Key Points
Clinical and biological features of therapy-related and de novo NPM1-mutated AML overlap.
Therapy-related NPM1-mutated AML most likely represents a de novo disease with a coincidental previous history of cytotoxic therapy.
Abstract
NPM1-mutated acute myeloid leukemia (AML) shows unique features. However, the characteristics of “therapy-related” NPM1-mutated AML (t-NPM1 AML) are poorly understood. We compared the genetics, transcriptional profile, and clinical outcomes of t-NPM1 AML, de novo NPM1-mutated AML (dn-NPM1 AML), and therapy-related AML (t-AML) with wild-type NPM1 (t-AML). Normal karyotype was more frequent in t-NPM1 AML (n = 78/96, 88%) and dn-NPM1 (n = 1986/2394, 88%) than in t-AML (n = 103/390, 28%; P < .001). DNMT3A and TET2 were mutated in 43% and 40% of t-NPM1 AML (n = 107), similar to dn-NPM1 (n = 88, 48% and 30%; P > 0.1), but more frequently than t-AML (n = 162; 14% and 10%; P < 0.001). Often mutated in t-AML, TP53 and PPM1D were wild-type in 97% and 96% of t-NPM1 AML, respectively. t-NPM1 and dn-NPM1 AML were transcriptionally similar, (including HOX genes upregulation). At 62 months of median follow-up, the 3-year overall survival (OS) for t-NPM1 AML (n = 96), dn-NPM1 AML (n = 2394), and t-AML (n = 390) were 54%, 60%, and 31%, respectively. In multivariable analysis, OS was similar for the NPM1-mutated groups (hazard ratio [HR] 0.9; 95% confidence interval [CI], 0.65-1.25; P = .45), but better in t-NPM1 AML than in t-AML (HR, 1.86; 95% CI, 1.30-2.68; P < .001). Relapse-free survival was similar between t-NPM1 and dn-NPM1 AML (HR, 1.02; 95% CI, 0.72-1.467; P = .90), but significantly higher in t-NPM1 AML versus t-AML (HR, 1.77; 95% CI, 1.19-2.64; P = .0045). t-NPM1 and dn-NPM1 AML have overlapping features, suggesting that they should be classified as a single disease entity.
Introduction
NPM1-mutated acute myeloid leukemia (AML)1 accounts for about 30% to 35% of all adult AML and is recognized by the World Health Organization (WHO) 2016 classification of lympho-hematopoietic tumors as a distinct leukemia entity with unique biological and clinico-pathological features.2 These include frequent normal karyotype,1 aberrant cytoplasmic localization of nucleophosmin,1 high expression of HOX genes,3,4 negativity/low expression of CD34,1 a distinct microRNA profile,5 high stability of NPM1 mutations at relapse (allowing them to be used as tracks of measurable residual disease),6 good response to chemotherapy and favorable outcome in the absence of FLT3-ITD.2,7
NPM1-mutated AML is closely associated with de novo origin.1 However, about 14% to 16% of therapy-related AML, as defined by a history of exposure to chemotherapy and/or radiotherapy,8-10 carry NPM1 mutations.11,12 These cases frequently harbor a normal karyotype and DNMT3A mutations11,13 and are rarely associated with chromosome aberrations typical of t-AML (eg, 7q-/-7),11 paralleling what is observed in de novo NPM1-mutated AML (dn-NPM1 AML).14 Cases of NPM1-mutated AML occurring after chemotherapy for previous lymphoid malignancies appear to arise from a background of DNMT3A- or TET2-driven clonal hematopoiesis (CH) rather than being the direct result of cytotoxic therapy,15,16 although it is difficult to formally exclude any influence of therapy on CH dynamics. Together, these findings raise the question of whether, in at least a proportion of cases, “therapy-related” NPM1-mutated AML (t-NPM1 AML) may represent de novo leukemia.11,17 However, this issue remains unresolved because of the few cases analyzed to date and the lack of information on the biology and outcome of t-NPM1 AML.11,13,18 Indeed, before recent updates,19,20 t-NPM1 AML was classified as t-AML rather than NPM1-mutated AML,21 with important clinical implications.
Here, we address this issue by comparing the karyotype, mutational landscape, transcriptional profile, and clinical outcome of a large series of t-NPM1 AML cases with those of dn-NPM1 AML and therapy-related AML with NPM1 wild-type (t-AML). Our results provide evidence that the biological and clinical features of t-NPM1 and dn-NPM1 AML overlap closely, suggesting a common disease entity.
Methods
Patients
Patients were identified from the UK National Cancer Research Institute AML17 trial, Toulouse-Bordeaux AML database (DATAML), Study Alliance Leukemia AML registry, and Munich Leukemia Laboratory/MLL (data extracted on/before 21 November 2021). Individual data were collected for patients with either NPM1-mutated AML or therapy-related AML, according to WHO 2016 criteria.21 Patients were excluded from the clinical analysis if they had acute promyelocytic leukemia, did not receive 7+3-like regimens containing anthracycline and cytarabine (with/without additional agents), or had no follow-up information (supplemental Figure 1, available on the Blood website). Cytogenetic results were assigned to risk categories according to Medical Research Council (MRC) criteria.22 Genetic risk stratification of NPM1-mutated AML by FLT3-ITD mutation and its allelic ratio (based on standard DNA fragment length analysis) was according to European Leukemia Net (ELN) 2017.23 Based on NPM1 mutation status and prior exposure to chemotherapy and/or radiotherapy, patients were divided into 3 groups: dn-NPM1 AML, t-NPM1 AML, and t-AML. This study was conducted following the Declaration of Helsinki. All patients signed informed consent forms related to their respective clinical trial.
Targeted DNA sequencing
Genomic DNA from all t-NPM1 AML cases with available materials was analyzed, including patients not receiving intensive chemotherapy. The cohort significantly overlapped, but was not identical to, patients with t-NPM1 AML in the clinical analysis. A total of 107 samples, including 12 from the UK National Cancer Research Institute AML17, 29 from DATAML, 40 from Study Alliance Leukemia, 17 from MLL, and 9 from Italy (including 2 previously published cases15,16), were subjected to targeted sequencing of genes recurrently mutated in myeloid neoplasms (supplemental Methods). As comparator groups, additional cases from MLL (dn-NPM1 AML, n = 88; t-AML, n = 163) were investigated for the most relevant gene mutations (listed in Table 1 and included in all targeted sequencing panels that were used – supplemental Table 1).
Genes mutated by targeted next generation sequencing (NGS) in greater than or equal to 5% of t-NPM1 AML and/or t-AML cases, in comparison to dn-NPM1 AML
| Gene . | t-NPM1, % (mutant/wild-type cases) . | t-AML, % (mutant/wild-type cases) . | P value∗ t-NPM1 vs t-AML . | dn-NPM1 AML, % (mutant/wild-type cases) . | P value∗ dn-NPM1 AML vs t-AML . |
|---|---|---|---|---|---|
| DNMT3A | 43 (46/107) | 13 (22/163) | <.0001 | 48 (42/88) | .56 |
| TET2 | 40 (43/107) | 11 (18/163) | <.0001 | 30 (26/88) | .134 |
| FLT3 | |||||
| Indel† | 16 (17/107) | 8 (12/158) | .044 | 30 (26/88) | .025 |
| TK | 16 (17/107) | 3 (4/127) | .0009 | 20 (17/86) | .569 |
| IDH2 | 19 (20/107) | 8 (13/156) | .0145 | 18 (16/88) | 1 |
| PTPN11 | 18 (19/107) | 0 (0/100) | <.0001 | 9 (8/88) | .097 |
| IDH1 | 15 (16/107) | 7 (10/155) | .034 | 7 (6/86) | .11 |
| SRSF2 | 10 (11/107) | 7 (9/134) | .35 | 7 (6/88) | .45 |
| NRAS | 8 (9/107) | 10 (15/153) | .83 | 10 (9/87) | .80 |
| KRAS | 7 (7/107) | 7 (10/153) | 1 | 2 (2/86) | .30 |
| WT1 | 6 (6/107) | 5 (6/119) | 1 | 6 (5/85) | 1 |
| STAG2 | 5 (5/107) | 8 (5/66) | .508 | 6 (5/81) | .748 |
| ASXL1 | 4 (4/107) | 11 (18/163) | .04 | 2 (2/88) | .69 |
| PPM1D | 4 (4/107) | 5 (3/60) | .70 | 1 (1/88) | .38 |
| BCOR | 3 (3/107) | 7 (8/120) | .224 | 2 (2/86) | 1 |
| TP53 | 3 (3/107) | 26 (42/161) | <.0001 | 0 (0/88) | .253 |
| RUNX1 | 1 (1/107) | 12 (19/154) | .0005 | 0 (0/88) | 1 |
| Gene . | t-NPM1, % (mutant/wild-type cases) . | t-AML, % (mutant/wild-type cases) . | P value∗ t-NPM1 vs t-AML . | dn-NPM1 AML, % (mutant/wild-type cases) . | P value∗ dn-NPM1 AML vs t-AML . |
|---|---|---|---|---|---|
| DNMT3A | 43 (46/107) | 13 (22/163) | <.0001 | 48 (42/88) | .56 |
| TET2 | 40 (43/107) | 11 (18/163) | <.0001 | 30 (26/88) | .134 |
| FLT3 | |||||
| Indel† | 16 (17/107) | 8 (12/158) | .044 | 30 (26/88) | .025 |
| TK | 16 (17/107) | 3 (4/127) | .0009 | 20 (17/86) | .569 |
| IDH2 | 19 (20/107) | 8 (13/156) | .0145 | 18 (16/88) | 1 |
| PTPN11 | 18 (19/107) | 0 (0/100) | <.0001 | 9 (8/88) | .097 |
| IDH1 | 15 (16/107) | 7 (10/155) | .034 | 7 (6/86) | .11 |
| SRSF2 | 10 (11/107) | 7 (9/134) | .35 | 7 (6/88) | .45 |
| NRAS | 8 (9/107) | 10 (15/153) | .83 | 10 (9/87) | .80 |
| KRAS | 7 (7/107) | 7 (10/153) | 1 | 2 (2/86) | .30 |
| WT1 | 6 (6/107) | 5 (6/119) | 1 | 6 (5/85) | 1 |
| STAG2 | 5 (5/107) | 8 (5/66) | .508 | 6 (5/81) | .748 |
| ASXL1 | 4 (4/107) | 11 (18/163) | .04 | 2 (2/88) | .69 |
| PPM1D | 4 (4/107) | 5 (3/60) | .70 | 1 (1/88) | .38 |
| BCOR | 3 (3/107) | 7 (8/120) | .224 | 2 (2/86) | 1 |
| TP53 | 3 (3/107) | 26 (42/161) | <.0001 | 0 (0/88) | .253 |
| RUNX1 | 1 (1/107) | 12 (19/154) | .0005 | 0 (0/88) | 1 |
TK, tyrosine-kinase domain mutations.
By Fisher exact test.
Insertions or deletions of ≥1 amino acid by standard variant callers (not specifically developed for FLT3-ITD; see for the latter gold-standard data by capillary electrophoresis in Table 2).
Whole transcriptome sequencing analysis
Samples for RNA-seq were identified from the MLL and 96 samples were analyzed (17 t-NPM1 AML, 16 dn-NPM1 AML, 14 t-AML, and an additional 49 de novo AML with wild-type NPM1). For this analysis, only patients from the MLL were used to minimize differences in specimen collection, processing, and storage, which could have a significant impact on the transcriptome results. Library preparation and data processing are described in the supplemental Methods.
Immunohistochemistry
Bone marrow (BM) paraffin sections were deparaffinized, antigen-unmasked (EDTA pH 9.0, 10 minutes), and immunostained in Leica Bond III using a monoclonal antibody (Clone 376) recognizing both the wild-type and mutant NPM1 proteins1 (10 minutes), followed by post primary Alkaline Phosphatase (10 minutes) and polymer Alkaline Phosphatase (25 minutes).The reaction was developed using Mixed Red Refine (10 minutes) followed by hematoxylin counterstaining (5 minutes).
Skin paraffin sections were subjected to antigen unmasking in EDTA pH 9.0 (40 minutes) and immunostained in a Leica Bond III using a monoclonal antibody specific for the NPM1 mutant (generated by V.A. and B.T.G., Bergen, Norway), followed by postprimary antibody (15 minutes) and peroxidase polymer (15 minutes). The reaction was developed using Mixed DAB Refine (10 minutes) followed by hematoxylin counterstaining (5 minutes).
Statistical analysis
Statistical comparisons were performed between the t-NPM1 AML group and each of the other 2 groups for patients who met the criteria for clinical analysis (supplemental Figure 1). Baseline variables were compared using Fisher exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. The median follow-up time was calculated using the reverse Kaplan-Meier method.24 Response criteria and outcome measures, including overall survival (OS) and relapse-free survival (RFS), were defined as per ELN 2017.23 OS and RFS were calculated using the Kaplan-Meier method. The cumulative incidence of relapse (CIR) and death in remission were measured from the time of achieving complete remission (CR), with each as a competing risk for the other.25 All survival probabilities were reported at 3 years. Multivariable mixed-effects Cox regression models were constructed to assess the impact of the prognostic factors on OS and RFS. To account for potential heterogeneity between cohorts, a cohort-specific random effects term was included. Similar models were constructed for day 60 mortality, CR, CIR, and death in remission using logistic regression and competing risk regression,26 respectively. The potential impact of the treatment era (supplemental Table 4) was accounted for by including the year of diagnosis in all the regression models. No imputations were performed for missing data. All odds and hazard ratios [HR] are listed with the t-NPM1 AML group as the reference. As there were 2 between-group comparisons made for each parameter, the alpha level for the assessment of significance was adjusted to 0.05 ÷ 2 = 0.025.
Results
Genetics
dn-NPM1 AML frequently carries a normal karyotype and comutations of other genes, especially FLT3, DNMT3A, and TET2,1,27 whereas t-AML mostly harbors an abnormal karyotype and mutations of genes associated with its pathogenesis, including TP5328 and PPM1D.29 Therefore, we compared the cytogenetic and mutational landscape of dn-NPM1 AML, t-NPM1 AML, and t-AML.
t-NPM1 and dn-NPM1 AML showed the same high frequency of normal karyotype (88% of 96 t-NPM1 cases; 88% of 2394 dn-NPM1 cases), unlike t-AML (28% of 390 cases; P < .001). Conversely, t-AML often carried complex or monosomal karyotypes (22% and 15%, respectively), which are rare in t-NPM1 AML (2.3% and 1.1%, respectively; P < .001) (Table 2).
Baseline characteristics, early mortality, achievement of CR/CRi, and timing of allogeneic transplant, by disease group
| Number∗ . | De novo (dn) NPM1 AML . | t-NPM1 AML . | t-AML . | P value . | |
|---|---|---|---|---|---|
| 2394 . | 96 . | 390 . | t-NPM1 AML vs dn-NPM1 AML . | t-NPM1 AML vs t-AML . | |
| Age, median (IQR) | 56.00 (47.00-64.00) | 65.00 (58.00-69.25) | 60.00 (52.00-67.00) | <.001 | <.001 |
| Female (%) | 1291 (54%) | 62 (65%) | 251 (64%) | .047 | >.99 |
| Previous therapy | NA | <.001 | |||
| Chemotherapy ± radiotherapy | NA | 55 (57%) | 312 (80%) | ||
| Radiotherapy alone | NA | 41 (43%) | 78 (20%) | ||
| Indication for cytotoxic therapy | NA | .18 | |||
| Hematological | NA | 17 (18%) | 89 (23%) | ||
| Solid tumor | NA | 63 (66%) | 254 (65%) | ||
| Nonmalignant | NA | 9 (9.4%) | 16 (4.1%) | ||
| Unknown | NA | 7 (7.3%) | 31 (7.9%) | ||
| Latency between chemo/radiotherapy and AML, median years (IQR) | NA | 5.00 (3.00-10.00) | 4.00 (2.00-9.00) | NA | .34 |
| Year of diagnosis | .90 | .32 | |||
| Before 2011 | 1300 (54%) | 52 (54%) | 204 (52%) | ||
| 2011-2015 | 706 (29%) | 27 (28%) | 135 (35%) | ||
| After 2015 | 388 (16%) | 17 (18%) | 51 (13%) | ||
| Blood counts at diagnosis | |||||
| WCC, median (IQR) | 28.40 (8.00-70.22) | 31.95 (10.03-63.60) | 4.82 (2.00-22.01) | .67 | <.001 |
| Missing (n) | 22 | 5 | 8 | ||
| Platelets, median (IQR) | 63.00 (37.00-111.00) | 63.00 (36.75-118.50) | 46.50 (25.00-90.00) | .92 | .012 |
| Missing (n) | 38 | 7 | 10 | ||
| BM blast %, median (IQR) | 74.00 (50.00-89.00) | 76.00 (54.50-86.00) | 50.00 (30.75-79.00) | .69 | <.001 |
| Missing (n) | 175 | 9 | 27 | ||
| Karyotype | |||||
| Normal karyotype | 1986 (88%) | 78 (88%) | 103 (28%) | .51 | <.001 |
| Favorable | 0 (0%) | 0 (0%) | 45 (12%) | ||
| Other intermediate | 254 (11%) | 9 (10%) | 87 (24%) | ||
| Adverse | 28 (1.2%) | 2 (2.3%) | 131 (36%) | ||
| Missing (n) | 126 | 7 | 24 | ||
| Chromosomal abnormalities | |||||
| (−5) / del(5q) | 1 (<0.1%) | 0 (0%) | 36 (9.8%) | >.99 | <.001 |
| (−7) | 1 (<0.1%) | 0 (0%) | 32 (8.7%) | >.99 | .002 |
| (+8) | 102 (4.5%) | 3 (3.4%) | 42 (11%) | >.99 | .027 |
| (−17)/17p abnormality | 3 (0.1%) | 1 (1.1%) | 22 (6.0%) | .14 | .10 |
| inv(16) | 0 (0%) | 0 (0%) | 21 (5.7%) | NA | .019 |
| (t8;21) | 0 (0%) | 0 (0%) | 24 (6.6%) | NA | .007 |
| t(9;11) | 0 (0%) | 0 (0%) | 32 (8.8%) | NA | .002 |
| t(v;11) | 1 (<0.1%) | 0 (0%) | 28 (7.7%) | >.99 | .005 |
| Complex | 17 (0.7%) | 2 (2.3%) | 79 (22%) | .15 | <.001 |
| Monosomal | 2 (<0.1%) | 1 (1.1%) | 54 (15%) | .11 | <.001 |
| FLT3-ITD (%) | 998 (42%) | 30 (32%) | 43 (12%) | .043 | <.001 |
| Missing (n) | 40 | 1 | 19 | ||
| FLT3-ITD allelic ratio | .98 | .75 | |||
| Low (<0.5) | 381 (38%) | 13 (43%) | 15 (35%) | ||
| High (≥0.5) | 502 (50%) | 17 (57%) | 23 (53%) | ||
| Missing (n) | 115 (12%) | 0 | 5 (12%) | ||
| ELN 2017 risk group∗ | .46 | <.001 | |||
| Favorable | 1635 (77%) | 70 (79%) | 45 (13%) | ||
| Intermediate | 470 (22%) | 17 (19%) | 163 (46%) | ||
| Adverse | 28 (1.3%) | 2 (2.3%) | 148 (42%) | ||
| Missing(n) | 261 | 7 | 34 | ||
| CR/CRi with chemotherapy | 89% | 80% | 70% | .006 | .001 |
| Refractory disease | 7.2% | 8.7% | 24% | ||
| Death before assessment | 3.8% | 11% | 5.9% | ||
| Day 60 mortality (95% CI) | 5% (4-6) | 15% (7-22) | 11% (8-14) | <.001 | .2 |
| Allogeneic transplant (%) | .19 | .10 | |||
| In first CR/CRi | 536 (22%) | 19 (20%) | 101 (26%) | ||
| At relapse or refractory | 375 (16%) | 10 (10%) | 62 (16%) | ||
| No transplant | 1483 (62%) | 67 (70%) | 227 (58%) | ||
| Number∗ . | De novo (dn) NPM1 AML . | t-NPM1 AML . | t-AML . | P value . | |
|---|---|---|---|---|---|
| 2394 . | 96 . | 390 . | t-NPM1 AML vs dn-NPM1 AML . | t-NPM1 AML vs t-AML . | |
| Age, median (IQR) | 56.00 (47.00-64.00) | 65.00 (58.00-69.25) | 60.00 (52.00-67.00) | <.001 | <.001 |
| Female (%) | 1291 (54%) | 62 (65%) | 251 (64%) | .047 | >.99 |
| Previous therapy | NA | <.001 | |||
| Chemotherapy ± radiotherapy | NA | 55 (57%) | 312 (80%) | ||
| Radiotherapy alone | NA | 41 (43%) | 78 (20%) | ||
| Indication for cytotoxic therapy | NA | .18 | |||
| Hematological | NA | 17 (18%) | 89 (23%) | ||
| Solid tumor | NA | 63 (66%) | 254 (65%) | ||
| Nonmalignant | NA | 9 (9.4%) | 16 (4.1%) | ||
| Unknown | NA | 7 (7.3%) | 31 (7.9%) | ||
| Latency between chemo/radiotherapy and AML, median years (IQR) | NA | 5.00 (3.00-10.00) | 4.00 (2.00-9.00) | NA | .34 |
| Year of diagnosis | .90 | .32 | |||
| Before 2011 | 1300 (54%) | 52 (54%) | 204 (52%) | ||
| 2011-2015 | 706 (29%) | 27 (28%) | 135 (35%) | ||
| After 2015 | 388 (16%) | 17 (18%) | 51 (13%) | ||
| Blood counts at diagnosis | |||||
| WCC, median (IQR) | 28.40 (8.00-70.22) | 31.95 (10.03-63.60) | 4.82 (2.00-22.01) | .67 | <.001 |
| Missing (n) | 22 | 5 | 8 | ||
| Platelets, median (IQR) | 63.00 (37.00-111.00) | 63.00 (36.75-118.50) | 46.50 (25.00-90.00) | .92 | .012 |
| Missing (n) | 38 | 7 | 10 | ||
| BM blast %, median (IQR) | 74.00 (50.00-89.00) | 76.00 (54.50-86.00) | 50.00 (30.75-79.00) | .69 | <.001 |
| Missing (n) | 175 | 9 | 27 | ||
| Karyotype | |||||
| Normal karyotype | 1986 (88%) | 78 (88%) | 103 (28%) | .51 | <.001 |
| Favorable | 0 (0%) | 0 (0%) | 45 (12%) | ||
| Other intermediate | 254 (11%) | 9 (10%) | 87 (24%) | ||
| Adverse | 28 (1.2%) | 2 (2.3%) | 131 (36%) | ||
| Missing (n) | 126 | 7 | 24 | ||
| Chromosomal abnormalities | |||||
| (−5) / del(5q) | 1 (<0.1%) | 0 (0%) | 36 (9.8%) | >.99 | <.001 |
| (−7) | 1 (<0.1%) | 0 (0%) | 32 (8.7%) | >.99 | .002 |
| (+8) | 102 (4.5%) | 3 (3.4%) | 42 (11%) | >.99 | .027 |
| (−17)/17p abnormality | 3 (0.1%) | 1 (1.1%) | 22 (6.0%) | .14 | .10 |
| inv(16) | 0 (0%) | 0 (0%) | 21 (5.7%) | NA | .019 |
| (t8;21) | 0 (0%) | 0 (0%) | 24 (6.6%) | NA | .007 |
| t(9;11) | 0 (0%) | 0 (0%) | 32 (8.8%) | NA | .002 |
| t(v;11) | 1 (<0.1%) | 0 (0%) | 28 (7.7%) | >.99 | .005 |
| Complex | 17 (0.7%) | 2 (2.3%) | 79 (22%) | .15 | <.001 |
| Monosomal | 2 (<0.1%) | 1 (1.1%) | 54 (15%) | .11 | <.001 |
| FLT3-ITD (%) | 998 (42%) | 30 (32%) | 43 (12%) | .043 | <.001 |
| Missing (n) | 40 | 1 | 19 | ||
| FLT3-ITD allelic ratio | .98 | .75 | |||
| Low (<0.5) | 381 (38%) | 13 (43%) | 15 (35%) | ||
| High (≥0.5) | 502 (50%) | 17 (57%) | 23 (53%) | ||
| Missing (n) | 115 (12%) | 0 | 5 (12%) | ||
| ELN 2017 risk group∗ | .46 | <.001 | |||
| Favorable | 1635 (77%) | 70 (79%) | 45 (13%) | ||
| Intermediate | 470 (22%) | 17 (19%) | 163 (46%) | ||
| Adverse | 28 (1.3%) | 2 (2.3%) | 148 (42%) | ||
| Missing(n) | 261 | 7 | 34 | ||
| CR/CRi with chemotherapy | 89% | 80% | 70% | .006 | .001 |
| Refractory disease | 7.2% | 8.7% | 24% | ||
| Death before assessment | 3.8% | 11% | 5.9% | ||
| Day 60 mortality (95% CI) | 5% (4-6) | 15% (7-22) | 11% (8-14) | <.001 | .2 |
| Allogeneic transplant (%) | .19 | .10 | |||
| In first CR/CRi | 536 (22%) | 19 (20%) | 101 (26%) | ||
| At relapse or refractory | 375 (16%) | 10 (10%) | 62 (16%) | ||
| No transplant | 1483 (62%) | 67 (70%) | 227 (58%) | ||
FLT3-ITD, internal tandem duplication of FLT3 as assessed by fragment length analysis; IQR, interquartile range; WCC, white cell count.
For the patients with t-AML (NPM1 wild-type), ELN risk was only assigned where full cytogenetic and mutation data was available. As comutations (beyond FLT3-ITD) do not alter the ELN 2017 risk category for those with an NPM1 mutation, the dn-NPM1 and t-NPM1 groups include patients with complete data for cytogenetics and FLT3-ITD allelic ratio
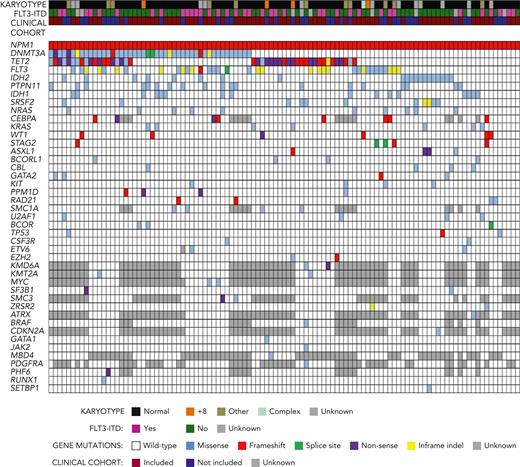
On targeted sequencing (supplemental Table 1), all 107 t-NPM1 AML showed NPM1 mutations: type A 78% (83/107), B 8% (9/107), and D 7% (7/107), similar to dn-NPM1AML.1 The most frequent comutated genes in t-NPM1 AML (Figure 1; Table 1 and supplemental Table 2) were DNMT3A (46/107, 43%), TET2 (43/107, 40%), FLT3 (34/107, 32%), IDH2 (20/107, 19%), PTPN11 (19/107, 18%), IDH1 (16/107, 15%), and SRSF2 (11/107, 10%), a pattern very similar to that of dn-NPM1 AML as reported in the literature,27 and as also observed here (with the exception of lower frequency of FLT3 indels; Tables 1 and 2). In particular, DNMT3A and TET2 mutations occurred in 43% and 40% of t-NPM1 AML cases, respectively, a frequency comparable to that of dn-NPM1 AML (48% and 30%, respectively, in 88 cases; P > .1) but significantly higher than that of t-AML (13% and 11%, respectively, in 163 cases; P < .001); DNMT3A and TET2 mutations also had similarly high variant allele frequencies (VAFs) in t-NPM1 and dn-NPM1 AML (medians: 45% and 43% for DNMT3A, respectively; 47% and 46% for TET2, respectively). The similar incidence and VAFs of DNMT3A and TET2 mutations in t-NPM1 AML and dn-NPM1 AML are consistent with the origin of DNMT3A- or TET2-mutant diagnostic AML clones (whether t-NPM1 or dn-NPM1) from a cell of a preleukemic DNMT3A- or TET2-mutant hematopoietic clone. The other genes with significantly more frequent mutations in t-NPM1 AML than in t-AML were FLT3, IDH2, PTPN11, and IDH1 (Table 1).
Myeloid gene comutations in t-NPM1 AML. 107 cases of t-NPM1 AML (columns) were subjected to targeted sequencing of genes recurrently mutated in myeloid neoplasms, which are shown in rows when mutated in at least 1 case. Above the gene mutation grid, each case is also annotated with karyotype, FLT3-ITD status as assessed by fragment length analysis, and inclusion in the clinical cohort. Color-coded figure keys are shown below the grid (regarding karyotype, cases denoted with “other” have chromosomal abnormalities other than those listed in Table 2).
Myeloid gene comutations in t-NPM1 AML. 107 cases of t-NPM1 AML (columns) were subjected to targeted sequencing of genes recurrently mutated in myeloid neoplasms, which are shown in rows when mutated in at least 1 case. Above the gene mutation grid, each case is also annotated with karyotype, FLT3-ITD status as assessed by fragment length analysis, and inclusion in the clinical cohort. Color-coded figure keys are shown below the grid (regarding karyotype, cases denoted with “other” have chromosomal abnormalities other than those listed in Table 2).
In contrast, genes typically mutated in t-AML, such as ASXL1, RUNX1, and TP53, were mutated in t-NPM1 AML at a significantly lower frequency, comparable to that of dn-NPM1 AML (Table 1). In particular, mutations in the DNA damage response genes TP53 and PPM1D, which are well known to be selected for by cytotoxic therapy, were rare in t-NPM1 AMLs, with only 3 cases (3%) showing a TP53 variant (1 likely being a benign polymorphism according to ClinVar) and 4 cases (4%) showing a PPM1D mutation (1 being a missense variant of unknown significance). The VAFs of TP53 mutations (52%-6%-68%) and PPM1D mutations (38%, 47%, 2%, 46%) were compatible with their occurrence within the NPM1-mutant clone (27%, 10%, and 3%, respectively, in the TP53-mutant cases; 34%, 23%, 35%, and 27%, respectively, in the PPM1D-mutant cases; supplemental Table 2), although we cannot exclude that the mutations with low VAF in TP53 (6%; n = 1 case) or in PPM1D (2%, n = 1 case) were present in a nonleukemic clone different from the leukemic clone harboring the NPM1 mutation (whose VAF was 10% and 35%, respectively).
Collectively, the cytogenetic and molecular data demonstrate that t-NPM1 AML shares the same genetic signature as dn-NPM1 AML, but lacks that of t-AML.
Transcriptome analysis
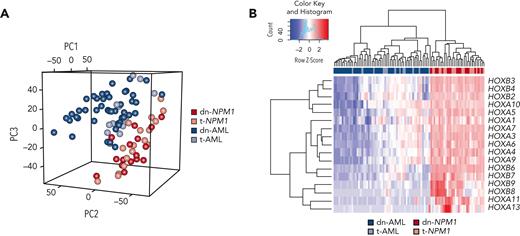
NPM1-mutated AML displays a unique gene expression profile, including overexpression of HOX genes (thought to maintain leukemic stemness3), which is associated to the cytoplasmic dislocation of nucleophosmin.4 Therefore, we compared the transcriptomes of t-NPM1 AML, dn-NPM1 AML, t-AML, and de novo AML with wild-type NPM1.
Unsupervised principal component analysis of the top 2066 most variable genes showed a clear separation between NPM1-mutated AML and AML with NPM1 wild-type, irrespective of the de novo or therapy-related origin (Figure 2A). Differential expression analysis identified 487 genes that were significantly altered (250 genes upregulated and 237 genes downregulated) between dn-NPM1 AML and dn-AML with NPM1 wild-type (supplemental Table 3). As many as 30% of these genes overlapped with those differentially expressed between t-NPM1 AML and t-AML (supplemental Table 3). Genes coding for CD133 and CD34 were commonly downregulated in NPM1-mutated AML (log2 fold change <−5, P < .0001), whereas multiple HOX genes displayed increased expression in NPM1-mutated AML (Figure 2B), diverging from AMLs with NPM1 wild-type. Moreover, dn-AML and t-AML were characterized by upregulation of MN1 and BAALC, as expected from their NPM1–wild-type status.30
Gene expression analysis of dn-NPM1 AML, t-NPM1 AML, dn-AML, and t-AML. (A) Principal component analysis of the most variable genes based on the median absolute deviation. (B) Gene expression of the most variable HOX genes.
Gene expression analysis of dn-NPM1 AML, t-NPM1 AML, dn-AML, and t-AML. (A) Principal component analysis of the most variable genes based on the median absolute deviation. (B) Gene expression of the most variable HOX genes.
The RNA-seq data demonstrated that t-NPM1 AML has a similar transcriptomic signature to that of dn-NPM1 AML, which is distinct from that of t-AML.
Immunohistochemistry
NPM1 mutated AML is typically characterized by the aberrant dislocation of mutant NPM1 from the nucleoli to the cytoplasm of leukemic cells.1 This is due to mutations occurring in the nucleolar localization signal and to the addition of a new nuclear export signal,31 and it is easily detectable by immunohistochemistry.1
Leukemic cells from 5 patients with t-NPM1 AML, for which BM (n = 5) and skin (n = 1) biopsies were available, showed typical cytoplasmic delocalization of nucleophosmin. This immunostaining pattern was identical to that observed in dn-NPM1 AML1 and clearly differed from the expected nucleus restricted expression of NPM1 in t-AML with wild-type NPM1 (not shown).1 Moreover, similarly to dn-NPM1 AML,1 t-NPM1 AML showed no/low expression of CD34. These results demonstrate that dn-NPM1 AML and t-NPM1 AML show the same immunohistochemical pattern, which is different from that of t-AML.
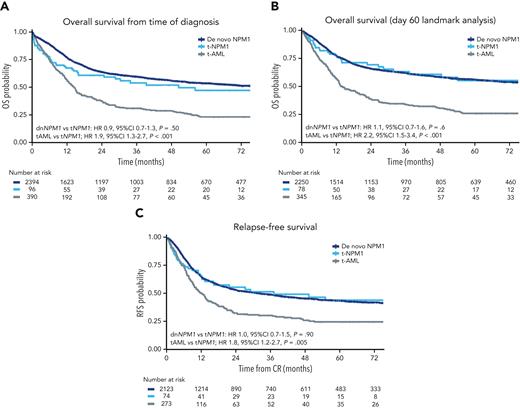
Survival curves. (A) OS from the time of diagnosis, by group. (B) OS, day 60 landmark analysis, by group. (C) RFS from the time of achieving CR.
Survival curves. (A) OS from the time of diagnosis, by group. (B) OS, day 60 landmark analysis, by group. (C) RFS from the time of achieving CR.
Clinical characteristics
A total of 2394 patients with dn-NPM1 AML, 96 with t-NPM1 AML, and 390 with t-AML who met the inclusion criteria were identified (supplemental Figure 1), spanning a period of AML diagnosis between 2005 to 2021 (supplemental Methods). The baseline characteristics are summarized in Table 2. The median follow- up was 62 months in the dn-NPM1 AML and 42 months and 54 months for the t-NPM1 and t-AML groups, respectively. The t-NPM1 AML group had baseline blood counts, BM blast percentages, and karyotypic abnormalities similar to those in the dn-NPM1 AML group; however, these were significantly different in the t-AML group. The median age (56 years) was lowest in patients with dn-NPM1 AML, higher in those with t-AML (60 years), and higher again in those with t-NPM1 AML (65 years, P < .001 for each comparison).
A history of prior chemotherapy (± radiotherapy) or radiotherapy alone was recorded in 57% and 43% of t-NPM1 AML cases and in 80% and 20% of t-AML cases, respectively (P < .001) (Table 2). Previously diagnosed neoplasms included solid tumors (66% of t-NPM1 AML and 65% of t-AML cases) or hematologic neoplasms (18% of t-NPM1 AML and 23% of t-AML cases) treated a median of 5 and 4 years before the onset of t-NPM1 AML and t-AML cases, respectively (interquartile range: 3-10 and 2-9, respectively), without major differences between t-NPM1 and t-AML (Table 2).
Early mortality was higher in the 2 treatment-related groups than in the dn-NPM1 AML (supplemental Table 5), with logistic regression demonstrating that both prior chemotherapy (± radiotherapy) and radiotherapy alone were associated with increased day 60 mortality (supplemental Table 6). As a consequence of higher early treatment-related deaths, patients with t-NPM1 AML had a lower CR/complete remission with incomplete hematologic recovery (CRi) rate than those with dn-NPM1 AML, although the rates of refractory disease were similar (Table 1). A multivariable analysis accounting for age, white blood cell and platelet counts, karyotype, and FLT3-ITD status showed no difference in the achievement of CR/CRi between the 2 NPM1-mutated groups (OR, 1.39; 95% confidence interval [CI], 0.75-2.59; P = .3, supplemental Table 7). Conversely, patients with t-NPM1 AML had a higher rate of CR/CRi than those with t-AML, which was maintained in the multivariable analysis (OR, 0.37; 95% CI, 0.19-0.75; P = .006) (supplemental Table 7).
The OS at 3 years for the t-NPM1 AML, dn-NPM1 AM L, and t-AML groups was 54% (95% CI 44-64), 60% (95% CI 57-62), and 31% (95% CI 26-37), respectively (supplemental Table 4). In multivariable analysis, survival was similar for the 2 NPM1 mutated AML groups (HR, 0.9; 95% CI, 0.65-1.25; P = .5) but better in t-NPM1 AML than in t-AML (HR, 1.86; 95% CI, 1.30-2.68; P < .001) (supplemental Table 7). Owing to the increased risk of early death during the first 60 days following induction chemotherapy, survival in the t-NPM1 AML group initially overlapped with that in the t-AML group (Figure 3A), but beyond this landmark became very similar to that in the dn-NPM1 AML group (Figure 3B), which was confirmed in a time-stratified regression model (supplemental Table 8).
RFS did not differ between the t-NPM1 AML and dn-NPM1 AML groups (HR, 1.02; 95% CI, 0.72-1.467; P = .90) but was significantly higher than that in the t-AML group (HR, 1.77; 95% CI, 1.19-2.64; P = .005) (Figure 3C; supplemental Table 6). The CIR at 3 years in the t-NPM1, dn-NPM1, and t-AML groups were 39%, 42%, and 51%, respectively, with competing risk regression demonstrating similar CIR for the t-NPM1 AML and dn-NPM1 AML groups, whereas the CIR was higher for t-AML. The presence of FLT3-ITD appeared to have an adverse impact on OS in the 2 NPM1 AML groups but not in the t-AML group, although it did not reach significance in the smaller t-NPM1 AML group (supplemental Figure 2; supplemental Table 9). For patients achieving CR, OS and RFS censored at allogeneic hematopoietic stem cell transplantation (allo-HSCT) in CR1 were not significantly different on multivariable analysis between the t-NPM1 and t-AML groups, which may be due to reduced numbers in each group from early censoring (supplemental Figure 3; supplemental Table 10).
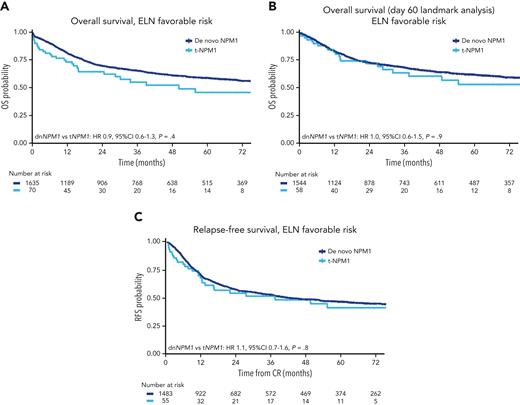
An analysis limited to patients with ELN 2017 favorable risk (NPM1 mutated, FLT3-ITD low or negative, nonadverse karyotype) showed no difference in OS or RFS between the t-NPM1 and dn-NPM1 groups (Figure 4; supplemental Table 11).
Survival curves of NPM1-mutated AML (de novo vs therapy-related) belonging to the ELN favorable risk (NPM1 mutated, FLT3-ITD low or negative, nonadverse karyotype).
Survival curves of NPM1-mutated AML (de novo vs therapy-related) belonging to the ELN favorable risk (NPM1 mutated, FLT3-ITD low or negative, nonadverse karyotype).
Finally, sensitivity analyses were performed by dividing the therapy-related groups into those with a history of chemotherapy (± radiotherapy) or radiotherapy alone. These results demonstrate a similar trend of better OS and RFS in the t-NPM1 AML group, particularly in the chemotherapy cohort. These differences were not significant because of the small number of samples analyzed (supplemental Figure 4).
Discussion
We demonstrated that t-NPM1 AML displays the same cytogenetics, mutational landscape, transcriptional profile, and clinical outcome as dn-NPM1 AML (with the exception of a slightly lower incidence of FLT3-ITD for unclear reasons); therefore, they should be considered a single disease entity. Although some role of cytotoxic drugs and/or radiotherapy in the development of t-NPM1 AML cannot be excluded, our findings support the interpretation that t-NPM1 AML represents a de novo leukemia or has a leukemogenic mechanism different from that of other t-AMLs.
The concept that t-NPM1 AML most likely represents de novo AML with a coincidental history of prior therapy is supported by several findings. Radiotherapy alone was much more common in the t-NPM1 AML group, with previous reports suggesting that t-AML/t-MDS arising after radiotherapy more closely resembles de novo disease.32 In this and other studies,11 the time between treatment and AML diagnosis was well outside the usual latency period for the development of t-AML, which occurred after >10 years in as many as 29% of cases. Moreover, there is no experimental evidence that chemoradiotherapy can induce mutations in NPM1.
It is unlikely that t-NPM1 AML was caused by chemoradiotherapy-driven selection and expansion of a pre-existing CH promoted by mutations in TP5333 or PPM1D,34,35 as has been reported in t-AML.28,36-39 In fact, TP53 or PPM1D mutations are detectable in ∼25% and up to 20% of t-AML, respectively,33,39 whereas they were found in only 3% and 4% of t-NPM1 AML, respectively. Conversely, t-NPM1 AML harbored TET2 and/or DNMT3A mutations at a similar frequency to dn-NPM1 AML, but significantly higher than t-AML with wild-type NPM1, and the allele load of these mutations was also similarly high in t-NPM1 AML and dn-NPM1 AML. Thus, we hypothesized that, similar to dn-NPM1 AML,2 t-NPM1 AML often develops when NPM1 mutations (gatekeeper mutations never detected in CH40) occur in the context of CH usually driven by TET2 and/or DNMT3A mutations.2,41 The transcriptional profile of t-NPM1 AML overlapped with that of dn-NPM1 AML and was distinct from t-AML, supporting the notion that the acquisition of an NPM1 mutation is the event driving the expression signature specific to this disease and that previous cytotoxic treatment does not seem to play the same pathogenetic role as in usual t-AMLs.
Along with the genomic and transcriptional findings, the clinical outcomes of t-NPM1 and dn-NPM1 AML were similar and better than those of the t-AML group. An increased early death rate was observed in both therapy-related groups, possibly related to their older age and the impact of previous cytotoxic therapies on their ability to tolerate intensive chemotherapy. However, OS in patients who survived for the first 60 days and RFS in patients who achieved CR were very similar between the 2 NPM1 AML groups. These findings suggest that, in patients considered fit, those with t-NPM1 should receive standard intensive chemotherapy, including an FLT3 inhibitor, where appropriate.
After remission, allo-HSCT is considered mandatory in patients with high-risk t-AML,42,43 whereas this issue in t-AML with a favorable karyotype (ie, RUNX1-RUNX1T112,44,45 or CBFB/MYH1112,46) remains controversial. Firm conclusions on the role of allo-HSCT in t-NPM1 AML cannot be made from our data, as the number of cases undergoing allogeneic stem cell transplantation in CR1 was small (n = 19) and selection biases are likely to be significant. However, with no difference in the relapse rate between t-NPM1 and dn-NPM1 AML cases, it may be suggested that in t-NPM1 AML belonging to the ELN favorable group, transplant decisions may be guided by molecular measurable disease (MRD) assessment, as in patients with dn-NPM1 AML.23 A similar favorable outcome of t-NPM1 and dn-NPM1 AML cases without FLT3-ITD (favorable risk) has been also recently observed in the cohort of patients from the Swedish registry.47
Finally, we predict that older patients with t-NPM1 AML who are not eligible for intensive chemotherapy will show the same good response to hypomethylating agents plus venetoclax, as observed in dn-NPM1 AML,7,48 and that in the future they could also benefit from menin49 and/or XPO1 inhibitors4 that are currently under development for dn-NPM1 AML.
Unlike the WHO 2016 classification of myeloid malignancies,21 which recommends the classification of patients with NPM1-mutated AML presenting with a previous history of chemotherapy and/or radiotherapy as t-AML rather than NPM1-mutated AML, the current 2022 International Consensus Classification20 and the fifth WHO classification of myeloid neoplasms19 both recommend classifying NPM1-mutated AML independent of the clinical history of the patient. Our clinical, molecular, and immunohistochemical analyses are the first to demonstrate that this interpretation is correct.
Our study has several limitations. A large proportion of the data are derived from real world registries, which are subject to selection bias and are limited by the quality and detail of the data entered. Information on the type and dose of previous chemotherapy and radiotherapy was unavailable for most patients. Importantly, molecular MRD data were not available for patients with NPM1 mutations, which would be useful for confirming that MRD in t-NPM1 AML has the same strong prognostic value as in dn-NPM1 AML. Additionally, very few patients in this study were treated with FLT3 inhibitors. Finally, as discussed above, we were not able to convincingly address the important issue of allo-HSCT in intermediate risk t-NPM1.
In conclusion, the overlapping features of t-NPM1 AML and dn-NPM1 AML suggest that they should be classified as a single disease entity and that t-NPM1 AML may in fact represent a biologically de novo leukemia.
Acknowledgments
The authors acknowledge Robert Hills, who provided statistical guidance.
This work has been supported by the European Research Council (ERC Adv grant 2016 no. 740230 to B.F. and ERC Cons grant 2016 no. 725725 to M.P.M.) and the Italian Association for Cancer Research (AIRC; grant no. 23604 to B.F. and grant Start-Up 2019 no. 22895 to L.B.), Blood Cancer UK, the UK National Institute of Health Research (ref. RP-PG-0108-10093), and Cancer Research UK (ref A29806 and grant no. CRUK/08/025), Haematology Society of Australia and New Zealand (J.O.) and RCPA Foundation (J.O.), the Norwegian Cancer Society (grants no. 303445 and 190175 to B.T.G.), the Trond Mohn Foundation (to V.A.).
Authorship
Contribution: B.F. had the original idea; B.F., N.R., and T.H. designed the study; J.O. analyzed all clinical data and carried out the statistical analysis; M.M. performed the RNA-Seq sequencing and analysis; E.T analyzed all mutational data and contributed to the design of the study; C.T, S.S., and A.V. performed the mutational analyses; R.S., M.B., H.S., C.M.-T., C.D.B. and C. Röllig provided the clinical data from the German Study Alliance Leukemia AML registry; R.D., A.F.G. and I.T. provided clinical data from the UK National Cancer Research Institute (NCRI) AML17 trial; C. Récher, S.B., P.Y.D, and A.P. provided clinical data from the French DATAML registry; M.T.V., E.D., A.B. and A.R. provided samples for mutational analysis; L.B. and V.M.P. were involved in immunohistochemical studies; M.P.M. contributed to data revision and critical discussion on data presentation; V.A. and B.T.G. provided the specific antibody against the NPM1 mutant; and B.F., N.R., T.H., J.O., M.M and E.T. wrote the manuscript with the input from all the other authors.
Conflict-of-interest disclosure: J.O. has no conflicts to declare. M.M. is employed by MLL Munich Leukemia Laboratory. E.T. declares no conflict of interest. C.T. is CEO and co-owner of AgenDix GmbH, received honoraria for scientific advisor and/or speakers bureau activities from Novartis, Jazz Pharmaceuticals, Astellas, Avencell. R.S. declares research support from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, PharmaMar, Roche, and consultancy with AbbVie, Astellas, Daiichi Sankyo, Jazz, Pfizer. R.D. declares research support from AbbVie and Amgen and consultancy with Astellas, Pfizer, Novartis, Jazz, Beigene, Shattuck, and AvenCell. S.S. and A.V. declares no conflict of interest. S.B. is advisor for AbbVie, Jazz Pharmaceuticals, Daiichi-Sankyo, Sanofi, Astellas, and BMS. E.D. declares no conflict of interest. P.Y.D. declares honoraria from Daiichi-Sankyo, Jazz Pharmaceutical, Astellas, AbbVie, Celgene, Janssen. A.P. declares grant/research support: Astellas, Roche; Speaker’s Bureau: Astellas, AbbVie, Gilead, Pfizer, Roche, Sanofi; Consultant: Jazz, AbbVie, Agios, BMS, Gilead, Novartis, Pfizer, Roche, and Takeda. A.B. is in the advisory boards for Daiichi-Sankyo and Novartis. A.F.G., I.T., and M.T.V. declares no conflict of interest. A.R. is consultant/advisor for Amgen, Omeros, Novartis, Astellas Pharma, Jazz Pharmaceuticals, F. Hoffmam La Roche, AbbVie, Janssen, Pfizer, Incyte, and Kite-Gilead. L.B. declares consultancy at scientific advisory boards for AbbVie and Amgen. V.P. and V.A. declares no conflict of interest. B.T.G. declares consultancy to BerGenBio, Novartis, Pfizer, and Sanofi Genzyme, received research support from Pfizer, and has stock ownership in Alden Cancer Therapy AS and KinN Therapeutics AS. M.P.M. declares honoraria from Rasna Therapeutics, Inc for scientific advisor activities and serves as consultant for scientific advisory boards of AbbVie, Amgen, Celgene, Janssen, Novartis, Pfizer, and Jazz Pharmaceuticals. C. Récher declares research grants from AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, Chugai, MaaT Pharma, Astellas, Roche, Daiichi-Sankyo and Iqvia; advisor for AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Pfizer, Roche, Servier, and Takeda. C. Röllig declares no conflict of interest. M.B. declares consultancy at advisory boards to Alexion, Jazz Pharmaceuticals, Merck as well as lecture honoraria from Jazz Pharmaceuticals, Novartis Celgene, and Amgen. H.S. declares consultancy and scientific support by Gilead and AbbVie. C.M.-T. and C.D.B. declares no conflict of interest. T.H. is part owner of MLL Munich Leukemia Laboratory. N.R. declares honoraria/ Consultancy, Pfizer, Astellas, AbbVie. Research Funding, Jazz Pharma, Pfizer. B.F. licensed a patent on NPM1 mutants (no. 102004901256449) and declares honoraria from Rasna Therapeutics, Inc for scientific advisor activities.
Correspondence: Brunangelo Falini, Section of Hematology, Department of Medicine and Surgery, University of Perugia, and Center for Hemato-Oncological Research (CREO), Santa Maria della Misericordia Hospital, Piazzale Menghini, 06132 Perugia, Italy; e-mail: brunangelo.falini@unipg.it.
References
Author notes
∗J.O., M.M., and E.T. contributed equally to this study.
†T.H., N.R., and B.F. are joint last authors.
RNA sequencing data have been deposited at the European Genome-phenome Archive (EGA) under accession number EGAS00001006835.
Data are available on request from the corresponding author, Brunangelo Falini (brunangelo.falini@unipg.it).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal