Key Points
A protein S-K196E mutation reduced its activated protein C cofactor activity in recombinant murine protein S-K196E and in K196E mutant mice.
Mice carrying a protein S-K196E mutation or heterozygous protein S deficiency were more vulnerable to venous thrombosis than wild-type mice.
Abstract
Protein S (PS) acts as an anticoagulant cofactor for activated protein C in regulation of blood coagulation. The K196E mutation in PS is a race-specific genetic risk factor for venous thromboembolism with a prevalence of ∼2% within the Japanese population. To evaluate the thrombosis risk of the PS-K196E mutation, we generated PS-K196E knockin mice and heterozygous PS-deficient mice. We analyzed their thrombotic states, comparing with mice carrying the factor V Leiden mutation (FV-R504Q), a race-specific genetic risk for venous thrombosis in whites. PS-K196E mice grew normally but had decreased activated protein C cofactor activity in plasma. Purified recombinant murine PS-K196E showed the same decreased activated protein C cofactor activity. A deep vein thrombosis model of electrolytic inferior vena cava injury and pulmonary embolism models induced by infusion of tissue factor or polyphosphates revealed that PS-K196E mice, heterozygous PS-deficient mice, and FV-R504Q mice were much more susceptible to venous thrombosis compared with wild-type mice. Transient middle cerebral artery ischemia-reperfusion injury model studies demonstrated that both PS-K196E mice and heterozygous PS-deficient mice had cerebral infarction similar to wild-type mice, consistent with human observations. Our in vitro and in vivo results support a causal relationship between the PS-K196E mutation and venous thrombosis and indicate that PS-K196E mice can provide an in vivo evaluation system to help uncovering racial differences in thrombotic diseases.
Introduction
Protein S (PS) is a vitamin K–dependent plasma glycoprotein that is mainly synthesized in liver and endothelial cells. PS acts as an anticoagulant cofactor for activated protein C (APC) in the proteolytic inactivation of factor Va (FVa) and factor VIIIa (FVIIIa) and negatively regulates blood coagulation.1,2 In human plasma, ∼60% of PS forms a complex with C4b binding protein (C4BP), and the formation of this complex abolishes the APC cofactor activity of PS. PS also acts as a cofactor for tissue factor pathway inhibitor during the inhibition of factor Xa (FXa) in human plasma, but this cofactor activity has not been detected in mouse plasma.3
PS deficiency is a risk factor for developing venous thromboembolism (VTE).4 Homozygous or compound heterozygous PS deficiency is an extremely rare disorder that causes purpura fulminans in affected newborns.5 Heterozygous PS deficiency is milder but firmly associated with an increased risk of VTE.4 The risk of thrombosis in individuals with PS deficiency is increased with other genetic or acquired factors predisposing to thrombosis.
A rare variant in the PS gene, PROS1, that is present in 1.8% of Japanese, which causes a Lys196 to Glu substitution in PS (PS-K196E, rs 121918474, c.586A>G, also known as PS Tokushima,6 PS-K155E in the mature protein numbering7 ) is a genetic risk factor for VTE in the Japanese population (odds ratio = 3.7-8.6).8-11 The K196E mutation in PS is located in the second epidermal growth factor (EGF)-like domain.2,7,9,11-14 We estimate that ∼1 out of every 12 000 Japanese individuals is homozygous for the 196E allele, representing a total of as many as 10 000 individuals.13 Thus, a substantial number of Japanese carry the mutant PS 196E allele, as a heterozygote or homozygote, and a likely risk of development of VTE. The phenotype-genotype analysis in individuals with PS-K196E mutation showed that heterozygotes had 16% lower PS anticoagulant activity than wild-type individuals, although the anticoagulant activities between heterozygotes and wild-types are substantially overlapped.12 PS-K196E mutation appears to be a genetic risk for deep vein thrombosis (DVT) during pregnancy,15 and PS-K196E mutation concomitant with other predisposing mutations for thrombosis was found in patients with VTE.16 This mutation, however, did not increase the risk for adverse pregnancy outcomes defined by 2 or more miscarriages, fetal growth restriction, and/or intrauterine fetal death.17 PS-K196E mutation seems to be Japanese specific, because the mutation has not been observed in the white, Chinese, or Korean populations.11,18,19
Racial differences in the genetic background of venous thrombosis have been well documented.20 As an example, the factor V (FV) Leiden mutation, R506Q, that is a VTE risk factor is predominantly found in white populations.21 This mutation renders FVa resistant to the inactivation by APC, resulting in a hypercoagulable state.22,23 A mouse model carrying the homologous murine FV Leiden mutation (R504Q) showed enhanced venous thrombosis, consistent with an increased incidence of VTE in human FV Leiden carriers.24,25 Thus, FV Leiden mice provide a valuable in vivo model of thrombosis-associated diseases in whites and have been studied under various stimulations or pathophysiologic conditions.25-27
Recent guidelines for establishing pathogenic causality of rare genetic variants emphasize that the strongest evidence for causality comes from disruption of the candidate gene in a model organism (eg, a mouse) that recapitulates the pathology in humans.20 Hence, we generated a mouse colony carrying the PS-K196E mutation. Based on PS-knockout (PS-KO) studies,3,28 homozygous PS deficiency in mice leads to embryonic lethality with a fulminant coagulopathy and hemorrhages. Heterozygous PS-deficient mice had reduced plasma PS antigen levels and APC cofactor activity, and they survived to adulthood but showed severe thrombosis after induction of pulmonary embolism (PE) by injection of tissue factor.
