Abstract
Protein S (PS) is an important natural anticoagulant with potentially multiple biologic functions. To investigate further the role of PS in vivo, we generated Pros+/− heterozygous mice. In the null (−) allele, the Pros exons 3 to 7 have been excised through conditional gene targeting. Pros+/− mice did not present any signs of spontaneous thrombosis and had reduced PS plasma levels and activated protein C cofactor activity in plasma coagulation and thrombin generation assays. Tissue factor pathway inhibitor cofactor activity of PS could not be demonstrated. Heterozygous Pros+/− mice exhibited a notable thrombotic phenotype in vivo when challenged in a tissue factor–induced thromboembolism model. No viable Pros−/− mice were obtained through mating of Pros+/− parents. Most E17.5 Pros−/− embryos were found dead with severe intracranial hemorrhages and most likely presented consumptive coagulopathy, as demonstrated by intravascular and interstitial fibrin deposition and an increased number of megakaryocytes in the liver, suggesting peripheral thrombocytopenia. A few E17.5 Pros−/− embryos had less severe phenotype, indicating that life-threatening manifestations might occur between E17.5 and the full term. Thus, similar to human phenotypes, mild heterozygous PS deficiency in mice was associated with a thrombotic phenotype, whereas total homozygous deficiency in PS was incompatible with life.
Introduction
Protein S (PS; encoded by the PROS1 gene) is a vitamin K–dependent protein whose role in physiologic anticoagulation and pathologic thrombosis has been extensively studied. Notably, the importance of PS as an anticoagulant is illustrated by the dramatic clinical manifestations observed in very few homozygous and compound heterozygous patients with severe PS deficiency reported to date.1-3 Life-threatening conditions such as purpura fulminans and disseminated intravascular coagulation (DIC) in the neonatal period are observed in such patients. Heterozygous PS deficiency is milder, but is firmly associated with an increased risk of thromboembolic events. Whereas some PS-deficient subjects will not experience thrombosis and will remain asymptomatic, symptomatic PS-deficient patients often present with recurrent deep vein thrombosis and pulmonary embolism at adult age, requiring long-term anticoagulation. Consistent with the multifactorial nature of the thromboembolic disease,4 the risk associated with PS deficiency is modulated by other risk factors for venous thromboembolism, either transient (surgery, trauma, immobilization, pregnancy/puerperium, oral contraceptive), environmental, or genetic (thrombophilic defects, such as the homozygous mutation factor V (FV)Leiden or inherited protein C deficiency). The importance of such determinants in the clinical expression of PS deficiency is further underlined by the case of a compound heterozygous patient with severe PS deficiency that only manifested late in childhood.5
PS deficiency can be acquired with transient decrease in PS levels, as observed in several pathophysiologic states such as vitamin K-antagonist therapy, oral contraceptives, pregnancy, liver disease, nephrotic syndrome, DIC, autoimmune disorders, and infections.6 Nevertheless, PS deficiency characterized by persistently low PS levels generally has a genetic basis, and much attention has focused on inherited PS deficiency and investigation of potential carriers of PROS1 mutations in families with thrombotic manifestations. With more than 200 PROS1 mutations reported in the literature,1 only few mutations have been functionally studied, allowing us to gain insight into the molecular basis of PS deficiency and structure/function relationships of PS.7
Molecular mechanisms governing the anticoagulant activity of PS are still not fully understood. PS has long been involved in regulating the anticoagulant activity of activated protein C (APC), but it is now recognized that the anticoagulant activity of PS might also be direct, that is, independent of APC (recently reviewed in Castoldi and Hackeng8 ). PS thus functions as a nonenzymatic cofactor for APC in proteolytic inactivation of FVa and FVIIIa, but also for tissue factor (TF) pathway inhibitor (TFPI) in the inhibition of factor Xa.9-11 In addition, PS might directly limit thrombin generation by binding to and inhibiting factor Xa and FVa in the prothrombinase complex.12,13 APC-cofactor activity of PS is consistent with the similar clinical manifestations associated with deficiencies in PS and protein C, but is in marked contrast with the weak potentiating effect of PS on APC proteolytic activity in vitro. Indeed, an only approximately 20-fold stimulation of APC-catalyzed cleavage of FVa by PS has been reported in purified systems.14 This supported the notion that the APC-independent anticoagulant activity of PS might contribute to its overall anticoagulant activity in plasma. However, no case of a PS-deficient subject with impaired direct anticoagulant activity of PS has been reported.
Besides anticoagulation, new potential roles for PS have recently emerged. Indeed, PS is highly homologous to growth arrest-specific gene 6 (Gas6), a ligand for the TAM subfamily of 3 tyrosine kinase receptors (namely Tyro3, Axl, and Mertk).15 Activation of TAM receptors by Gas6 has been implicated in inhibition of inflammation in dendritic cells and macrophages, promotion of phagocytosis of apoptotic cells, or maturation of natural killer cells.16 Furthermore, Gas6 was found to be a major player in innate immunity during systemic inflammatory syndrome (L. Burnier, R. Sugamele, D. Le Roy, T. Roger, T. Fumeaux, S. Clauser, M. Chanson, S. Rignault, P. Carmeliet, J. Pugin, G. Lemke, G. Matsushima, H. S. Earp, F. Feihl, D. Borgel, L. Liaudet, R. Chioléro, M.S., T. Calandra, A.A.-S., manuscript submitted). Importantly, there is increasing evidence that PS might bind to and activate TAM receptors. Accordingly, PS was demonstrated to be a potent agonist of Mertk receptor in the retinal pigment epithelium,17 while stimulating both the phagocytosis of apoptotic cells by macrophages and the autophosphorylation of Mertk in macrophages in a separate study.18
Whereas Gas6,19 TAM receptors,20 and anticoagulant proteins such as TFPI21 or components of the protein C pathway22-24 have been knocked out and have benefited from several animal studies, a mouse model of PS deficiency was still awaited to clarify the PS anticoagulant activity and to document further the role of PS as a ligand for TAM receptors in vivo. We report in this study the generation and the characterization of the thrombotic phenotype of mice with heterozygous and homozygous deficiency in PS.
