Abstract
A polymorphism in coagulation factor V, factor V Leiden (FVL), is the major known genetic risk factor for thrombosis in humans. Approximately 10% of mutation carriers experience clinically significant thrombosis in their lifetime. In a small subset of patients, thrombosis is associated with coinheritance of other prothrombotic gene mutations. However, the potential contribution of additional genetic risk factors in the majority of patients remains unknown. To gain insight into the molecular basis for the variable expressivity of FVL, mice were generated carrying the homologous mutation (R504Q [single-letter amino acid codes]) inserted into the endogenous murine Fv gene. Adult heterozygous (FvQ/+) and homozygous (FvQ/Q) mice are viable and fertile and exhibit normal survival. Compared with wild-type mice, adult FvQ/Q mice demonstrate a marked increase in spontaneous tissue fibrin deposition. No differences in fetal development or survival are observed among FvQ/Q,FvQ/+ or control littermates on the C57BL/6J genetic background. In contrast, on a mixed 129Sv-C57BL/6J genetic background,FvQ/Q mice develop disseminated intravascular thrombosis in the perinatal period, resulting in significant mortality shortly after birth. These results may explain the high degree of conservation of the R504/R506 activated protein C cleavage site within FV among mammalian species and suggest an important contribution of other genetic factors to the thrombosis associated with FVL in humans.
Introduction
Factor V (FV) together with the serine protease factor Xa forms the prothrombinase complex that converts prothrombin to active thrombin. Deficiency of FV results in a major bleeding disorder in humans,1 and genetically engineered mice that are completely deficient in FV exhibit partial lethality at mid-embryogenesis, with the remaining animals dying of hemorrhage at birth.2 FV plays a central regulatory role in hemostasis. It is synthesized as an inactive precursor and is activated to FVa by thrombin cleavage.3 FVa is subsequently inactivated by the natural anticoagulant activated protein C (APC), which cleaves FVa at amino acids R506 (single-letter amino acid code), R306, and R679 in the heavy chain.4,5 Kinetic studies have demonstrated that cleavage occurs first at R506, an event required for efficient cleavage at the other 2 sites. The substitution of Q for R506 in FV, also known as FV Leiden (FVL), has a prevalence of 2% to 7% in most European populations6,7 and is identified in 20% to 50% of patients with venous thrombo-embolic disease.8-10 The lifetime incidence of thrombosis is approximately 10% in heterozygotes and 80% in homozygotes.11-13 Despite the negative evolutionary selection that might be expected from this potentially fatal disorder, the variant allele is present at a remarkably high frequency in European populations (≈ 0.03) and appears to have arisen from a single founder, who is estimated to have lived 21 000 to 34 000 years ago.14
To explore the molecular basis for the incomplete penetrance and variable expressivity of the FVL mutation, we generated mice carrying the homologous mutation (R504Q) by a gene-targeting “knock-in” approach. Homozygous FVL (FvQ/Q) mice exhibit biochemical evidence for spontaneous fibrin deposition in multiple tissues. In addition, a marked variability in the thrombophilic phenotype is observed dependent on strain background, identifying one or more modifier genes in the 129Sv strain that interact with FVL to produce fatal thrombosis in the perinatal period.
Materials and methods
Introduction of the R504Q mutation into murine embryonic stem cells by homologous recombination
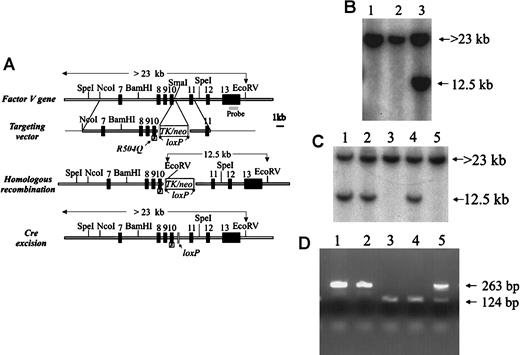
A portion of the murine Fv gene was cloned from a 129Sv library as previously described.2 The homologous arms of the targeting vector were assembled from a 15-kilobase (kb) lambda phage clone containing exons 7 to 11 of the murine Fvgene (Figure 1A). The R504Q mutation was introduced by site-directed mutagenesis (Muta-Gene mutagenesis kit, Bio-Rad, Hercules, CA), according to the manufacturer's instructions, into an approximately 5-kbSalI(vector-derived)/BamHI fragment containing exons 8 through 11 subcloned into pBluescript. The mutagenesis oligonucleotide (antisense) was 5′-CCTGTACACCCTG(CT)CTGGTCCAGG-3′, in which the underlined nucleotides represent the mutation, with the native sequence in parentheses. An approximately 1-kb SphI/HpaI fragment containing exons 9 and 10 and the introduced mutation was then subcloned back into an 11.8-kb Fv gene fragment (from theNcoI site in intron 6 to a vector-derived [plasmid vector pSL301] SalI site in intron 11) cloned in Bluescript (Figure 1A). The presence of the mutation and the integrity of the entire SphI/HpaI mutagenesis-derived fragment were confirmed by DNA sequence analysis. A 3.7-kbSalI/XhoI TK/neo-cassette flanked byloxP sites from the vector pFlox (gift of J. Marth, Univ of California, San Diego, La Jolla, CA) was subcloned into theSmaI site in intron 10 of the above-mentioned mutagenizedFv genomic segment. The resulting construct containsFv exons 7 through 11 with the R504Q mutation inserted into exon 10, the TK/neo selectable marker in intron 10, with a 9.4-kb homologous 5′ arm and a 2.4-kb 3′ arm (Figure 1A). This targeting vector was linearized with SfiI and introduced into 129Sv-derived D3 embryonic stem (ES) cells (kindly provided by T. Doetschman, University of Cincinnati) by electroporation, and stable transfectants were selected as previously described.2 Individual ES clones were screened for homologous recombination by Southern blot analysis with an exon 13 probe (Figure 1B). The presence of a single-copy TK/neo gene insertion at the expected site was also confirmed by Southern blotting with a neo probe (data not shown). We obtained 5 correctly targeted ES cell clones carrying the TK/neo-cassette from 50neo (G418) resistant colonies, yielding a 10% targeting efficiency. However, only 3 of the 5 targeted clones contained the R504Q mutation as detected by Mn1I digestion of the polymerase chain reaction (PCR) product amplified from exon 10 of ES cell DNA (data not shown).
Generation of the
FvR504Q “knock-in” allele by gene targeting.(A) Structure of the FV gene (from exons 7 through 13), targeting vector carrying the R504Q mutation in exon 10 and selectableTK/neo cassette flanked by loxP sites, and the expected results of successful homologous recombination and Cre excision of the TK/neo cassette. (B) Southern blot analysis—using the exon 13 probe indicated in panel A—of DNA prepared from targeted ES cells following digestion with EcoRV. Lanes 1 and 2 show only the germline band at 23 kb. Lane 3 shows a successfully targeted ES clone with the predicted 12.5-kb band from the recombined allele and the 23-kb band from the remaining allele. (C) Southern blot analysis using same probe as in panel 1B to detect ES clones that had undergone Cre-mediated excision as shown in lanes 3 and 5 by the absence of the 12.5-kb EcoRV fragment. (D) PCR analysis of wild-type and mutant Fv alleles using primers that cross the insertion site in intron 10. Amplification of DNA from the wild-type allele yields a 124-bp fragment, and DNA from the mutant allele produces a 263-bp fragment.
Generation of the
FvR504Q “knock-in” allele by gene targeting.(A) Structure of the FV gene (from exons 7 through 13), targeting vector carrying the R504Q mutation in exon 10 and selectableTK/neo cassette flanked by loxP sites, and the expected results of successful homologous recombination and Cre excision of the TK/neo cassette. (B) Southern blot analysis—using the exon 13 probe indicated in panel A—of DNA prepared from targeted ES cells following digestion with EcoRV. Lanes 1 and 2 show only the germline band at 23 kb. Lane 3 shows a successfully targeted ES clone with the predicted 12.5-kb band from the recombined allele and the 23-kb band from the remaining allele. (C) Southern blot analysis using same probe as in panel 1B to detect ES clones that had undergone Cre-mediated excision as shown in lanes 3 and 5 by the absence of the 12.5-kb EcoRV fragment. (D) PCR analysis of wild-type and mutant Fv alleles using primers that cross the insertion site in intron 10. Amplification of DNA from the wild-type allele yields a 124-bp fragment, and DNA from the mutant allele produces a 263-bp fragment.