In the present study, we generated PS-K196E knockin mice and analyzed their phenotypes, comparing with heterozygous PS-deficient mice and FV-R504Q knock-in mice. Our data show that the PS-K196E mutation in mice increases the risk of thrombotic diseases, proving the pathogenic causality of this mutation beyond doubt. The PS K196E mice may be valuable for future pathogenic studies related to venous thrombosis.
Materials and methods
Generation of PS-K196E knockin mice and PS-KO mice, genetic analysis, and RNA analysis
The targeting vector was introduced into 129/SvEvTac-derived embryonic stem cells by electroporation.29-32 The male chimeras were bred to wild-type C57BL/6J females and F1 offspring with the PS-K196E mutation or PS deficiency were backcrossed to C57BL/6J mice (Japan SLC, Hamamatsu, Japan) for 10 generations. The detailed materials and methods are described in the supplemental Data (available on the Blood Web site). All animal procedures were approved by the Animal Care and Use Committee of the National Cerebral and Cardiovascular Center and performed in accordance with institutional and national guidelines and regulations.
Plasma APC cofactor activity and PS antigen assays
Blood was collected from anesthetized mice via the retro-orbital plexus into tubes containing a 0.1 volume of 3.8% sodium citrate. Plasma was prepared from blood by centrifugation at 1000g for 10 minutes.
Plasma APC cofactor activity was measured by an activated partial thromboplastin time (APTT)-based clotting assay using a recombinant mouse APC.33 Plasma samples (40 µL) were pretreated with 450 nM recombinant mouse APC or Owren-Koller buffer (10 µL) for 1 min at 37°C. STA-Cephascreen reagent (50 µL; Diagnostica Stago, Asnières-sur-Seine, France) was added and incubated for 3 min at 37°C. The clotting time was recorded using a coagulometer (STart4; Diagnostica Stago) after adding 25 mM CaCl2 (50 µL). The prolongation of clotting time by the addition of APC to wild-type plasma was considered as 100% APC cofactor activity.
Plasma PS antigens were measured by enzyme-linked immunosorbent assay using antibodies against human PS. Plasma samples in 0.5% bovine serum albumin were applied to rabbit anti–human PS-coated (Dako, Glostrup, Denmark) microwell plates for 2 hours at room temperature. Bound PS was detected by incubation with peroxidase-conjugated rabbit anti-human PS prepared using Peroxidase Labeling Kit-NH2 (Dojindo, Kumamoto, Japan) for 1 hour. Bound antibody was detected using SureBlue Reserve TMB Microwell Peroxidase Substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) as absorbance change at 450 nm. The PS level measured in wild-type mouse plasma was arbitrarily defined as 100%.
APC cofactor activity analysis of recombinant mouse PS proteins in vitro
Recombinant mouse wild-type PS and PS-K196E mutant proteins were made as described.33 SDS-PAGE analysis under reducing conditions revealed that the recombinant proteins were ∼95% pure (data not shown). APC cofactor activity of recombinant PS was determined by modified APTT and FXa 1-stage clotting assays as described previously.33 In a modified APTT assay, 10 µL normal mouse pooled plasma (BioreclamationIVT, Baltimore, MD), 5 µL human fibrinogen (2 mg/mL; Enzyme Research Laboratories, South Bend, IN), 25 µL recombinant mouse APC (36 nM), 25 µL recombinant mouse PS (0, 3.38, 6.75, 13.5, and 27 nM), and 25 µL APTT-XL (Thermo Fisher Scientific, Waltham, MA) were mixed and incubated for 3 minutes at 37°C. The clotting time was recorded after adding 25 µL CaCl2 (25 mM). In an FXa 1-stage clotting assay, 10 µL normal mouse pooled plasma, 25 µL of a mixture (4:1) of phospholipid vesicles (25 µM, 80% 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine/20% 1,2-dioleoyl-sn-glycero-3-phosphatidylserine; Avanti Polar Lipids, Alabaster, AL) plus human fibrinogen (2 mg/mL), 25 µL recombinant mouse APC (53 nM), 25 µL recombinant mouse PS (0, 0.63, 1.25, 2.5, 5, and 10 nM), and 25 µL mouse FXa (Haematologic Technologies, Essex Junction, VT) were mixed and incubated for 3 minutes at 37°C. The clotting time was recorded after adding 25 µL CaCl2 (25 mM). Each assay was performed in triplicate. The APC cofactor activity of recombinant mouse PS was evaluated based on the slope of a linear increase in clotting time with the dosage of recombinant mouse PS.
DVT model
A mouse DVT model of electrolytic inferior vena cava (IVC) injury was performed according to the method of Diaz et al, with minor modifications.34,35 Mice were anesthetized with 2.5% 2,2,2-tribromoethanol and kept around 37°C using a heating pad (Bio Research Center, Nagoya, Japan). The IVC was exposed through a midline laparotomy, and all side branches between the renal and iliac veins were ligated with a 7-0 polypropylene suture. An anode of 27G stainless-steel needle electrode (NE-115B; Nihon Koden, Tokyo, Japan) was inserted into the caudal IVC and attached to the anterior wall. A cathode was inserted subcutaneously to complete a circuit. A direct current of 200 µA was applied for 10 minutes using an electric stimulator (SEN-3041; Nihon Koden) with an isolator unit (SS-203J; Nihon Koden). After current load, the needle was gently removed from the IVC and the abdomen was closed by polyglycolic acid suture and cyanoacrylate glue. At 2 days postsurgery, blood was collected and platelet counts were determined using an automatic cell counter (KX-21NV; Sysmex, Kobe, Japan). Thrombi formed in the IVC were removed, weighed in wet condition, and photographed using a digital microscope (VHX-1000; Keyence, Osaka, Japan). Plasma thrombin-antithrombin complex (TAT) and interleukin-6 (IL-6) levels were measured using an Enzygnost TAT micro kit (Siemens AG, Munich, Germany) and a BD OptEIA mouse IL-6 ELISA kit (BD Biosciences, San Jose, CA), respectively.
PE models
A recombinant human tissue factor (TF) reagent (Dade Innovin) containing phospholipids and calcium was purchased from Siemens AG. A high-molecular-weight polyphosphate (HMW polyP) was prepared as reported by Smith et al.36 Sodium metaphosphate (Sigma-Aldrich Japan, Tokyo, Japan) was washed twice with purified water and solubilized in 250 mM LiCl. The soluble HMW polyP (40-1200 phosphate units long) was precipitated by adding 2.5 volumes of acetone and dissolved in purified water. The concentrations of HMW polyP were determined as phosphate monomer using BIOMOL Green reagent (Enzo Life Sciences, Farmingdale, NY) after complete hydrolysis in 1 N HCl.
Mice were anesthetized with 2.5% 2,2,2-tribromoethanol, and 15 µL/g body weight of the TF reagent (1/30 dilution) or HMW polyP (1.67 g/L) was infused into the IVC.37 The dose of TF and HMW polyP was chosen such that ∼20% of wild-type mice survived after the infusion. Survival time was recorded until 20 minutes after the infusion, while defining death as respiratory arrest that persisted for at least 2 minutes. Two minutes after the respiratory arrest (but while the heart was still beating) or at the completion of the 20-minute observation period, mice were perfused with 0.5 mL 1% Evans blue via right ventricle. Lungs were excised, photographed, and scored for Evans blue perfusion defects using a scoring scale (from 0 for no occlusion to 4 for complete occlusion). The scores were evaluated by 2 individuals anonymously.
Transient middle cerebral artery (MCA) occlusion model
Cerebral ischemia-reperfusion injury was induced using the 3-vessel occlusion technique as described previously.38,39 In brief, the left common carotid artery was isolated and occluded by a vascular clip (1-vessel occlusion [1-VO]) under halothane inhalation-anesthesia. A skin incision was made at the midpoint between the left orbit and the external auditory canal. After the removal of the zygomatic bone and downward retraction of the mandibular bone, a craniectomy was made using an electric drill. The distal M1 portion of left MCA, peripheral to the perforating arteries of the basal ganglia, was permanently occluded (2-vessel occlusion [2-VO]) by electrocauterization and cut at the lateral edge of the olfactory tract. Finally, the right common carotid artery was occluded (3-vessel occlusion [3-VO]) using a vascular clip. After 15-minute focal ischemia induced by the 3-VO, 2 clips at the common carotid arteries were removed to establish reperfusion through the collateral arteries (the source of the vascular reserve) distal to the 1-VO in the cortex. During the operation, blood pressure was monitored by an indirect blood pressure meter (BP-98A; Softron, Tokyo, Japan) and rectal temperature was regulated within the range of 36.5 to 37.5°C by a temperature controller (NS-TC10; Neuroscience, Tokyo, Japan). After 24 hours, neurologic deficits were assessed using a scoring scale (from 0 to 4) as described previously,38,39 and the brains were excised and stained with 2,3,5-triphenyl tetrazolium chloride. The infarcted and the total hemispheric areas of each section were measured using a computer-assisted image-analysis system (WinROOF; Mitani, Tokyo, Japan).31,39 The infarct volume was adjusted for edema by dividing the volume by the edema index (left hemisphere volume/right hemisphere volume).
Statistical analysis
Statistical significance was assessed by the 1-way analysis of variance followed by the post hoc Bonferroni’s multiple comparison test. Data for nonnormal and nonparametric distributions were assessed by the Kruskal-Wallis test followed by the post hoc Dunn’s multiple comparison test. Survival rates were analyzed by the Mantel-Cox log-rank test. Differences were considered to be significant at P < .05.
Results
Generation of PS-K196E and PS-KO mice
To generate PS-K196E mice, we introduced the K196E mutation into the endogenous Pros1 gene by homologous recombination (supplemental Figure 1A). We confirmed the expected structure of the targeted locus by polymerase chain reaction (data not shown) and Southern blotting (supplemental Figure 1B). Pros1 messenger RNA was detected in PS-K196E mice with normal sizes (∼3.5 kb) and amounts by the northern blot analysis of liver total RNA (supplemental Figure 1C). Expression of the mutant messenger RNA in PS-K196E mice was verified by the appearance of 238-bp and 171-bp fragments in reverse-transcription polymerase chain reaction products after digestion with NruI (supplemental Figure 1D) and by direct sequencing of the products. To generate PS-KO mice, we disrupted the Pros1 gene in a similar manner using a targeting vector that eliminated exon 3 (supplemental Figure 2).
Genotyping of 299 offspring by intercrosses in heterozygous PS-K196E (Pros1+/E) mice showed the expected 1:2:1 Mendelian distribution of Pros1+/+ (82/299, 27.4%), Pros1+/E (149/299, 49.8%), and Pros1E/E (68/299, 22.7%). Pros1E/E mice were viable and fertile. In contrast, no Pros1−/− pups were obtained in a total of 316 pups from Pros1+/− intercrosses, as previously reported.3,28 Pros1+/+ and Pros1+/− mice were born in the 1:2 distribution (109/316 [34.5%] and 207/316 [65.5%], respectively) by Pros1+/− mice intercrosses, and Pros1+/− mice grew normally. Thus, although PS is essential for embryonic development in mice, the homozygous PS-K196E mutation did not cause embryonic lethality. In addition, Pros1E/− mice were viable when Pros1E/E mice were bred with Pros1+/− mice.
Plasma APC cofactor activities and PS antigens in PS-K196E and PS-KO mice
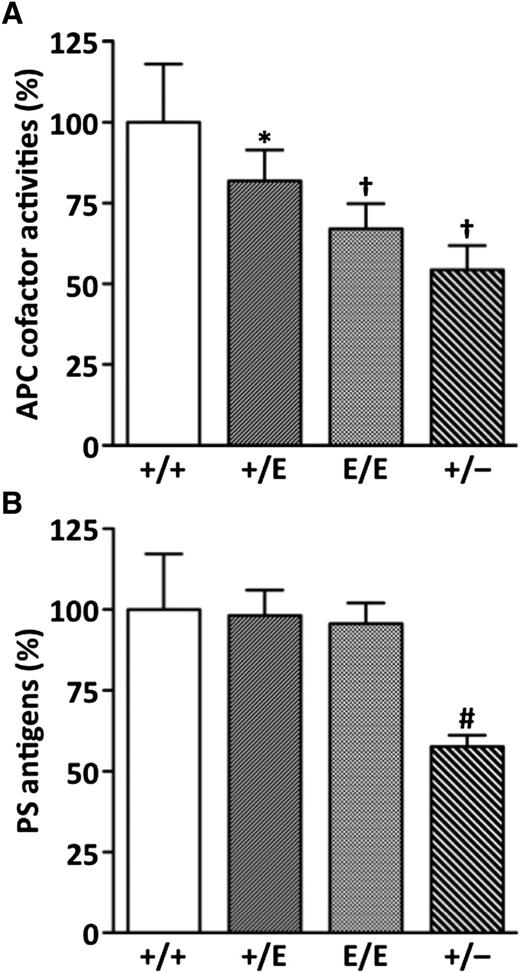
We measured plasma APC cofactor activity by an APTT-based clotting assay in the absence and presence of recombinant mouse APC as previously reported.33 The APC cofactor activity determined from the prolongation of clotting time by APC was significantly reduced in Pros1+/E, Pros1E/E, and Pros1+/− mice compared with Pros1+/+ mice (Figure 1A). Similar to human Pros1+/E subjects, Pros1+/E mice had ∼18% lower activity (82.0% ± 9.5%, mean ± SD) than Pros1+/+ mice (100% ± 18.0%). More severe reductions in the activity were observed in Pros1E/E (67.1% ± 7.8%) and Pros1+/− (54.3% ± 8.3%) mice. Basal APTT values in the absence of added APC were not different among the mouse groups (Pros1+/+, 23.3 ± 0.6 s; Pros1+/E, 23.3 ± 0.6 s; Pros1E/E, 23.3 ± 0.6 s; Pros1+/−, 23.3 ± 0.6 s).
Plasma APC anticoagulant cofactor activity and PS antigen levels. (A) APC cofactor activities. Data are mean ± standard deviation (SD) of Pros1+/+ (n = 10), Pros1+/E (n = 10), Pros1E/E (n = 10), and Pros1+/− (n = 8) mice. (B) PS antigens. Data are mean ± SD of 5 mice for each genotype. *P < .05 in comparison with Pros1+/+ mice. †P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E mice. #P < .001 in comparison with Pros1+/+, Pros1+/E, and Pros1E/E mice. The levels measured in Pros1+/+ mice were defined as 100%.
Plasma APC anticoagulant cofactor activity and PS antigen levels. (A) APC cofactor activities. Data are mean ± standard deviation (SD) of Pros1+/+ (n = 10), Pros1+/E (n = 10), Pros1E/E (n = 10), and Pros1+/− (n = 8) mice. (B) PS antigens. Data are mean ± SD of 5 mice for each genotype. *P < .05 in comparison with Pros1+/+ mice. †P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E mice. #P < .001 in comparison with Pros1+/+, Pros1+/E, and Pros1E/E mice. The levels measured in Pros1+/+ mice were defined as 100%.
The plasma PS antigen levels were normal in Pros1+/E mice (98.2% ± 7.9%) and Pros1E/E (95.7% ± 6.4%) mice, indicating that PS-K196E mutant was normally secreted into blood but had reduced APC anticoagulant cofactor activity (Figure 1B). In contrast, the PS antigen levels were reduced in Pros1+/− mice (57.7% ± 3.5%), corresponding to around 50% reduction in the APC cofactor activity.
In vitro APC cofactor activity of recombinant mouse PS-K196E mutant
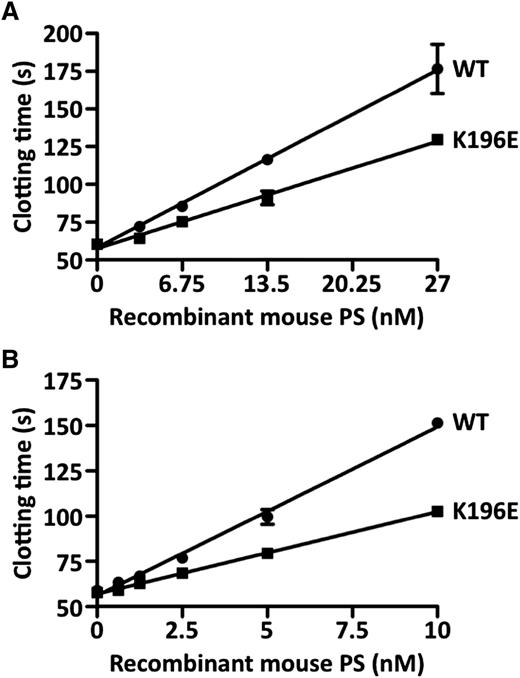
To characterize further the effect of K196E mutation on the anticoagulant activity of PS, we prepared recombinant mouse wild-type PS and the PS-K196E mutant and compared their APC cofactor activities using 2 different in vitro assays. In a modified APTT assay, PS-K196E showed 60% APC cofactor activity compared with wild-type PS (Figure 2A). In an FXa 1-stage clotting assay, PS-K196E similarly showed 49% APC cofactor activity compared with wild-type PS (Figure 2B). Thus, the purified PS-K196E mutant had 49% to 60% of normal PS APC cofactor activity.
APC anticoagulant cofactor activity of recombinant mouse PS. (A) Modified APTT assay. Increasing concentrations of recombinant mouse wild-type (WT) PS (●) or PS-K196E (▪) were added to normal mouse pooled plasma, and a modified APTT clotting assay was performed in the presence of recombinant mouse APC. (B) FXa 1-stage clotting assay. Increasing concentrations of recombinant mouse WT PS (●) or PS-K196E (▪) were added to normal mouse pooled plasma, and an FXa 1-stage clotting assay was performed in the presence of recombinant mouse APC. Each assay was performed in triplicate, and data are shown as mean ± SD.
APC anticoagulant cofactor activity of recombinant mouse PS. (A) Modified APTT assay. Increasing concentrations of recombinant mouse wild-type (WT) PS (●) or PS-K196E (▪) were added to normal mouse pooled plasma, and a modified APTT clotting assay was performed in the presence of recombinant mouse APC. (B) FXa 1-stage clotting assay. Increasing concentrations of recombinant mouse WT PS (●) or PS-K196E (▪) were added to normal mouse pooled plasma, and an FXa 1-stage clotting assay was performed in the presence of recombinant mouse APC. Each assay was performed in triplicate, and data are shown as mean ± SD.
Exacerbation of VTE in PS-K196E mice
To investigate the effects of PS-K196E mutation on VTE, we performed DVT and PE model experiments. In the DVT model experiment, we applied the electrolytic IVC injury to Pros1+/+, Pros1+/E, Pros1E/E, Pros1+/−, and homozygous FV-R504Q (FvQ/Q) mice. In this model, thrombus formation under continuous blood flow is induced by endothelial activation at the site of anodal electrolysis and thrombi grow to a maximum size at 2 days after the injury.34 In this study, we applied milder electrolytic stimulation (200 µA, 10 minutes) than that of the original method (250 µA, 15 minutes) to adequately assess prothrombotic states in mice. We measured thrombus weight in IVC at 2 days after the injury and found enhanced thrombus formation in Pros1E/E, Pros1+/−, and FvQ/Q mice compared with Pros1+/+mice (Figure 3A). Accompanying the increase in thrombus weight, peripheral platelet counts were decreased, and plasma TAT and IL-6 levels were increased in Pros1E/E, Pros1+/−, and FvQ/Q mice compared with Pros1+/+ mice (Figure 3B-D). In Pros1+/E mice, thrombus weight, plasma TAT, and IL-6 levels were modestly, although not significantly, increased and platelet counts were significantly decreased compared with Pros1+/+ mice. Platelet counts, plasma TAT, and IL-6 levels in nontreated mice were not different among the mouse groups. IL-6 has been shown to play a key role in promoting inflammation in mouse DVT models.40,41 Thus, these results suggest that PS-K196E mutation in mice promotes venous thrombus formation accompanied by enhancement of coagulation and inflammatory responses.
DVT model of electrolytic IVC injury. (A) Thrombus weight (TW) in IVC. (B) Platelet counts (PLT) in peripheral blood in mice with IVC injury. Platelet counts of nontreated control mice were not different among the groups (Pros1+/+, 118.4 ± 4.5 × 104/µL; Pros1+/E, 131.9 ± 13.1 × 104/µL; Pros1E/E, 110.5 ± 18.7 × 104/µL; Pros1+/−, 103.0 ± 14.5 × 104/µL; FvQ/Q, 111.9 ± 17.4 × 104/µL; mean ± standard error of the mean [SEM] of 3 mice). (C) TAT in plasma. TAT of nontreated control mice was not different among the groups (Pros1+/+, 3.2 ± 0.5 µg/L; Pros1+/E, 3.0 ± 0.3 µg/L; Pros1E/E, 2.7 ± 0.5 µg/L; Pros1+/−, 3.3 ± 0.8 µg/L; FvQ/Q, 3.5 ± 0.9 µg/L; mean ± SEM of 3 mice). (D) Interleukin-6 (IL-6) in plasma. IL-6 was not detected in nontreated mice of all genotypes (n = 3). Data are mean ± SEM of 12 mice for Pros1+/+, Pros1+/E, Pros1E/E, Pros1+/−, and FvQ/Q mice. *P < .05 in comparison with Pros1+/+ mice. **P < .001 in comparison with Pros1+/+ mice. †P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E mice.
DVT model of electrolytic IVC injury. (A) Thrombus weight (TW) in IVC. (B) Platelet counts (PLT) in peripheral blood in mice with IVC injury. Platelet counts of nontreated control mice were not different among the groups (Pros1+/+, 118.4 ± 4.5 × 104/µL; Pros1+/E, 131.9 ± 13.1 × 104/µL; Pros1E/E, 110.5 ± 18.7 × 104/µL; Pros1+/−, 103.0 ± 14.5 × 104/µL; FvQ/Q, 111.9 ± 17.4 × 104/µL; mean ± standard error of the mean [SEM] of 3 mice). (C) TAT in plasma. TAT of nontreated control mice was not different among the groups (Pros1+/+, 3.2 ± 0.5 µg/L; Pros1+/E, 3.0 ± 0.3 µg/L; Pros1E/E, 2.7 ± 0.5 µg/L; Pros1+/−, 3.3 ± 0.8 µg/L; FvQ/Q, 3.5 ± 0.9 µg/L; mean ± SEM of 3 mice). (D) Interleukin-6 (IL-6) in plasma. IL-6 was not detected in nontreated mice of all genotypes (n = 3). Data are mean ± SEM of 12 mice for Pros1+/+, Pros1+/E, Pros1E/E, Pros1+/−, and FvQ/Q mice. *P < .05 in comparison with Pros1+/+ mice. **P < .001 in comparison with Pros1+/+ mice. †P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E mice.
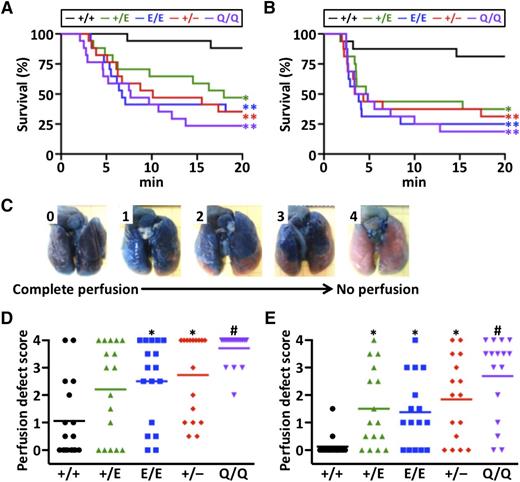
In the PE model experiments, we infused recombinant human TF or HMW polyP into mouse IVC. PolyP is a linear polymer of inorganic phosphates that is ubiquitous from bacteria to humans. PolyP acts as a natural negatively charged surface that activates the intrinsic pathway of blood coagulation.36 After the induction of PE in mice by TF or HMW polyP, we evaluated the 20-minute survival (Figure 4A-B, respectively). Using a scoring scale from no occlusion (score 0) to complete occlusion (score 4) (Figure 4C), we determined the degree of lung vascular occlusion by perfusion with Evans blue (Figure 4D-E). The survival was significantly reduced in Pros1+/E, Pros1E/E, Pros1+/−, and FvQ/Q mice compared with Pros1+/+ mice in both TF-induced and HMW polyP-induced PE models. The lung perfusion defect score was inversely correlated with survival and significantly increased in Pros1E/E, Pros1+/−, and FvQ/Q mice after TF-induced PE, and in Pros1+/E, Pros1E/E, Pros1+/−, and FvQ/Q mice after HMW polyP-induced PE. These results suggest that the PS-K196E mutation in mice increases lung vascular occlusion and mortality after induction of PE.
TF-induced PE and HMW-polyP–induced PE models. (A) Survival curve after TF infusion (n = 17/group). (B) Survival curve after HMW-polyP infusion (n = 16/group). (C) Scale to measure lung perfusion defect scores. Score of 0 indicates complete perfusion of Evans blue with no vascular occlusion, and score of 4 indicates no Evans blue perfusion with complete vascular occlusion. (D) Lung perfusion defect scores after TF infusion. (E) Lung perfusion defect scores after HMW-polyP infusion. Symbols represent data from a single mouse. Bars represent the mean values of groups. *P < .05 in comparison with Pros1+/+ mice. **P < .005 in comparison with Pros1+/+ mice. #P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E and Pros1E/E mice.
TF-induced PE and HMW-polyP–induced PE models. (A) Survival curve after TF infusion (n = 17/group). (B) Survival curve after HMW-polyP infusion (n = 16/group). (C) Scale to measure lung perfusion defect scores. Score of 0 indicates complete perfusion of Evans blue with no vascular occlusion, and score of 4 indicates no Evans blue perfusion with complete vascular occlusion. (D) Lung perfusion defect scores after TF infusion. (E) Lung perfusion defect scores after HMW-polyP infusion. Symbols represent data from a single mouse. Bars represent the mean values of groups. *P < .05 in comparison with Pros1+/+ mice. **P < .005 in comparison with Pros1+/+ mice. #P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E and Pros1E/E mice.
No exacerbation of ischemic stroke in PS-K196E mice
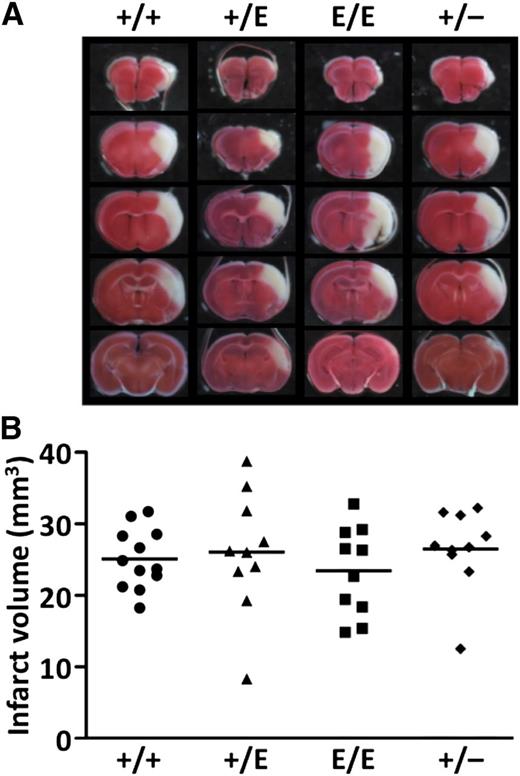
To examine the effects of PS-K196E mutation on arterial ischemic diseases, transient MCA ischemia-reperfusion injury was applied in mice using the 3-VO technique. We previously observed that both FvQ/+ and FvQ/Q mice showed increased infarct volumes compared with wild-type mice 24 hours after the ischemia-reperfusion injury induced by the same technique.42 However, infarct volumes 24 hours after ischemia in Pros1+/E, Pros1E/E, and Pros1+/− mice were not different from those in Pros1+/+ mice (Figure 5). The edema index and the neurologic deficit score were also not different among the mouse groups (data not shown). These results suggest that PS-K196E mutation in mice does not cause aggravation of ischemic stroke, unlike FV Leiden mutation.
Transient MCA occlusion model using the 3-VO technique. (A) Representative images of coronal sections of Pros1+/+, Pros1+/E, Pros1E/E, and Pros1+/− mouse brains. (B) Infarct volumes. No significant differences (P > .05) were observed among the groups. Symbols represent data from a single mouse. Bars represent the mean values of groups.
Transient MCA occlusion model using the 3-VO technique. (A) Representative images of coronal sections of Pros1+/+, Pros1+/E, Pros1E/E, and Pros1+/− mouse brains. (B) Infarct volumes. No significant differences (P > .05) were observed among the groups. Symbols represent data from a single mouse. Bars represent the mean values of groups.
Discussion
To assess the pathogenic causality of the PS K196E mutation for venous thrombosis, we established colonies of PS-K196E knockin mice and PS-KO mice.43 Plasma from PS-K196E homozygous mice had 67% of normal APC cofactor activity, similar to purified recombinant murine PS-K196E that had 49% to 60% APC cofactor activity. Mouse C4BP does not contain the PS-binding subunit, the β-chain, which is a pseudogene in mice,44 so interpretation of APC cofactor activity is uncomplicated by C4BP considerations. The susceptibility of PS-K196E mice to venous thrombosis was determined in multiple models, including (1) an electrolytic IVC model of venous thrombosis that produces a nonocclusive and consistent IVC thrombus in the presence of constant blood flow, (2) TF-initiated pulmonary thrombosis, and (3) HMW-polyP–initiated pulmonary thrombosis. All thrombotic biomarkers or parameters in these thrombosis injury models, including mortality, showed that the PS-K196E mutation caused increased venous thrombosis, generally very similar to PS heterozygosity and to the murine FV Leiden mutation. Heterozygosity for this PS mutation gave a milder thrombotic phenotype than PS mutant homozygosity. These results unambiguously demonstrate a causal link between the PS-K196E mutation and thrombophilia, strongly supporting the PS-K196E mutation as a human genetic risk factor for VTE.8-11
The PS lysine residue 196 is located in the second EGF-like domain, and it is highly conserved in PS from human, chimpanzee, rhesus monkey, mouse, rat, opossum, cattle, dog, pig, chicken, and Xenopus, indicating its importance for the PS function. Based on modeling of the tertiary structure of the second EGF-like domain of PS, Lys-196 is on the surface of the molecule,2 where it likely interacts with APC. Consistent with this interpretation for the molecular defect of PS-K196E, monoclonal antibodies were made that recognize the PS-196E epitope,46 indicating that residue 196 is likely exposed on the PS surface.
Currently, the extent to which the PS-K196E mutation or PS deficiency are risk factors for arterial occlusive diseases is not clear. To explore the arterial occlusive risk of these PS genetic variations, we used a cerebral focal ischemia-reperfusion model. Because of some limitations of the nylon-thread–induced ischemia/reperfusion MCA occlusion brain injury methodology,46 we employed the 3-VO technique for rodent ischemic stroke.38,39 This method consists of temporary occlusion of both common carotid arteries in conjunction with permanent unilateral occlusion of an MCA. The method does not use any foreign materials to occlude the lumen of the vessels and consistently produces focal ischemia, regional cerebral blood flow that is >10% but <20%, and adequate reperfusion in the cortex, achieving good reproducibility for the homogeneous development of cortical infarction.39 When this method was used to evaluate the susceptibility of PS-K196E mice and PS-KO mice to cerebral ischemia-reperfusion injury, we found that these PS-modified mice did not show any increase in brain infarct volume compared with wild-type control mice. PS protects neurons from ischemic injury in mouse stroke models.47 Therefore, mice with decreased APC cofactor activity might have been expected to show an aggravation of ischemic stroke, but it was not the case, indicating that the PS-K196E mutation and heterozygosity for wild-type PS do not compromise endogenous PS neuroprotective mechanisms. Because the neuroprotective effects of PS are mediated through its sex-hormone–binding globulin-like region,48 we speculate that the PS-K196E mutant with the intact sex-hormone–binding globulin-like region retains its neuroprotective actions. Moreover, the neuroprotective effects of pharmacologic APC are based on APC’s cell-signaling actions, not on its anticoagulant actions, so loss of APC anticoagulant cofactor activity might not compromise endogenous neuroprotection.49
Five rare genetic race-specific variants linked to VTE risk include PS-K196E in Japanese, FV Leiden and prothrombin II ntG20210A in whites, and R189W-protein C and del-Lys193-protein C in Chinese.11 Whether these mutations have been deleterious or advantageous during evolution is unclear. Each mutation, whether a gain of function or a loss of function, causes increased thrombin generation that may prevent bleeding and achieve hemostasis or increase thrombosis risk. Even today, as in ancient history, bleeding is the leading cause of maternal death linked to childbirth in the absence of modern medical care.50 Thus, the PS-K196E, like the other 4 VTE-linked mutations, on balance may have benefited the Japanese population historically by reducing maternal death, although it increased VTE risk. If so, this PS-K196E Japanese mutation has been advantageous since its first occurrence after divergence of the Japanese population from other populations and during its subsequent adaptive evolution.
In summary, the murine PS-K196E mutation similarly reduces its APC anticoagulant cofactor activity in plasma and in purified systems, and PS-K196E mice and heterozygous PS deficiency are more vulnerable to venous thrombosis than wild-type mice, proving pathogenic causality for the K196E mutation. Thus, PS-K196E mice may provide a novel murine resource for studies of thrombosis in vivo that may assist defining race-dependent, PS-dependent pathophysiological mechanisms for thrombosis in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Stephanie A. Smith and Dr James H. Morrissey (University of Illinois) for providing the protocol for preparation of HMW polyP.
This work was supported in part by grants-in-aid from the Ministry of Health, Labour and Welfare of Japan, the Japan Society for the Promotion of Science, the Mitsubishi Pharma Research Foundation, the Japan Cardiovascular Research Foundation, the Uehara Memorial Foundation, the National Institutes of Health, National Heart, Lung, and Blood Institute (HL31950 and 21544) (J.H.G.), and the Takeda Scientific Foundation.
Authorship
Contribution: F.B. designed research, performed most of the experiments, analyzed and interpreted data, and wrote the paper; T.K. performed the MCA ischemia-reperfusion model and analyzed data; J.A.F. performed the recombinant mouse PS experiments and analyzed data; H.Y. established the MCA ischemia-reperfusion model using the 3-vessel occlusion technique and interpreted data; Y.T. analyzed data of the PE model experiments; K.K. constructed the targeting vector for generating the PS-K196E and PS-KO mice; J.H.G. made recombinant murine APC and PS, interpreted data, and wrote the paper; and T.M. designed research, interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshiyuki Miyata, Department of Cerebrovascular Medicine, National Cerebral and Cardiovascular Center, 5-7-1 Fujishirodai, Suita, Osaka 565-8565, Japan; e-mail: miyata@ncvc.go.jp.

![Figure 3. DVT model of electrolytic IVC injury. (A) Thrombus weight (TW) in IVC. (B) Platelet counts (PLT) in peripheral blood in mice with IVC injury. Platelet counts of nontreated control mice were not different among the groups (Pros1+/+, 118.4 ± 4.5 × 104/µL; Pros1+/E, 131.9 ± 13.1 × 104/µL; Pros1E/E, 110.5 ± 18.7 × 104/µL; Pros1+/−, 103.0 ± 14.5 × 104/µL; FvQ/Q, 111.9 ± 17.4 × 104/µL; mean ± standard error of the mean [SEM] of 3 mice). (C) TAT in plasma. TAT of nontreated control mice was not different among the groups (Pros1+/+, 3.2 ± 0.5 µg/L; Pros1+/E, 3.0 ± 0.3 µg/L; Pros1E/E, 2.7 ± 0.5 µg/L; Pros1+/−, 3.3 ± 0.8 µg/L; FvQ/Q, 3.5 ± 0.9 µg/L; mean ± SEM of 3 mice). (D) Interleukin-6 (IL-6) in plasma. IL-6 was not detected in nontreated mice of all genotypes (n = 3). Data are mean ± SEM of 12 mice for Pros1+/+, Pros1+/E, Pros1E/E, Pros1+/−, and FvQ/Q mice. *P < .05 in comparison with Pros1+/+ mice. **P < .001 in comparison with Pros1+/+ mice. †P < .001 in comparison with Pros1+/+ mice, and P < .05 in comparison with Pros1+/E mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/19/10.1182_blood-2015-06-653162/4/m_2247f3.jpeg?Expires=1767697793&Signature=mHx8tbxIR1UddMNBrBtyXmW9CJBf7YGgMm0vwWx2tDONKkb1X9qog0Ykjj0sw73VdyqN2S6VAy-h8mJLlmNrkgVmQ4iH7s0v9SgRZQnZfwoWRR9wauzgFKKOT6QM-ZA0aJDaHsw~~eICXND1OZA2Srk5NwQSPeJMbXIUOL9VL7eZyaY26Z-AiBuNbXDHssuc3~hiei3DTSYLGFUZod~4Ce8ugb3BZsiWoA-475xtJ8iChyFHI-UMj40fvgwRE7NmHsIAVYnLrdlnihSjGGSjUDmPf0GL8gNT1NPFtO8x5O28Pcgdft8i1LTqphQWf3-Qh2iq5I6KSflZoAUvxtGsCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)