Methods
Generation of mice with one null PROS allele
A conditional gene-targeting vector was constructed from a 129Sv mouse bacterial artificial chromosome by a recombineering approach,25 essentially as described previously26 (see supplemental methods, available on the Blood website; see the Supplemental Materials link at the top of the online article). Briefly, the targeting vector consisted of a single loxP site 5′ of Pros exon 3, and was marked with a StuI site for genotyping purposes, together with an Frt-neo-Frt-loxP cassette 3′ of Pros exon 7 marked with a DraIII site for genotyping purposes (Figure 1A). This targeting vector was linearized and electroporated into 129Sv-derived embryonic stem (ES) cells (see supplemental methods). One correctly targeted clone was injected into C57BL/6J blastocysts, and male chimeras were obtained. Germline transmission of the targeted allele (floxNeo) was verified by crossing the male chimeras to female C57BL/6J mice, to generate mice with one conditional targeted floxNeo allele (F1ProsfloxNeo/+ mice). ProsfloxNeo/+ mice were kept on a mixed 129Sv-C57BL/6J genetic background.
Targeted disruption of the Pros gene. (A) A 23.7-kb targeting vector was constructed that contains a single loxP site 5′ of Pros exon 3, and an Frt-neo-Frt-loxP cassette 3′ of exon 7 (loxP and Frt sites are depicted by red and blue triangles, respectively). Restriction sites StuI (S) and DraIII (D) were introduced at these sites for genotyping purposes. The long 5′ homology arm was 4 kb, and the short 3′ homology arm was 2 kb. Homologous recombination in 129Sv ES cells resulted in a floxNeo allele that was detected by long-range PCR and Southern blotting analysis, as described in supplemental Figure 1. One correctly targeted ES cell clone was injected into blastocysts of C57BL/6J mice to generate chimeras, which in turn transmitted the floxNeo allele to F1 ProsfloxNeo/+ mice. These mice were genotyped by PCR (see supplemental Figure 1). Cre-mediated recombination through crossing of ProsfloxNeo/+ mice to a general Cre deleter strain (Nes-Cre1) resulted in the generation of a null (−) allele. Germline transmission of this null allele yielded Pros+/− mice in a mixed 129Sv-C57BL/6J genetic background. (B) WT, Pros+/−, and Pros−/− mice were genotyped by Southern blotting. Genomic tail DNA of E17.5 embryos from the 3 genotypes were digested by StuI and hybridized with the 3′DraIII probe (Figure 1A). The WT band was 12.2 kb, and the null (−) band was 4.4 kb (arrows). (C) WT, Pros+/−, and Pros−/− mice were genotyped by 2 separate PCRs with mouse tail genomic DNA as a template and primer couples PROS-Fwd/PROS-Rv (F and R, respectively [A]) for the WT band (234 pb), and PROS-Fwd/PROSnull-Rv (F and Rnull, respectively [A]) for the null band (571 pb). (D) Reduced levels of PS in the plasma of Pros+/− mice compared with WT mice were demonstrated after polyacrylamide gel electrophoresis of nonreduced plasma samples by Western blotting with a rabbit polyclonal antibody raised against mouse PS. Mouse plasma PS migrated at approximately 80 kDa (arrow).
Targeted disruption of the Pros gene. (A) A 23.7-kb targeting vector was constructed that contains a single loxP site 5′ of Pros exon 3, and an Frt-neo-Frt-loxP cassette 3′ of exon 7 (loxP and Frt sites are depicted by red and blue triangles, respectively). Restriction sites StuI (S) and DraIII (D) were introduced at these sites for genotyping purposes. The long 5′ homology arm was 4 kb, and the short 3′ homology arm was 2 kb. Homologous recombination in 129Sv ES cells resulted in a floxNeo allele that was detected by long-range PCR and Southern blotting analysis, as described in supplemental Figure 1. One correctly targeted ES cell clone was injected into blastocysts of C57BL/6J mice to generate chimeras, which in turn transmitted the floxNeo allele to F1 ProsfloxNeo/+ mice. These mice were genotyped by PCR (see supplemental Figure 1). Cre-mediated recombination through crossing of ProsfloxNeo/+ mice to a general Cre deleter strain (Nes-Cre1) resulted in the generation of a null (−) allele. Germline transmission of this null allele yielded Pros+/− mice in a mixed 129Sv-C57BL/6J genetic background. (B) WT, Pros+/−, and Pros−/− mice were genotyped by Southern blotting. Genomic tail DNA of E17.5 embryos from the 3 genotypes were digested by StuI and hybridized with the 3′DraIII probe (Figure 1A). The WT band was 12.2 kb, and the null (−) band was 4.4 kb (arrows). (C) WT, Pros+/−, and Pros−/− mice were genotyped by 2 separate PCRs with mouse tail genomic DNA as a template and primer couples PROS-Fwd/PROS-Rv (F and R, respectively [A]) for the WT band (234 pb), and PROS-Fwd/PROSnull-Rv (F and Rnull, respectively [A]) for the null band (571 pb). (D) Reduced levels of PS in the plasma of Pros+/− mice compared with WT mice were demonstrated after polyacrylamide gel electrophoresis of nonreduced plasma samples by Western blotting with a rabbit polyclonal antibody raised against mouse PS. Mouse plasma PS migrated at approximately 80 kDa (arrow).
The null allele was obtained by crossing ProsfloxNeo/+ mice to a Nestin-Cre transgenic mouse line27 (Nes-Cre1; see supplemental methods). Cre-mediated recombination of the floxNeo allele results in a shift in the reading frame and introduction of several stop codons, thus generating a null (−) allele. Heterozygous Pros+/− mice carrying one wild-type (WT) allele and one null allele (−) were kept on a mixed 129Sv-C57BL/6J genetic background.
Animal experiments were approved by the Swiss Federal Veterinary Office.
Genotyping
Pros+/− heterozygous mice were genotyped by 2 separate polymerase chain reactions (PCRs) amplifying the WT allele and the null (−) allele. Primer combinations PROS-Fwd/PROS-Rv and PROS-Fwd/PROSnull-Rv were used for amplifying the WT and null alleles, respectively (for primer sequences and PCR conditions, see supplemental methods; Figure 1A).
Preparation of plasma samples
Mice were anesthetized with pentobarbital, and whole blood was drawn from the inferior vena cava into 3.13% citrate (1 vol anticoagulant/9 vol blood). Blood was centrifuged at 2400g (for 10 minutes at room temperature) to obtain platelet-poor plasma used for plasma coagulation or thrombography assays.
Blood cell counts and measurement of hemostatic parameters
Blood (300 μL) was drawn from the retroorbital vein of mice anesthetized by intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (16 mg/kg), and blood cell counts were carried out with a Sysmex XE-2100 Alpha automated cell counter (Sysmex Digitana).