Effect of inserted loxP sequence on intron 10 splicing efficiency
The targeting vector shown in Figure 1A was transformed intoEscherichia coli strain K1062(Cre), resulting in excision of the TK/neo cassette and an intron 10 structure identical to that predicted for the targeted ES clones followingCre-mediated excision (see below). We subcloned 5-kbBamHI/NotI genomic fragments from theCre-excised vector and the original genomic clone (containing exons 10 and 11, and intron 10 with or without theloxP element), into the vector pSPL3 and transiently transfected into COS-1 cells by Lipofectamine (Gibco BRL), according to the manufacturer's instructions. PCR primers specific to exons 10 and 11 amplified the same single band corresponding to the expected correctly spliced product, with similar quantities observed in either the presence or the absence of theloxP fragment (data not shown). These data indicate that the presence of the 139–base pair (bp) insertion in intron 10 (containing the single loxP site and flanking vector-derived linker sequences) does not significantly alter Fv intron 10 splicing.
Production of FvQ/Q mice
To remove the TK/neo-cassette from intron 10 in the targeted ES cells, 10μg of supercoiled pMC-Cre plasmid DNA (gift of J. Marth) was transiently transfected into the targeted ES clones that contained the R504Q mutation by electroporation under the same conditions as for the stable transfectants. After electroporation, the mixture was immediately diluted with medium and then plated out at 100, 103, and 104 colonies per 100-mm plate, assuming a 50% killing rate. The cells were grown for 9 to 10 days in the absence of antibiotic selection, and individual colonies were isolated for screening by Southern blot analysis by means of the same probe as above. Of the unselected clones, 15% were found to have undergone the TK/neo excision event. Successfully targeted ES clones carrying the FvR504Q mutation and the excisedTK/neo cassette were injected into C57BL/6J mouse blastocysts as previously described.2 The presence of the mutation in representative mice derived from each of the original 3 independent ES cell colonies was verified by DNA sequencing of mouse genomic DNA amplified by PCR with primers 5′-TTGCCTCTGGGCTGATAGGG-3′ (in exon 10) and 5′- CCTAATCTGTGCCAGCG-3′ (in intron 10). Since the R504Q mutation is separated from the intron 10 loxP insertion by only 400 bp, the presence of the latter was subsequently used as a size marker for rapid PCR genotyping. The intron 10 primers 5′-CCTCTGGACTCTGACTGCAG- 3′ and 5′-TATTCTGGACTACAAGAGTGAG-3′ flank the 139-bp insertion containing the loxP site, amplifying fragments of 263 bp and 124 bp from the R504Q and wild-type Fvalleles, respectively (Figure 1D).
Chimeric founder males were bred to C57BL/6J females (The Jackson Laboratory, Bar Harbor, ME) to generate F1 heterozygous offspring. Homozygous R504Q mice were obtained from F1 intercrosses. For most experiments, R504Q mice were backcrossed to C57BL/6J mice for 4 generations (N4), and N4 R504Q heterozygous mice were intercrossed to produce homozygous offspring. For additional analysis of mutant mice on a mixed 129Sv-C57BL/6J genetic background, C57BL/6J N4 R504Q homozygous mice were crossed back to 129Sv/J or 129SvIm/J (The Jackson Laboratory), and the heterozygous offspring were then intercrossed.
Analysis of thrombotic phenotype
FV procoagulant activity and APC resistance assays were determined as previously described15 with the use ofFvQ/Q, FvQ/+ and Fv+/+ littermates (on a mixed 129Sv-C57BL/6J genetic background).2 Quantitation of tissue fibrin from multiple homogenized tissues was performed in 8-week-old mice as previously described.16 These animals (Figure 3) were littermates from an intercross of FvQ/+ (N4 backcrossed to C57BL/6J).
Fetuses and neonates were photographed at autopsy, fixed in 1% glutaraldehyde in 0.1 mol/L phosphate buffer for 1 hour or 10% neutral buffered formalin overnight at room temperature. Fetuses and neonates were decalcified for 15 hours in formic acid, and then heads were removed and bodies were split sagittally just off midline. Heads were embedded in paraffin to provide coronal sections, and bodies were embedded in paraffin for sagittal sectioning. All fetuses and neonates were step-sectioned (3 to 6 μm thick) for light microscopic evaluation taken every 250 μm. Embryo/fetuses and adult tissues were stained with hematoxylin and eosin.
Results and discussion
Generation of mice carrying the Fv R504Q mutation
We introduced the R504Q mutation into the endogenous murineFv gene by homologous recombination. This mutation in murine FV has previously been shown to result in partial APC resistance in vitro, comparable to the effect of the homologous R506Q mutation in human FV.15 17 A targeting vector carrying the R504Q mutation in exon 10 and a TK/neo-expression cassette flanked by loxP sites in intron 10 (Figure 1A) was transfected into 129Sv ES cells. Successfully targeted clones were identified by Southern blotting (Figure 1B). We obtained 5 correctly targeted ES cell clones, 3 of which contained R504Q, indicating that homologous recombination in these clones had occurred upstream of this mutation in the 5′ arm of the targeting vector (data not shown). The occurrence of 2 homologous recombination events in the 400-bp region between the mutation and the TK/neo-cassette and only 3 events in the 9400-bp region upstream of the mutation suggests a possible recombination “hot spot” at the junction of the genomic and synthetic sequences.
Transfection of successfully targeted ES clones with a Creexpression plasmid (pMC-Cre) catalyzed recombination between theloxP sites, resulting in excision of theTK/neo-cassette, leaving behind only a 139-bp fragment within intron 10 containing a single loxP element (Fig.1A,C,D). Reverse transcription PCR (RT-PCR) analysis of COS-1 cells transfected with modified Fv genomic fragments demonstrated that the efficiency of intron 10 splicing was not altered by the presence of this small loxP segment insertion (see “Materials and methods”).
FV activity and APC resistance in FVL mice
Chimeric male mice generated from ES cells carrying the R504Q mutation after excision of the TK/neo-cassette were bred to C57BL/6J females, and F1 heterozygous (FvQ/+) offspring were intercrossed to generate homozygous R504Q (FvQ/Q) mice. No obvious differences were observed among litters obtained from chimeric founders corresponding to each of the original 3 independently targeted ES clones.
Progeny derived from a single founder were selected to establish the colony
FV clotting activities measured in adult FvQ/Q andFvQ/+ mice were indistinguishable from those of wild-typeFv+/+ mice. Taken together with our previous data that the R504Q mutation does not affect the procoagulant activity of FV in vitro,15 these results indicate that gene expression from the targeted Fv allele is equivalent to wild-type and that the biosynthesis, processing, and clearance of murine FV are not altered by the FVL mutation. However, plasma APC resistance was conferred by the R504Q mutation in a dose-dependent manner, similar to that observed in humans with FV Leiden (APC resistance ratio, 2.1 ± 0.3 for Fv+/+, 1.5 ± 0.1 for FvQ/+, and 1.3 ± 0.1 for FvQ/Q mice).
Spontaneous thrombosis and increased tissue fibrin content inFvQ/Q mice
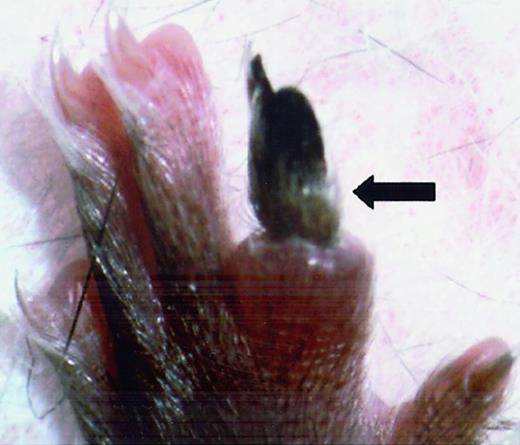
FvQ/+ mice were grossly indistinguishable from their normal littermates except for a rare, sporadic thrombo-embolic event as illustrated in Figure 2. This pattern is comparable to the mild phenotype observed in human FVL patients in whom similar sporadic events occur with a lifetime penetrance of ≈ 10%.2
Spontaneous vascular thrombosis.
Spontaneous vascular thrombosis in 6-week-oldFvQ/+ mouse resulted in necrotic toe (N4 C57BL/6J background).
Spontaneous vascular thrombosis.
Spontaneous vascular thrombosis in 6-week-oldFvQ/+ mouse resulted in necrotic toe (N4 C57BL/6J background).
Because of the potentially confounding effect of the mixed 129Sv and C57BL/6J background, FvQ/+ mice were serially backcrossed to the C57BL/6J strain for 4 generations (N4), and intercrosses of these N4 animals were used to generate the FVQ/Q and FvQ/+ mice employed in subsequent experiments. Adult FvQ/Q mice on both the mixed 129Sv-C57BL/6J and the C57BL/6J N4 backgrounds appeared healthy and routinely survived to more than 1 year of age, with females exhibiting normal fertility and uncomplicated pregnancies. Routine histopathologic analysis of 6- to 8-week-old FvQ/Q mice revealed occasional focal hepatic fibrosis but was otherwise unremarkable.
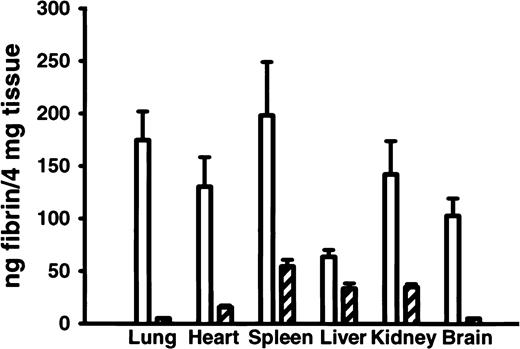
Analysis of tissue fibrin content in 8-week-old adult FvQ/Q mice16 showed biochemical evidence for chronic, low-grade thrombin generation leading to enhanced fibrin deposition in multiple tissues (Figure 3). Increased tissue fibrin deposition has also been demonstrated by the use of these methods in mice carrying a mutant thrombomodulin gene with reduced capacity to generate APC.16 These results suggest that similar subclinical chronic fibrin deposition may be occurring in human FVL patients, with the potential for long-term organ damage. This latter hypothesis is supported by the observation of increased circulating thrombin-antithrombin complexes in some human studies18,19 although it is not confirmed in others.20
Tissue fibrin deposition in various organs in
FvQ/Q and Fv+/+ mice. Tissue fibrin deposition in various organs is elevated in FvQ/Q mouse (n = 5) compared withFv+/+ mice (n = 5). Open bars represent FvQ/Q mice, and hatched bars represent Fv+/+ mice.
Tissue fibrin deposition in various organs in
FvQ/Q and Fv+/+ mice. Tissue fibrin deposition in various organs is elevated in FvQ/Q mouse (n = 5) compared withFv+/+ mice (n = 5). Open bars represent FvQ/Q mice, and hatched bars represent Fv+/+ mice.
Lethal perinatal thrombosis in FvQ/Q mice on 129Sv genetic background
Although intercrosses between F1 FvQ/+ mice on the original mixed 129Sv-C57BL/6J background produced viableFvQ/Q offspring that survived to adulthood, the expected number of FvQ/Q pups observed at 3 weeks of age was significantly decreased from what was expected (Table1).
Genotype distribution of progeny at 3 weeks of age or at 18.5 days postconception
| . | Total . | +/+ . | Q/+ . | Q/Q . |
|---|---|---|---|---|
| Mixed 129Sv-C57BL/6J genetic background | ||||
| Expected (FvQ/+XFvQ/+) | 100% | 25% | 50% | 25% |
| Day 21 | 135 | 37 (27%) | 80 (59%) | 18* (13%) |
| 18.5 DPC | 112 | 21 (19%) | 58 (52%) | 33 (29%) |
| C57BL/6J (N4) genetic background | ||||
| Day 21 (FvQ/+XFvQ/+) | 107 | 29 (27%) | 53 (50%) | 25 (23%) |
| FvQ/+XFvQ/Q Expected | 100% | 50% | 50% | |
| Day 21 | 137 | 72 | 64 | |
| C57BL/6J(N4) | 53% | 47% |
| . | Total . | +/+ . | Q/+ . | Q/Q . |
|---|---|---|---|---|
| Mixed 129Sv-C57BL/6J genetic background | ||||
| Expected (FvQ/+XFvQ/+) | 100% | 25% | 50% | 25% |
| Day 21 | 135 | 37 (27%) | 80 (59%) | 18* (13%) |
| 18.5 DPC | 112 | 21 (19%) | 58 (52%) | 33 (29%) |
| C57BL/6J (N4) genetic background | ||||
| Day 21 (FvQ/+XFvQ/+) | 107 | 29 (27%) | 53 (50%) | 25 (23%) |
| FvQ/+XFvQ/Q Expected | 100% | 50% | 50% | |
| Day 21 | 137 | 72 | 64 | |
| C57BL/6J(N4) | 53% | 47% |
DPC indicates days postconception.
P < .01 (chi square).
When offspring from F1 intercrosses were retrieved and genotyped at 18.5 days postconception (DPC) (approximately 1 to 2 days before birth), the expected number of FvQ/Q fetuses was observed (Table 1), indicating that the deficiency of FvQ/Q pups at 3 weeks is due not to embryonic loss, but rather to increased mortality in the perinatal or newborn period.
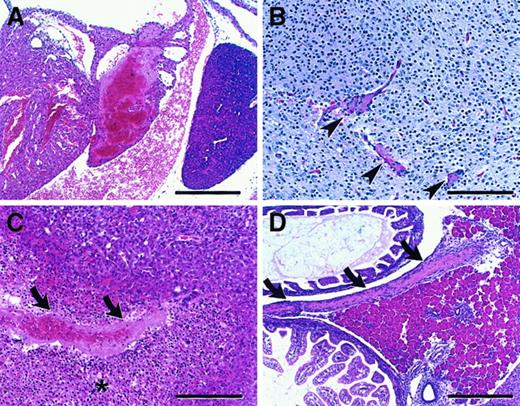
Intercrosses performed between surviving adult F2 FvQ/Q mice generated litters of entirely FvQ/Q offspring, with a subset appearing pale and lethargic at birth. Histological analysis demonstrated widespread thrombosis affecting multiple organs, including brain, liver, heart, pancreas, and spleen, in all FvQ/Q newborns, though of varying severity (Figure4).
Microscopic findings in neonatal homozygous FvQ/Q mice.
(A) Atrial thrombosis involving predominantly the left atrium with small right atrial thrombus attached to atrial septum (bar = 300μ). (B) Brain microthrombi (arrowheads), thalamic region (bar = 150μ). (C) Hepatic thrombus (arrows) with associated area of infarction (asterisk) (bar = 150μ). (D) Large thrombus (arrows) in mesentery adjacent to pancreas (bar = 300μ). All sections are stained with hematoxylin and eosin (H&E) (sections are from mice on a mixed 129Sv–C57BL/6J).
Microscopic findings in neonatal homozygous FvQ/Q mice.
(A) Atrial thrombosis involving predominantly the left atrium with small right atrial thrombus attached to atrial septum (bar = 300μ). (B) Brain microthrombi (arrowheads), thalamic region (bar = 150μ). (C) Hepatic thrombus (arrows) with associated area of infarction (asterisk) (bar = 150μ). (D) Large thrombus (arrows) in mesentery adjacent to pancreas (bar = 300μ). All sections are stained with hematoxylin and eosin (H&E) (sections are from mice on a mixed 129Sv–C57BL/6J).
Thrombosis was also seen in FvQ/Q fetuses examined at 18.5 DPC although it was much less pronounced. In contrast, histologic evidence for thrombosis was not observed in FvQ/+ orFv+/+ newborns (data not shown). Thus, homozygosity for FVL is associated with spontaneous thrombosis during late fetal development that is accentuated at the time of birth, leading to perinatal mortality in a subset of mice.
These data are in striking contrast to humans, where individuals with FVL exhibit normal survival.21 Although some reports suggest enhanced early pregnancy loss in FVL heterozygous females,22-24 the prevalence of homozygosity for FVL is consistent with Hardy-Weinberg equilibrium,25arguing against significant selective loss of FVL homozygotes from human populations. Although neonatal thrombosis in human FVL appears to be rare, the pathology observed in newborn FvQ/Q mice closely resembles the lethal neonatal purpura fulminans observed in humans with homozygous protein C deficiency,26 a more severe defect in the same natural anticoagulant pathway. Homozygous protein C deficiency in mice (Pc−/−) causes a profound consumptive coagulopathy that is uniformly lethal at or before birth, also appearing somewhat more severe than its human counterpart, though this difference may be due to low levels of residual protein C activity in most human patients.27 Histologic findings in Pc−/− mice resemble the disseminated thrombosis observed in FvQ/Q mice (Figure 4), though more widespread and with a more pronounced hemorrhagic component. The dramatically more severe consequences of FvQ/Q in mice than in humans may result from differences in hemostatic balance during a critical perinatal period. A recent evolutionary shift in this balance may account for the apparent tolerance of the R506Q mutation in humans, despite the high degree of conservation of the R504/R506 APC cleavage site among other mammalian species.
Evidence for one or more genetic modifier genes for FVL among inbred mouse strains
In contrast to the selective loss of FvQ/Q pups observed in the mixed 129Sv-C57BL/6J genetic background, an intercross of FvQ/+ mice from an N4 backcross to C57BL/6J produced the expected number of FvQ/Q offspring (Table 1). These results suggest the influence of strain-specific modifying factors accounting for this marked variation in neonatal mortality. To confirm the presence of a genetic modifier and exclude the influence of an unrecognized environmental factor, FvQ/Q N4 C57BL/6J mice were again crossed with Fv wild-type mice of the 129Sv strain, and intercrosses of heterozygous F1 offspring examined. The observed genotypes were again consistent with perinatal loss of approximately 50% of FvQ/Q progeny. It is interesting to note that the 129 background is also associated with increased fetal loss in tissue factor–deficient mice,28 though presumably through a different mechanism that either perturbs yolk sac vascular development or exacerbates the hemorrhagic defect, in contrast to the prothrombotic effect of the 129 modifier on FVL. Analysis of similar mouse models have demonstrated the presence of significant genetic modifiers for other important human diseases, including cystic fibrosis29 and hereditary hemorrhagic telangiectasia.30
Taken together, these data demonstrate that one or more variable genetic loci in the mouse exert a profound modifying influence on the FVL thrombotic phenotype. The incomplete penetrance of thrombosis in humans with FVL also suggests an important role for genetic modifiers. Consistent with this hypothesis, co-inheritance of mutations in other prothrombotic genes, such as protein C,31 protein S,32 antithrombin III,33 and prothrombin,34 has been shown to increase the incidence of vascular thrombosis in FVL humans. However, most of these latter genetic risk factors are uncommon, and co-inheritance with FVL can account for only a small subset of the thrombosis that occurs in carriers of the FVL mutation.
Our results demonstrate the presence of genetic variation in the mouse similar to that observed in humans and suggest that characterization of these murine modifier genes could have important implications for understanding the incomplete penetrance of FVL in humans. Yin et al35 recently demonstrated that the murine FVL mutation reported here unmasks a thrombotic phenotype in protein Z–deficient mice, providing the first direct evidence for the in vivo antithrombotic function of protein Z. In addition to protein Z, potential candidates for both the murine and the human genetic modifiers of FVL include nearly all of the known components of hemostasis. Subtle alterations at one of more of these loci could prove difficult to detect in complex human populations but may be more easily approached in the mouse.36
Supported by National Institutes of Health grants HL-035989 and 036195 (D.E.), P01-41484 (R.D.R. and P.D.C.), and HL-57345 (D.G.). D.G. is a Howard Hughes Medical Institute investigator.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David Ginsburg, Howard Hughes Medical Institute, University of Michigan Medical Center, 4520 MSRB I, 1150 West Medical Center Dr, Ann Arbor, MI 48109-0650; ginsburg@umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal