effect, then simply blocking IL-6 or STAT3 activation may lead to increased tumor growth and progression. However, the Bruton tyrosine kinase inhibitor ibrutinib has been shown recently to inhibit TLR-mediated proliferation of CLL cells in the presence of stromal cells.47 If JAK inhibitors like ruxolitinib allow enhanced TLR signaling, then the combination of ruxolitinib and ibrutinib may turn out to have complementary and synergistic activity in the treatment of CLL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (MOP130479) (D.E.S.) and (110952) (D.E.S. and Y.B-.D.), and the Canadian Cancer Society Research Institute (701630) (D.E.S.).
Authorship
Contribution: Y.L. and D.E.S. conceived the project and wrote the manuscript; F.Z. and R.G. performed in vivo mouse studies; G.S.D. performed tritiated thymidine uptake experiments; B.Y. provided miR reagents; Y.S., L.M.C., and Y.-J.L., obtained CLL cells, and performed cell culture and molecular biology studies; and Y.S., L.M.C., Y.B-.D., B.Y., and R.G. helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David E. Spaner, Biology Platform, S-116A, Research Building, Sunnybrook Research Institute, 2075 Bayview Ave, Toronto, Ontario M4N 3M5, Canada; e-mail: spanerd@sri.utoronto.ca.

![Figure 3. Effect of IL-6 on responses of CLL cells to resiquimod. (A) Purified CLL cells (2 × 106 cells/ml) were cultured overnight alone or with IL-6 (100 ng/ml) with or without actemra (15 μg/ml). Some cells were then stimulated with resiquimod and membrane TNF-α, and CD83 expression determined by flow cytometry after 4 hours. An example for patient #9 is shown with similar results obtained for 6 other samples. Numbers in the top right quadrants indicate percentages of mTNF-α+CD83+ cells. (B-C) TLR7 (B) and TNFA (C) transcript levels were determined in the remaining cells by real-time PCR and normalized to HPRT transcripts. The average and standard deviation (SD) of 4 independent experiments for 4 different patient samples are shown. (D-E) Purified CLL cells (104 cells) were cultured alone or with IL-2 and resiquimod in the presence or absence of IL-6 or stromal supernatant with or without actemra or ruxolitinib. Viable cells were counted after 8 to 12 days (D). [3H]thymidine uptake was measured after 48 hours (E). Averages and standard errors from triplicate wells or 3 separate cell counts are shown for 4 different patient samples. IL-6 tolerized CLL cells to TLR7 agonists and slowed the growth of activated CLL cells. *P < .05; **P < .01; ***P < .001. Ru, ruxolitinib.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/6/10.1182_blood-2014-12-618678/4/m_766f3.jpeg?Expires=1769824590&Signature=YOTr32fqvmIu56f3un7GHRq66x7MjKUQPYM-zi6xdtpau6a7VXQD0MWxPl~BVdQfl0VRZL2c8kbPMn~VztJZgVsnM-2olLdMQF7Jwj7B8lcmSf7k23RA2zIZX7bn98eRmQ6NzqD00C2vBe2X8zKJyxWAk3f2bkgOHytRLFekN-d6d1WzHgVm~AYm30emQNXrGont1jS~-0hGaZexpWkWKmzbRRhNLPEThUzSmR-kl3XGerb87I~NwuSCedXI63T2QkFn5XqVeB85~mIAXXrNuuWqwOZiSnDltHvi7-ccAWcEi-wD-XjnmqNlm0h8~JzzbK0U0X7LSaksLkKnEKmzQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)