Hemostatic parameters were measured on an automated Sysmex CA-7000 coagulation analyzer (Sysmex Digitana). Hemostatic parameters included the following: fibrinogen, prothrombin, and factors V, VII, VIII, IX, X, XI, and XII. Prothrombin time and activated partial thromboplastin time (APTT) were measured on a coagulometer (Amelung KC4Amicro; Heinrich Amelung).
Measurement of thrombin-antithrombin levels
Thrombin-antithrombin (TAT) measurement was performed in duplicate for each plasma sample using a commercially available enzyme-linked immunosorbent assay (Enzygnost TAT micro; Dade Behring), according to the manufacturer's instructions.
Western blotting experiments
PS was detected in mouse plasma (2 μL/lane) by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under nonreducing conditions, followed by Western blotting with a rabbit polyclonal antibody raised against mouse PS as primary antibody (final concentration: 1 μg/mL in phosphate-buffered saline, pH 7.3, containing 0.05% Tween 20 and 5% milk). For densitometric analysis, band intensities were calculated using ImageJ program.
APC-cofactor activity in a plasma coagulation assay
The APC-cofactor activity of mouse plasma PS was investigated in an APTT assay in the presence of WT recombinant mouse APC (wt-rmAPC).29 Mouse plasma (50 μL) was preincubated with 0.9 nM (final concentration) wt-rmAPC (in 10 μL of 50 mM Tris, 150 mM NaCl, pH 7.4 [Tris-buffered saline] containing 1 mg/mL gelatin and 5 mg/mL bovine serum albumin) in the presence of 5 μL rabbit polyclonal anti–human PS (DakoCytomation; 0.13 mg/mL, final concentration) for 1 minute at 37°C in a coagulometer. Plasma samples were then incubated for 3 minutes with Dade Actin (50 μL; Dade Behring), and clotting was initiated with the addition of 25 mM CaCl2 (50 μL).
APC-cofactor activity in a calibrated automated thrombography assay
PS APC-cofactor activity was assessed in a calibrated automated thrombography (CAT)–based APC resistance test in mouse plasma, as previously described.30 Briefly, 20 μL mouse plasma (preincubated or not with 5 μL rabbit polyclonal anti-human PS for 10 minutes at 37°C) was mixed with 10 μL human recombinant TF (hrTF)/phospholipid mixture, in the presence or absence of 10 μL wt-rmAPC, and thrombin generation was initiated at 33°C with 15 μL fluorogenic substrate/CaCl2 mixture. Final concentrations were as follows: 33.3% mouse plasma, 6 pM hrTF, 60 μM phospholipids, 8.2 mM CaCl2, and 0.42 mM fluorogenic substrate plus or minus 3 nM wt-rmAPC.
Thrombin generation was measured in duplicate; mean thrombin generation curves were calculated using the Thrombinoscope software (Thrombinoscope BV); and the area under the curve (endogenous thrombin potential [ETP]) was measured in the presence (ETP+APC) and absence (ETP−APC) of wt-rmAPC. For each plasma genotype, APC resistance was assessed by calculating normalized APC sensitivity ratios (nAPCsr),30 which were defined as the ratio of ETP+APC and ETP−APC normalized against the same ratio determined in the same experiment with an in-house mouse pooled normal plasma obtained from 8 WT mice.
TFPI-cofactor activity in a CAT assay
PS TFPI-cofactor activity was investigated in mouse plasma by measuring the effects of anti-PS and anti-TFPI antibodies on thrombin generation in CAT, as has been previously described for human plasma.10 A total of 20 μL mouse plasma, to which 2.4 μL corn trypsin inhibitor (Haematologic Technologies) was added, was preincubated or not with 10 μL rabbit polyclonal anti–mouse TFPI (American Diagnostica) and 5 μL rabbit polyclonal anti–human PS (from DakoCytomation) for 10 minutes at 37°C, and mixed with 7.6 μL mouse thromboplastin (mouse TF, mTF)/phospholipids mixture. Mouse thromboplastin was purified and quantified, as previously described.31 Thrombin generation was initiated at 33°C with 15 μL fluorogenic substrate/CaCl2 mixture. Final concentrations were as follows: 33.3% mouse plasma, 40 μg/mL corn trypsin inhibitor, 0.03 pM mTF, 30 μM phospholipids, 8.2 mM CaCl2, and 0.42 mM fluorogenic substrate plus or minus 0.17 mg/mL anti–mouse TFPI antibody and 0.35 mg/mL anti–human PS antibody.
TF-induced pulmonary embolism
A model of venous thromboembolism was adapted from Weiss et al,32 with minor modifications. Mice, aged 5 to 6 weeks (18-25 g), were anesthetized by intraperitoneal injection of a mixture of ketamine (80 mg/kg) and xylazine (16 mg/kg), and hrTF (Innovin; Dade Behring) was injected intravenously (2 μL/g body weight) via the inferior vena cava. Two dilutions of TF (1:2 and 1:8 in 0.9% NaCl) were used. The time to the onset of respiratory arrest that lasted at least 2 minutes was recorded and chosen as the time to death. Experiments were terminated at 20 minutes.
Two minutes after the onset of respiratory arrest or at the completion of the 20-minute observation period, lungs, livers, and kidneys were excised, and fixed in 10% formol. Lung sections (5 μm) were stained with hematoxylin and eosin (H&E) and examined.
Timed matings and embryo harvesting
Timed natural matings of Pros+/− heterozygous mice were set to generate late-stage E17.5 embryos. Embryos were harvested and photographed, and DNA was extracted from the whole tails for genotyping. E17.5 embryos were fixed in 10% formol, and were dehydrated and embedded in paraffin. Sagittal sections (10 μm) throughout the body were stained with H&E or immunostained with a polyclonal antibody raised against mouse fibrinogen (Nordic Immunologic Laboratories) at a working dilution of 1/200.
Statistical analysis
Values were expressed as mean plus or minus SEM. Survival data in the model of TF-induced venous thromboembolism were plotted using the method of Kaplan-Meier. Log-rank test was used to compare statistically the curves (Prism 5.01; GraphPad). The other data were analyzed by Student t test with GraphPad Prism 5.01. A value of P less than .05 was considered statistically significant.
Results
As the clinical manifestations are dramatically severe and life threatening in the extremely rare homozygous or compound heterozygous PS-deficient patients, we chose a conditional gene-targeting approach to generate mice with deficiency in PS. A region of the Pros gene encompassing the exons 3 to 7 was flanked with loxP sites in the conditional gene-targeting vector, so that Cre-mediated recombination would result in a null allele by introducing a shift in the reading frame and stop codons throughout the remaining exons (Figure 1A).
A novel recombineering approach25 allowed us to readily and rapidly generate a 23.7-kb targeting vector comprising a single loxP site 5′ of exon 3 and an Frt-neo-Frt-loxP cassette 3′ of exon 7 (Figure 1A). Although the distance between the 2 loxP sites was long (approximately 17.5 kb), a very high rate of Cre-mediated recombination and correct excision of the floxed DNA fragment was obtained in an inducible Escherichia coli strain capable of Cre-mediated recombination (EL350 cells).33 After electroporation of the purified linearized targeting vector in 129Sv embryonic stem (ES) cells, a total of 576 G418-resistant clones was screened by PCR and Southern blotting for correct and single incorporation into the Pros genomic locus (see supplemental methods). Five clones were found to be correctly targeted, and one was injected into C57BL/6J blastocysts to finally generate 3 male chimeras. Germline transmission of the targeted floxNeo allele to F1ProsfloxNeo/+ mice (carrying one copy of a WT allele and one copy of a floxNeo allele) was evidenced by PCR and Southern blotting (results not shown), as described in supplemental methods.
In the present study, we sought to validate our methodologic approach by generating and characterizing the phenotype of Pros null mice. To this aim, we first generated Pros+/− heterozygous mice carrying one copy of a WT allele and one copy of a Cre-recombined null (−) allele, by crossing F1ProsfloxNeo/+ mice to a general Cre-deleter strain (Nes-Cre1; see supplemental methods).27,34 Pros+/− mice were genotyped by Southern blotting and PCR (Figure 1B-C). Pros+/− mice showed reduced levels of plasma PS in Western blots (Figure 1D). Semiquantitative Western blotting analysis was made to quantify PS antigen levels in WT (n = 3) and Pros+/− (n = 3) mouse plasmas, based on densitometric analysis of PS band intensity values, with the mean value for WT plasmas taken as 100%. The Western blotting results showed that PS levels were 100.0% (± 16.3%) and 39.6% (± 6.8%) for WT and Pros+/− plasmas, respectively (P = .03).
Pros+/− mice were viable and did not present any gross anatomical abnormalities. Blood cell counts were normal in Pros+/− mice compared with WT mice (data not shown). Hemostatic parameters were also measured in WT (n = 5) and Pros+/− (n = 5) plasmas. Prothrombin times were 10.4 (± 0.2) seconds and 10.6 (± 0.3) seconds for WT and Pros+/− plasmas, respectively (P = n.s.), and APTT were 31.1 (± 1.3) seconds and 32.4 (± 1.1) seconds for WT and Pros+/− plasmas, respectively (P = n.s.). Levels of coagulation factors were indistinguishable between WT and Pros+/− plasmas (P = n.s.).
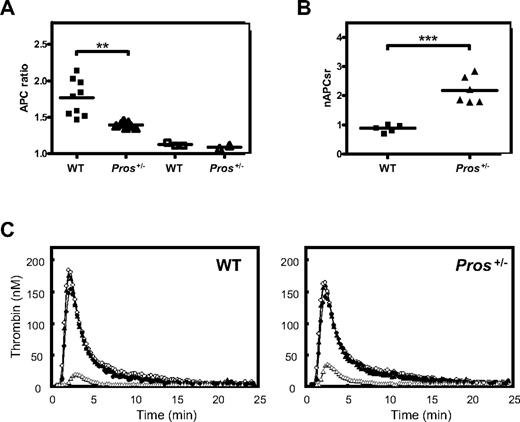
To test whether Pros+/− mice exhibited defective functional PS APC-cofactor activity, we used an APTT assay in the absence and presence of 0.9 nM wt-rmAPC (final concentration). Clotting times were recorded for WT (n = 9) and Pros+/− plasmas (n = 7), and APC-cofactor activities were inferred from the ratio of clotting times with wt-rmAPC and without wt-rmAPC (APC ratio). Mean APC ratios were 1.77 (± 0.08) and 1.39 (± 0.01) for WT and Pros+/− plasmas, respectively (P = .001; Figure 2A). This indicated an impaired sensitivity to wt-rmAPC in Pros+/− plasmas, compared with WT plasmas. When plasmas were preincubated with saturating amounts of rabbit polyclonal anti–human PS antibody, mean APC ratios were 1.12 (± 0.01) and 1.09 (± 0.02) for WT (n = 3) and Pros+/− (n = 2) plasmas, respectively (Figure 2A), indicating a strong dependency of wt-rmAPC anticoagulant activity on PS under the assay conditions. Thus, a decrease in ratio from 1.77 to 1.39 may be taken to reflect an approximately half-normal level of PS in Pros+/− plasmas.
Measurement of PS APC-cofactor activity in mouse plasma coagulation and mouse thrombin generation assays. (A) PS APC-cofactor activity in a mouse plasma coagulation assay. Prolongation of clotting times was measured for WT (■) or Pros+/− (▲) plasmas, to which 0.9 nM wt-rmAPC (final concentration) was added. An APC ratio was calculated as follows: (clotting times with wt-rmAPC)/(clotting times without wt-rmAPC). Mean APC ratios were 1.77 ± 0.08 for WT plasmas (n = 9) and 1.39 ± 0.01 for Pros+/− plasmas (n = 7; P = .001). For 3 WT (□) and 2 Pros+/− (△) plasmas, clotting times were recorded in the presence of saturating amounts of anti-PROS antibodies (0.13 mg/mL, final concentration). Mean APC ratios were 1.12 ± 0.01 and 1.09 ± 0.02 for WT and Pros+/− plasmas, respectively. (B) The APC-cofactor activity of PS from WT or Pros+/− plasmas was investigated in a CAT assay adapted for mouse plasma (see “Methods”). Mean thrombin generation curves were measured for WT (n = 5) and Pros+/− (n = 6) plasmas and for each genotype, nAPCsr were calculated, as described in “Methods.” The nAPCsr were 0.88 ± 0.06 for WT (n = 5) and 2.18 ± 0.19 for Pros+/− (n = 6) plasmas (P < .001). (C) For each genotype, thrombin generation curves were from pooled data, and mean thrombin generation curves represent the following conditions: the absence (○) and presence (△) of wt-rmAPC (3 nM, final concentration); the absence of wt-rmAPC, but in the presence of anti–human PS antibodies (0.35 mg/mL, final concentration; ▲); and the presence of 3 nM wt-rmAPC and anti–human PS antibodies (0.35 mg/mL, final concentration; ●).
Measurement of PS APC-cofactor activity in mouse plasma coagulation and mouse thrombin generation assays. (A) PS APC-cofactor activity in a mouse plasma coagulation assay. Prolongation of clotting times was measured for WT (■) or Pros+/− (▲) plasmas, to which 0.9 nM wt-rmAPC (final concentration) was added. An APC ratio was calculated as follows: (clotting times with wt-rmAPC)/(clotting times without wt-rmAPC). Mean APC ratios were 1.77 ± 0.08 for WT plasmas (n = 9) and 1.39 ± 0.01 for Pros+/− plasmas (n = 7; P = .001). For 3 WT (□) and 2 Pros+/− (△) plasmas, clotting times were recorded in the presence of saturating amounts of anti-PROS antibodies (0.13 mg/mL, final concentration). Mean APC ratios were 1.12 ± 0.01 and 1.09 ± 0.02 for WT and Pros+/− plasmas, respectively. (B) The APC-cofactor activity of PS from WT or Pros+/− plasmas was investigated in a CAT assay adapted for mouse plasma (see “Methods”). Mean thrombin generation curves were measured for WT (n = 5) and Pros+/− (n = 6) plasmas and for each genotype, nAPCsr were calculated, as described in “Methods.” The nAPCsr were 0.88 ± 0.06 for WT (n = 5) and 2.18 ± 0.19 for Pros+/− (n = 6) plasmas (P < .001). (C) For each genotype, thrombin generation curves were from pooled data, and mean thrombin generation curves represent the following conditions: the absence (○) and presence (△) of wt-rmAPC (3 nM, final concentration); the absence of wt-rmAPC, but in the presence of anti–human PS antibodies (0.35 mg/mL, final concentration; ▲); and the presence of 3 nM wt-rmAPC and anti–human PS antibodies (0.35 mg/mL, final concentration; ●).
The functional effects of low PS levels in Pros+/− mice were also assessed in a CAT-based APC resistance assay.30 In this assay, APC titration showed that the addition of 3 nM wt-rmAPC was able to reduce thrombin generation by approximately 90% in mouse pooled normal plasma (data not shown). Thus, thrombin generation curves were recorded for WT (n = 5) and Pros+/− (n = 6) plasmas in the presence of 3 nM wt-rmAPC. The results demonstrated that thrombin generation was reduced to 7.3% (± 0.4%) and 17.7% (± 1.5%) of basal thrombin generation (ie, in the absence of wt-rmAPC) for WT and Pros+/− plasmas, respectively (P < .001; Figure 2C). This indicated that Pros+/− plasmas were significantly less responsive to wt-rmAPC than WT plasmas. Data from CAT-based assays were normalized relative to a control mouse pooled normal plasma and gave mean nAPCsr values of 0.88 (± 0.06) for WT plasmas and 2.18 (± 0.19) for Pros+/− plasmas (P < .001; Figure 2B). As in the APTT assays above, addition of anti-PS antibodies to plasmas from both WT and Pros+/− genotypes ablated wt-rmAPC anticoagulant effects, indicating their strong dependence on PS under these assay conditions (Figure 2C). In the absence of wt-rmAPC, no significant difference was found in thrombin generation between Pros+/− and WT plasmas in either the absence or presence of anti-PS antibodies (Figure 2C). Taken together, these results demonstrated that Pros+/− mice exhibited reduced APC-cofactor activity in plasma, compared with WT mice.
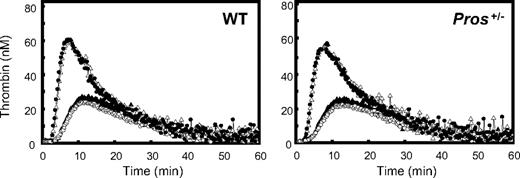
We then investigated the PS TFPI-cofactor activity in a mouse thrombin generation assay. Thrombin generation was initiated with 0.03 pM mTF to achieve basal thrombin generation peak heights between 20 and 40 nM thrombin. These peak heights in human plasma would provide optimal sensitivity for assessment of TFPI-cofactor activity of PS. In mouse plasma, however, there was no difference in the peak height of basal thrombin generation between WT and Pros+/− plasmas. If mouse PS had significant TFPI-cofactor activity, then an increase of basal thrombin generation in Pros+/− plasmas would be expected based on the assumption that mouse PS enhances TFPI activity. In agreement with an absence of TFPI-cofactor activity of mouse PS, addition of anti-PS antibodies that neutralized APC-cofactor activity had no effect on the peak height of basal thrombin generation in both WT and Pros+/− plasmas (Figure 3). Addition of anti-TFPI antibodies increased the peak height of thrombin generation to the same extent in WT and Pros+/− plasmas, confirming the anticoagulant role of TFPI in plasma, but addition of anti-PS antibodies did not further increase the peak height of thrombin generation in the presence of anti-TFPI antibodies alone (Figure 3). Thus, it appears that TFPI-cofactor activity of PS could not be demonstrated in mouse plasma using CAT-based assays that do demonstrate a TFPI-cofactor function for PS in human plasma.10
Measurement of PS TFPI-cofactor activity in thrombin generation assays. The TFPI-cofactor activity of PS from WT or Pros+/− plasmas was investigated in a CAT assay with very low levels of mouse TF (see “TFPI-cofactor activity in a CAT assay”). Mean thrombin generation curves of WT (n = 5) and Pros+/− (n = 6) plasmas were measured under the following conditions: in the absence of anti-PS and anti–mouse TFPI antibodies (○); in the presence of DakoCytomation anti-PS antibodies (0.35 mg/mL, final concentration; ▲); in the presence of anti–mouse TFPI antibodies (0.17 mg/mL, final concentration; ●); and in the presence of both DakoCytomation anti-PS (0.35 mg/mL, final concentration) and anti–mouse TFPI (0.17 mg/mL, final concentration) antibodies (△).
Measurement of PS TFPI-cofactor activity in thrombin generation assays. The TFPI-cofactor activity of PS from WT or Pros+/− plasmas was investigated in a CAT assay with very low levels of mouse TF (see “TFPI-cofactor activity in a CAT assay”). Mean thrombin generation curves of WT (n = 5) and Pros+/− (n = 6) plasmas were measured under the following conditions: in the absence of anti-PS and anti–mouse TFPI antibodies (○); in the presence of DakoCytomation anti-PS antibodies (0.35 mg/mL, final concentration; ▲); in the presence of anti–mouse TFPI antibodies (0.17 mg/mL, final concentration; ●); and in the presence of both DakoCytomation anti-PS (0.35 mg/mL, final concentration) and anti–mouse TFPI (0.17 mg/mL, final concentration) antibodies (△).
We next assessed whether quantitative and qualitative PS defects in Pros+/− mice could result in a thrombotic phenotype in vivo. To date, the oldest Pros+/− mice were 12 months old and did not present abnormal mortality and signs of spontaneous localized or widespread thrombosis. TAT levels were measured in the plasma of Pros+/− and WT mice aged 3 months and 9 to 12 months. They were indistinguishable between both genotypes and did not increase with aging. In 3-month-old mice, TAT levels were 13.5 (± 5.9) μg/L for WT mice (n = 9) and 11.6 (± 3.9) μg/L for Pros+/− mice (n = 9; P = n.s.), whereas in 9- to 12-month-old mice TAT levels were 14.2 (± 4.3) μg/L for WT mice (n = 5) and 10.2 (± 0.7) μg/L for Pros+/− mice (n = 5; P = n.s.). Pros+/− and WT mice were subsequently tested in a TF-induced venous thromboembolism model.32 Two doses of hrTF were injected via the inferior vena cava. With a lethal TF dose (2 μL/g body weight of TF diluted 1/2 in 0.9% NaCl), mice from both genotypes died within the 20-minute observation period. However, the mean time to death was 2.2 (± 0.1) minutes for Pros+/− mice (n = 12), and 4.6 (± 1.1) minutes for WT mice (n = 13; P = .047). We used a lower TF dose (2 μL/g body weight of TF diluted 1/8 in 0.9% NaCl) to achieve a survival rate at 20 minutes of 87.5% in WT mice (n = 8). In contrast, Pros+/− mice (n = 8) were more sensitive to the TF challenge, with a survival rate at 20 minutes of 25%. Survival curves of Pros+/− and WT mice were significantly different (P = .02; Figure 4A). Histologic analysis demonstrated an increased number of thrombi in the lungs of Pros+/− mice that died within the 20-minute observation period (Figure 4B), compared with WT (Figure 4B) and Pros+/− mice that survived the challenge, consistent with a higher thrombotic potential in Pros+/− mice. No thrombi were found in the liver or kidneys of both Pros+/− and WT mice that died from respiratory arrest within 20 minutes (results not shown), in agreement with a previous report.35
TF-induced venous thromboembolism model. (A) Anesthetized mice were injected intravenously with recombinant TF (1/8 dilution of Innovin in 0.9% NaCl; 2 μL/g body weight) via the inferior vena cava. The time to the onset of respiratory arrest that lasted at least 2 minutes was recorded for WT (■; n = 8) and Pros+/− (△; n = 8) mice. Experiments were terminated at 20 minutes. Pros+/− mice showed higher mortality than WT mice (P = .02), and 87.5% WT mice and 25% Pros+/− mice survived the TF challenge. (B) In the low-TF challenge and 2 minutes after onset of respiratory arrest or at the completion of the 20-minute observation period, lungs were excised and stained with H&E. Pros+/− mice that died within the 20-minute observation period had an increased number of thrombi (arrows, right panel), compared with WT mice that survived the TF challenge (left panel).
TF-induced venous thromboembolism model. (A) Anesthetized mice were injected intravenously with recombinant TF (1/8 dilution of Innovin in 0.9% NaCl; 2 μL/g body weight) via the inferior vena cava. The time to the onset of respiratory arrest that lasted at least 2 minutes was recorded for WT (■; n = 8) and Pros+/− (△; n = 8) mice. Experiments were terminated at 20 minutes. Pros+/− mice showed higher mortality than WT mice (P = .02), and 87.5% WT mice and 25% Pros+/− mice survived the TF challenge. (B) In the low-TF challenge and 2 minutes after onset of respiratory arrest or at the completion of the 20-minute observation period, lungs were excised and stained with H&E. Pros+/− mice that died within the 20-minute observation period had an increased number of thrombi (arrows, right panel), compared with WT mice that survived the TF challenge (left panel).
Collectively, these results indicated that Pros+/− mice had reduced plasma levels and anticoagulant activity of PS (ie, APC-cofactor activity). Furthermore, they presented a thrombotic phenotype in vivo when challenged in a TF-induced thromboembolism model. Whereas Pros+/− mice reproduced normally, no Pros−/− mice could be obtained from 5 different breeding pairs of Pros+/− parents. Indeed, genotyping of successive litters (a total of 196 pups) showed that 61 mice (31%) were WT and 135 (69%) were Pros+/− mice. This strongly suggested that homozygous (total) deficiency in PS was lethal.
Female Pros+/− mice were time mated with male Pros+/− mice to harvest late-stage E17.5 embryos. Genotyping of 27 E17.5 embryos demonstrated that 7 (26%) were Pros−/−, 12 (44%) were Pros+/−, and 8 (30%) were WT. Macroscopic examination clearly revealed that the majority of Pros−/− embryos were severely bruised in the head, with localized signs of bleeding and/or thrombosis throughout the body, especially on the back (Figure 5A). Pros−/− exhibited pallor in the feet and the nose, suggesting anemia secondary to bleeding. These massive bleedings were notably visible in macroscopic histologic analysis of a whole section of E17.5 Pros−/− embryos (Figure 5C). No developmental defect or difference in size and in gross anatomy was noted between Pros−/− embryos and their Pros+/− and WT littermates (Figure 5B). Less severe bleedings were observed in the brain of 2 (of 7) Pros−/− embryos that were found to be alive when harvested. However, localized signs of bleeding and/or thrombosis throughout the body (especially on the back) were still evident in these embryos. This indicated that the dramatic phenotype of Pros−/− embryos might be delayed and might manifest at later gestational stages before full-term.
Macroscopic examination of homozygous E17.5 Pros−/− embryos. (A) Most E17.5 Pros−/− embryos were found dead when harvested, and were severely bruised in the head (left panel), together with localized signs of bleedings and/or thrombosis throughout the body, especially on the back (arrows). (B) E17.5 Pros−/− embryos did not show any differences in development and gross anatomy with E17.5 Pros+/− or WT embryos. (C) Extensive intracranial hemorrhages (ICH) and secondary bleedings localized throughout the body (B) were visible in macroscopic histologic analysis of a whole section of E17.5 Pros−/− embryos.
Macroscopic examination of homozygous E17.5 Pros−/− embryos. (A) Most E17.5 Pros−/− embryos were found dead when harvested, and were severely bruised in the head (left panel), together with localized signs of bleedings and/or thrombosis throughout the body, especially on the back (arrows). (B) E17.5 Pros−/− embryos did not show any differences in development and gross anatomy with E17.5 Pros+/− or WT embryos. (C) Extensive intracranial hemorrhages (ICH) and secondary bleedings localized throughout the body (B) were visible in macroscopic histologic analysis of a whole section of E17.5 Pros−/− embryos.
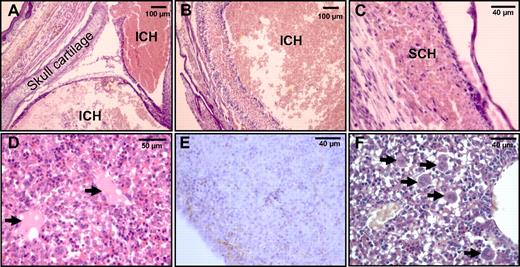
Histologic analyses further demonstrated a necrotic phenotype and the presence of intracranial hemorrhages in Pros−/− brains (Figure 6A-B), together with subcutaneous hemorrhages (Figure 6C). In the liver of Pros−/− embryos, signs of tissue necrosis and loss of hepatocytes were found by histologic analysis (Figure 6D-F). Pros−/− embryos also presented a thrombotic phenotype, mainly in the liver. This was evidenced by the presence of thrombi (Figure 6D) and fibrin deposition (Figure 6E) in their livers. Moreover, histologic analysis showed an increased number of megakaryocytes in Pros−/− livers (Figure 6F), suggesting peripheral thrombocytopenia. Taken together, these observations indicated that Pros−/− embryos exhibited consumptive coagulopathy.
Thrombotic phenotype in homozygous E17.5 Pros−/− embryos. Histologic analysis of E17.5 Pros−/− embryos demonstrated extensive intracranial hemorrhages (ICH; A-B) and subcutaneous hemorrhages (SCH; C), together with the presence of thrombi (arrows) in the liver (D). Fibrin deposition in E17.5 Pros−/− livers was evidenced by immunostaining with a polyclonal antibody raised against mouse fibrinogen (E). Histologic analysis also demonstrated an increased number of megakaryocytes in E17.5 Pros−/− livers (F), secondary to peripheral thrombocytopenia. Together with interstitial fibrin deposition, this suggested consumptive coagulopathy in E17.5 Pros−/− embryos.
Thrombotic phenotype in homozygous E17.5 Pros−/− embryos. Histologic analysis of E17.5 Pros−/− embryos demonstrated extensive intracranial hemorrhages (ICH; A-B) and subcutaneous hemorrhages (SCH; C), together with the presence of thrombi (arrows) in the liver (D). Fibrin deposition in E17.5 Pros−/− livers was evidenced by immunostaining with a polyclonal antibody raised against mouse fibrinogen (E). Histologic analysis also demonstrated an increased number of megakaryocytes in E17.5 Pros−/− livers (F), secondary to peripheral thrombocytopenia. Together with interstitial fibrin deposition, this suggested consumptive coagulopathy in E17.5 Pros−/− embryos.
Thus, homozygous PS deficiency in mice resulted in dramatically severe thrombotic and bleeding phenotype, not compatible with life at the neonatal period, as there was no occurrence of full-term Pros−/− mice to date.
Discussion
An increasing number of mouse models have been developed in the field of thrombosis research, but a model of PS deficiency was long awaited. As for any animal model mimicking human disease, whether our mouse model of PS deficiency can adequately reflect its human counterpart is questionable.
Inherited heterozygous PS deficiency in humans is associated with an increased risk of venous thrombosis. Symptomatic patients can present with unprovoked thromboembolic events and are subject to recurrent events. This risk pattern is not readily reflected or easily documented with our Pros+/− mice. No sign of spontaneous thrombosis has yet been noted in these mice, and TAT levels did not increase with aging. In humans, TAT levels are not markedly elevated in PS-deficient heterozygotes. The in vivo thrombotic phenotype we document in this study was provoked by injection of TF. Spontaneously, thrombotic PS-deficient murine phenotypes mimicking the human situation may likely require the generation of mice with more severe PS deficiency, as reported for protein C-deficient mice.36 To characterize such mice with more severe PS deficiency, quantitation of PS levels by enzyme-linked immunosorbent assay or related methods will be needed, as to date no assay for normal PS plasma levels in mice has been reported in the literature.37 This is mainly due to the lack of a sensitive and specific immunoassay, as has been developed for detecting mouse plasma APC.38 In the present study, we were able to use our rabbit polyclonal anti–mouse PS antibody to detect mouse plasma PS on Western blots. Semiquantitative Western blotting data indicated that our heterozygous Pros+/− mice have approximately 40% PS antigen in plasma.
PS-deficient patients who experience venous thromboembolism often have coinherited other prothrombotic gene mutations (thrombophilic defects such as the common homozygous FVLeiden or prothrombin G20210A mutations), precluding from systematically identifying an important role of PS alone in thrombosis. Pros+/− mice are theoretically free of prothrombotic gene mutations, but the thrombotic potential of Pros+/− mice might be aggravated by crossing the latter mice to genetically engineered mice with thrombophilic defects (eg, mice carrying the homozygous FVLeiden mutation [FvQ/Q mice]39 or mice deficient in protein C22).
In human plasma, C4b-binding protein (C4BP) is a major, but subtle regulator of PS anticoagulant activity,8 rather than an absolute inhibitor of APC-cofactor activity of PS because the PS-C4BP complex stimulates the APC-cleavage of FVa at R306, while inhibiting the APC cleavage at R506.40 Thus, there is most likely an overall inhibition of FVa inactivation when C4BP is added to plasma41 ; however, the human PS-C4BP complex retains direct PS anticoagulant activity42 and is still a TFPI cofactor in the inhibition of TF pathway.9 In contrast, PS does not form a complex with C4BP in mouse plasma,43 indicating that a mouse model of PS deficiency will reflect a model for deficiency of free PS relative to pathophysiology. Thus, PS deficiency murine models will allow us to investigate the effect of free PS on thrombosis, but any potentially important roles of PS bound to C4BP will not be evaluated.
Moreover, whether a mild heterozygous PS deficiency in mice results in a thrombotic phenotype, for example, when mice are subjected to mild prothrombotic injuries, merits future investigations. Although heterozygous Pros+/− mice had a significant, but moderate reduction in APC-cofactor activity in vitro, they exhibited an apparently strong thrombotic phenotype in the TF-induced thromboembolism model. The determinants in this model are not precisely known, and notably, the importance of the anticoagulant protein C pathway in this acute thrombosis model has not been investigated with genetically modified mice. However, this model could be related to a model of DIC with elevated plasma TAT levels and platelet consumption, and it has been found to be highly dependent on thrombin generation.35 This indicated that PS is an important negative regulator of thrombin generation in vivo in mice, and that mild deficiency in PS does exhibit hypercoagulable state in severely challenged mice. The physiologic role for the antithrombotic activity of PS is also evidenced by the dramatically severe phenotype incompatible with life observed in homozygous Pros−/− mice.
Interestingly, our mouse model of PS deficiency can be compared with other mouse models targeting proteins (protein C and TFPI) implicated in the PS anticoagulant activity in humans. As observed in humans, deficiencies in PS and protein C seem to be similar in mice. This is illustrated by the comparable phenotypes of mice with a homozygous deficiency in PS and protein C (Proc−/− mice).22 Similarly to Proc−/− mice, E17.5 Pros−/− embryos are characterized by a necrotic and thrombotic phenotype in the brain and liver, respectively. Such phenotypes might also manifest at later gestational stages for Pros−/− embryos, but there was no occurrence of Pros−/− newborns in our hands. Whereas severe consumptive coagulopathy has been evidenced in Proc−/− newborns in the neonatal period,22 consumptive coagulopathy was only inferred in E17.5 Pros−/− embryos by interstitial and intravascular fibrin deposition accompanied by an increased number of megakaryocytes in the liver, suggesting peripheral thrombocytopenia. Although we demonstrated that Pros+/− mice had reduced APC-cofactor activity, we were unable to demonstrate any TFPI-cofactor activity in mouse plasma, whereas it has been readily detected in human plasma.9,10 Further studies in purified systems will be required to investigate the apparent lack of such an activity in mouse plasma and its species-specific molecular determinants. Whereas Pros−/− and Proc−/− mice appear to exhibit similar thrombotic phenotypes, total disruption of Tfpi gene leads to a more severe thrombotic phenotype, with intrauterine lethality and a small proportion of Tfpi−/− embryos surviving at late gestational stages.21
To conclude, we have generated and validated a mouse model of PS deficiency. Total deficiency in PS was lethal, and mild PS deficiency was associated with a thrombotic phenotype in vivo. Further studies are underway to investigate whether Pros+/− mice might be defective in systems involving activation of TAM receptors by PS. In the future, our conditional gene-targeting approach will also allow us to generate tissue-specific or inducible repression of PS expression to gain insight into the intriguing biologic functions of PS in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Xiao Xu and Elena Guirao for excellent technical assistance, and the transgenic animal facility of the Faculty of Biology and Medicine and the University Hospitals in Lausanne for help in generating the PS-deficient mice. We also are grateful to Myriam Guidoux for her help in realizing figures of embryos.
This work was supported by Swiss National Foundation for Scientific Research Grant PP00B-106690/1 (to A.A.-S.), National Institutes of Health Grant HL031950 (to J.H.G.), and the Pierre Mercier pour la Science Foundation (to A.A.-S.). R.C. was supported by Swiss National Foundation for Scientific Research Grant PP00A-106714.
National Institutes of Health
Authorship
Contribution: F.S. performed the research and wrote the paper; A.C.B., M.A., S.N.T., and T.M.H. performed the research; T.M.H. and M.S. participated in writing the paper; R.C. contributed to the experimental design and construction of the conditional gene-targeting vector, and participated in writing the paper; J.A.F. and J.H.G. produced wt-rmAPC and anti–mouse PS antibody and participated in writing the paper; and A.A.-S. designed and organized the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Angelillo-Scherrer, Service and Central Laboratory of Hematology, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Rue du Bugnon 46, CH-1011 Lausanne, Switzerland; e-mail: Anne.Angelillo-Scherrer@chuv.ch.

![Figure 1. Targeted disruption of the Pros gene. (A) A 23.7-kb targeting vector was constructed that contains a single loxP site 5′ of Pros exon 3, and an Frt-neo-Frt-loxP cassette 3′ of exon 7 (loxP and Frt sites are depicted by red and blue triangles, respectively). Restriction sites StuI (S) and DraIII (D) were introduced at these sites for genotyping purposes. The long 5′ homology arm was 4 kb, and the short 3′ homology arm was 2 kb. Homologous recombination in 129Sv ES cells resulted in a floxNeo allele that was detected by long-range PCR and Southern blotting analysis, as described in supplemental Figure 1. One correctly targeted ES cell clone was injected into blastocysts of C57BL/6J mice to generate chimeras, which in turn transmitted the floxNeo allele to F1 ProsfloxNeo/+ mice. These mice were genotyped by PCR (see supplemental Figure 1). Cre-mediated recombination through crossing of ProsfloxNeo/+ mice to a general Cre deleter strain (Nes-Cre1) resulted in the generation of a null (−) allele. Germline transmission of this null allele yielded Pros+/− mice in a mixed 129Sv-C57BL/6J genetic background. (B) WT, Pros+/−, and Pros−/− mice were genotyped by Southern blotting. Genomic tail DNA of E17.5 embryos from the 3 genotypes were digested by StuI and hybridized with the 3′DraIII probe (Figure 1A). The WT band was 12.2 kb, and the null (−) band was 4.4 kb (arrows). (C) WT, Pros+/−, and Pros−/− mice were genotyped by 2 separate PCRs with mouse tail genomic DNA as a template and primer couples PROS-Fwd/PROS-Rv (F and R, respectively [A]) for the WT band (234 pb), and PROS-Fwd/PROSnull-Rv (F and Rnull, respectively [A]) for the null band (571 pb). (D) Reduced levels of PS in the plasma of Pros+/− mice compared with WT mice were demonstrated after polyacrylamide gel electrophoresis of nonreduced plasma samples by Western blotting with a rabbit polyclonal antibody raised against mouse PS. Mouse plasma PS migrated at approximately 80 kDa (arrow).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/11/10.1182_blood-2009-03-209031/4/m_zh89990941030001.jpeg?Expires=1765895495&Signature=HztmuD1gQMlnsSFdoXvdaQHMTTXyslu6dDevuLCmCmHWgF7T5u1tWBay9iHcuVYXXhPdrXrlB9daQlEFC~dcWhPyGcx-PT6xjWNJWbg9NH0M-iR4COErxHdzMK6FL9z1RhHok3R93EqyQJrIIPd4WFeAYLW15EZ~CWrOJdYsgQNCKiDZVLGU5e5OF822Qqo6x7og7HP1WIV3BXe5-TAnZj5S~0INTaqrOlXNC0fWFH2t-gINfPiukuqraZbczsjGN4QQPza9L0iI4GYzz7gySLZZw05IxdB3m-XhS5K91aQo14KiALy893scYLv7l7zE5vxSe6CPNtfkOiebkIVcNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal