Abstract
ADAMTS13 is a plasma metalloproteinase that regulates platelet adhesion and aggregation through cleavage of von Willebrand factor (VWF) multimers. In humans, genetic or acquired deficiency in ADAMTS13 causes thrombotic thrombocytopenic purpura (TTP), a condition characterized by thrombocytopenia and hemolytic anemia with microvascular platelet thrombi. In this study, we report characterization of mice bearing a targeted disruption of the Adamts13 gene. ADAMTS13-deficient mice were born in the expected mendelian distribution; homozygous mice were viable and fertile. Hematologic and histologic analyses failed to detect any evidence of thrombocytopenia, hemolytic anemia, or microvascular thrombosis. However, unusually large VWF multimers were observed in plasma of homozygotes. Thrombus formation on immobilized collagen under flow was significantly elevated in homozygotes in comparison with wild-type mice. Thrombocytopenia was more severely induced in homozygotes than in wild-type mice after intravenous injection of a mixture of collagen and epinephrine. Thus, a complete lack of ADAMTS13 in mice was a prothrombotic state, but it alone was not sufficient to cause TTP-like symptoms. The phenotypic differences of ADAMTS13 deficiencies between humans and mice may reflect differences in hemostatic system functioning in these species. Alternatively, factors in addition to ADAMTS13 deficiency may be necessary for development of TTP.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening systemic disease, characterized by anemia, thrombocytopenia, and microvascular thrombosis.1-4 Hemolysis, the cause of the anemia, generates pointed red cell fragments, schistocytes. Thrombocytopenia is caused by the consumption of platelets in thrombi, which cause renal and neurologic dysfunction. Without treatment, the mortality rate of affected patients exceeds 90%, but plasma exchange reduces the death rate to approximately 20%.5
Our understanding of TTP pathophysiology increased considerably with the identification of ADAMTS13, which specifically cleaves the Tyr1605-Met1606 peptidyl bond of von Willebrand factor (VWF).6-10 VWF is a large glycoprotein that mediates platelet adhesion to vascular lesions. It is mainly synthesized in endothelial cells and secreted into the blood as “unusually large” VWF (UL-VWF) multimers, the highly active forms for platelet adhesion and aggregation.11,12 ADAMTS13 cleaves UL-VWF multimers into smaller forms under flow, limiting platelet thrombus formation under normal conditions. Severe deficiency in ADAMTS13 activity is observed in most patients with TTP, allowing UL-VWF multimers to persist in the circulation.1-4 UL-VWF multimers mediate enhanced platelet clumping under shear stress, which is thought to cause the clinical symptoms of TTP. Congenital TTP is associated with mutations in the ADAMTS13 gene, whereas acquired TTP results from the production of autoantibodies against ADAMTS13. A number of causative mutations for congenital TTP have been identified within the ADAMTS13 gene.3,4 In vitro expression studies have confirmed the deleterious effects of mutant ADAMTS13 on proteolytic activity or secretion.13-15
Here, we generated a mouse model of ADAMTS13 deficiency by a gene-targeting approach, to further understand the pathophysiologic process of TTP. We found that the complete deficiency in ADAMTS13 is not sufficient to produce in mice the typical TTP phenotype. Other triggers may be needed to provoke the disease.
Materials and methods
Generation of ADAMTS13-deficient mice
The isolation of λ phage genomic clones containing Adamts13 has been previously described.16 The targeting vector was constructed from a 12.3-kb fragment including exons 3-12, in which the 3.6-kb SalI-EcoRI region containing exons 3-6 was replaced by a neomycin resistance cassette. A diphtheria toxin A fragment expression cassette was inserted into downstream of the 3′-homologous region. The vector was introduced into 129/Sv-derived R-CMTI-1A embryonic stem cells by electroporation. Cells were selected in medium containing G418 (Invitrogen, Carlsbad, CA) and screened by polymerase chain reaction (PCR) and Southern blot analyses. Targeted cells were microinjected into C57BL/6 blastocysts to generate chimeric mice. The resulting male chimeras were bred to wild-type 129/Sv females to produce heterozygous F1 offspring on the 129/Sv genetic background. Heterozygotes were interbred to obtain homozygous mice. Male mice aged 8 to 12 weeks were used for phenotypic analyses. Pregnant female mice aged 8 months were used for renal histology analysis. Female mice aged 15 to 20 weeks (20-30 g) were used for in vivo thrombosis experiments. All animal procedures were performed in accordance with institutional guidelines and were approved by the Animal Care and Use Committee of the National Cardiovascular Center Research Institute.
Genotypic analysis
gDNA, isolated from ear or kidney, was used for genotyping by PCR or Southern blot analyses. For PCR analysis, DNA amplification was performed using a mixture of 3 primers: an intron 2–specific forward primer (5′-ACCCTATCTCTGGCCTGTATTCCT-3′), an intron 3–specific reverse primer (5′-TACTGACTTGTGACCACAAGCCCT-3′), and a neo cassette-specific reverse primer (5′-ATCGAGTCTAGCTTGGCTGGACGT-3′). For Southern blot analysis, a 580-bp fragment upstream of the 5′-homologous region was generated by PCR with primers 5′-TGTCTGCAAGTGCAGTGAGAGGCA-3′ and 5′-AATGAAGATGGCACCAGTGAGGAT-3′ and used for the synthesis of a fluorescein-labeled probe. The probe was hybridized to HindIII-digested gDNA and detected using a CDP-Star detection module (Amersham, Piscataway, NJ).
RT-PCR analysis
Total RNA was prepared from liver using ISOGEN reagent (Nippon Gene, Tokyo, Japan) and subjected to 1-step reverse transcription-PCR (RT-PCR; Qiagen, Hilden, Germany). An exon 21/22-specific sense primer (5′-TTGTGGGAGAGGTCTGAAGGAACT-3′) and an exon 24/25-specific antisense primer (5′-ACAGGAGACAGAGCACTCTGTCCA-3′) were used to amplify ADAMTS13 mRNA.
In situ hybridization
In situ hybridization was performed as described.17 A 435-bp mouse Adamts13 cDNA fragment (nucleotides: 679-1113) was used to synthesize digoxigenin-labeled sense and antisense RNA probes by in vitro transcription with a DIG RNA labeling mix (Roche, Basel, Switzerland). The probe was hybridized to liver sections and detected using an anti-DIG AP conjugate (Roche) and NBT/BCIP solution (Roche). Sections were counterstained with Kernechtrot solution.
Measurement of plasma ADAMTS13 activity
With the mice under ether anesthesia, blood was collected from the retro-orbital plexus into tubes containing a 0.1 volume of 3.8% sodium citrate. Plasma was prepared from blood by centrifugation at 800g for 15 minutes at room temperature. ADAMTS13 activity was measured using a recombinant substrate, GST-mVWF73-H, as described.16,18 Activity was also measured using a fluorogenic substrate, FRETS-VWF73 (Peptide Institute, Minoh, Japan).19
VWF multimer analysis
Plasma samples, diluted in sodium dodecyl sulfate (SDS) sample buffer (10 mM Tris-HCl, 2% SDS, 2 mM EDTA, 0.02% bromphenol blue, and 43.5% glycerol, pH 6.8) were electrophoresed on a 1% agarose gel (Agarose IEF; Amersham) at a constant current of 15 mA at 4°C. After transfer to a nitrocellulose membrane (Bio-Rad, Hercules, CA) by capillary blotting, the membrane was incubated in peroxidase-conjugated rabbit anti–human VWF (1:500, Dako, Glostrup, Denmark) in 5% skim milk to detect VWF multimers. Bound antibody was detected with Western Lighting Chemiluminescence Reagent Plus (Perkin-Elmer, Boston, MA) on an image analyzer (Fujifilm, Tokyo, Japan). The chemiluminescent intensities of each lane were scanned using Image Gauge software (Fujifilm); the relative intensity profiles were shown.
Hematologic analysis
Blood cell counts and hematocrit were determined using an automatic cell counter (KX-21NV; Sysmex, Kobe, Japan). Peripheral blood smears were stained with May-Grünwald-Giemsa and examined under light microscopy. Plasma haptoglobin levels were analyzed using a mouse haptoglobin enzyme-linked immunosorbent assay (ELISA) test kit (Life Diagnostics, West Chester, PA).
Plasma VWF antigen was measured by ELISA using antibodies against human VWF. Plasma samples in 1% BSA were applied to rabbit anti–human VWF-coated (Dako) ELISA plates for 2 hours at room temperature. Bound VWF was detected by incubation with peroxidase-conjugated rabbit anti–human VWF (1:4000, Dako) in 1% BSA for 1 hour. Bound antibody was detected using a SureBlue Reserve TMB Microwell Peroxidase Substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD); the absorbance at 450 nm was measured. A standard curve was constructed from the pooled plasma of 129/Sv mice.
Plasma fibrinogen levels were also measured by ELISA using rabbit anti–human fibrinogen (Dako) and peroxidase-conjugated goat anti–mouse fibrinogen (Nordic Immunological Laboratories, Tilburg, The Netherlands) antibodies. Plasma factor VIII (FVIII) activity was measured using a Testzym FVIII Kit (Daiichi Pure Chemicals, Tokyo, Japan). To assess the ELISA and FVIII activity data, the levels measured in wild-type mice were arbitrarily defined as 100%.
Histologic analysis
The kidneys of pregnant female mice were fixed in phosphate-buffered 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin and eosin or periodic acid-Schiff reagent. VWF antigen was detected using an ENVISION+ system (Dako) with rabbit anti–human VWF (Dako).
Coagulation tests and bleeding assay
The prothrombin time (PT) and activated partial thromboplastin time (APTT) of plasma samples were determined using Thrombocheck PT (Sysmex) and Thrombocheck APTT (Sysmex) reagents, respectively. Bleeding analysis was performed on mice anesthetized with sodium pentobarbital (50 μg/g). Tails were amputated 3 mm from the tip and immersed in 1 mL PBS at 37°C for 15 minutes. Blood loss was estimated from the comparison of the absorbance of the PBS at 562 nm with that of PBS containing known volumes of mouse blood.
Platelet aggregation analysis
Platelet aggregation was measured using an aggregometer (MC Medical, Tokyo, Japan) as described.20 Platelet counts in platelet-rich plasma (PRP) were adjusted to 3.0 × 105/μL by adding platelet-poor plasma (PPP). Aggregation was initiated by addition of acid-insoluble type I collagen (MC Medical) or botrocetin to PRP. PPP was used as a standard indicating 100% aggregation.
Perfusion assay with a parallel plate flow chamber
Platelet thrombus formation in flowing blood on immobilized collagen was analyzed using a parallel plate flow chamber as described.21,22 Acid-insoluble type I collagen-coated (Sigma, St Louis, MO) glass coverslips were placed in a flow chamber. The chamber was mounted on a fluorescence microscope (Axiovert S100; Carl Zeiss, Oberkochen, Germany) equipped with a 40 ×/0.75 numeric aperture objective lens (Carl Zeiss) and a CCD camera system (DXC-390; Sony, Tokyo, Japan). Blood was collected into tubes containing argatroban (240 μM; Mitsubishi Chemical Corporation, Tokyo, Japan). The fluorescent dye mepacrine (10 μM; Sigma) was added to the blood. Whole blood samples were aspirated through the chamber and across the collagen-coated coverslip by a syringe pump (Harvard Apparatus, South Natic, MA) at a constant flow rate producing a wall shear rate of 750 s–1 The shear rate was calculated from the assumption that the viscosity of mouse blood is equal to that of human blood. To analyze the cumulative thrombus volume, image sets at 1.0-μm z-axis intervals within a defined area (156.4 × 119.6 μm) was captured using MetaMorph software (version 6.1.4; Universal Imaging, West Chester, PA). After blind deconvolution of image sets processed by AutoDeblur software package (version 8.0.2; AutoQuant Imaging, Troy, NY), 3-dimensional volumetric measurements of thrombi were accomplished using VoxBlast software (version 3.0; Vartek, Fairfield, IA).
In vivo thrombosis model
A mixture of 600 ng/g collagen (Nycomed, Roskilde, Denmark) and 60 ng/g epinephrine (Sigma) was injected into tail vein of mice.23 Blood was collected 15 minutes after the injection and platelet counts were determined.
Statistical analysis
Statistical significance was assessed by the Student t test or the χ2 test. Differences were considered to be significant at P below .05.
Results
Generation of ADAMTS13-deficient mice
We previously reported 2 strain-specific forms of the mouse Adamts13 gene.16 In the 129/Sv strain, the Adamts13 gene contains 29 exons, as in human ADAMTS13, encoding a protein with a similar domain organization as human ADAMTS13. Several strains of mice, including the C57BL/6 strain, harbor a retrotransposon insertion, encoding a variant form of ADAMTS13 that lacks the C-terminal domains. Therefore, we generated and analyzed ADAMTS13-deficient mice on a 129/Sv genetic background.
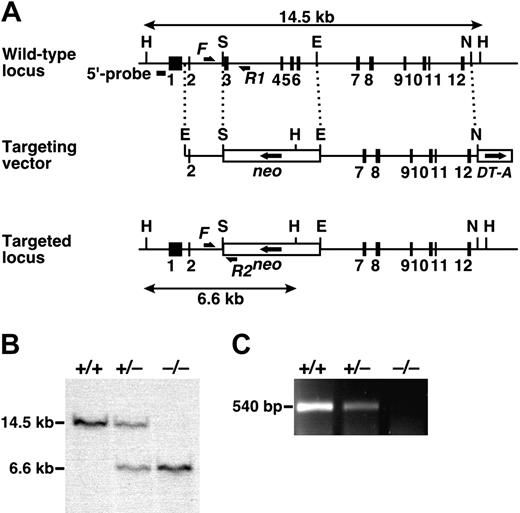
Targeted disruption of the mouse Adamts13 gene. (A) Structure of the targeted locus in the mouse Adamts13 gene. Exons are represented by filled boxes. A neomycin-resistance cassette (neo), in the opposite transcriptional orientation, and a forward-oriented diphtheria toxin A fragment expression cassette (DT-A) are indicated. Homologous fragments are indicated by dotted lines; the HindIII fragments detected by Southern analysis of the wild type and targeted alleles are indicated by double-headed arrows. The sites of primers used for the genotyping PCR (F, R1, and R2) are indicated by arrows. H indicates HindIII; S, SalI; E, EcoRI; N, NcoI. (B) Southern blot analysis. gDNA from offspring obtained from heterozygous intercrosses was digested with HindIII and detected with the 5′-specific probe (wild type: 14.5 kb; targeted allele: 6.6 kb). (C) RT-PCR analysis. Total RNA isolated from mouse liver was reverse-transcribed and amplified using the Adamts13-specific primer set to generate a 540-bp fragment.
Targeted disruption of the mouse Adamts13 gene. (A) Structure of the targeted locus in the mouse Adamts13 gene. Exons are represented by filled boxes. A neomycin-resistance cassette (neo), in the opposite transcriptional orientation, and a forward-oriented diphtheria toxin A fragment expression cassette (DT-A) are indicated. Homologous fragments are indicated by dotted lines; the HindIII fragments detected by Southern analysis of the wild type and targeted alleles are indicated by double-headed arrows. The sites of primers used for the genotyping PCR (F, R1, and R2) are indicated by arrows. H indicates HindIII; S, SalI; E, EcoRI; N, NcoI. (B) Southern blot analysis. gDNA from offspring obtained from heterozygous intercrosses was digested with HindIII and detected with the 5′-specific probe (wild type: 14.5 kb; targeted allele: 6.6 kb). (C) RT-PCR analysis. Total RNA isolated from mouse liver was reverse-transcribed and amplified using the Adamts13-specific primer set to generate a 540-bp fragment.
In situ hybridization analysis of ADAMTS13 mRNA. Liver sections from Adamts13+/+ (top panels) and Adamts13–/– (bottom panels) mice were hybridized to the antisense (left panels) or sense (right panels) Adamts13 RNA probes. The hybridized sections were counterstained with Kernechtrot solution. Typical positive signals are indicated by arrows.
In situ hybridization analysis of ADAMTS13 mRNA. Liver sections from Adamts13+/+ (top panels) and Adamts13–/– (bottom panels) mice were hybridized to the antisense (left panels) or sense (right panels) Adamts13 RNA probes. The hybridized sections were counterstained with Kernechtrot solution. Typical positive signals are indicated by arrows.
The Adamts13 gene was disrupted using a targeting vector that eliminated exons 3-6, encoding the catalytic domain (Figure 1A). The expected structure of the targeted locus was confirmed by PCR (data not shown) and Southern blotting (Figure 1B). Elimination of ADAMTS13 mRNA in Adamts13–/– mice was verified by RT-PCR of total RNA from liver (Figure 1C), the primary site of synthesis.16 In situ hybridization analysis also confirmed the loss of ADAMTS13 mRNA in Adamts13–/– mice (Figure 2). Because ADAMTS13 is expressed in hepatic stellate cells,24,25 we detected hybridization with an antisense probe in the nonparenchymal liver cells of Adamts13+/+ mice. According to their morphology, these cells were hepatic stellate cells. Specific hybridization was not detected in sections from Adamts13–/– mice.
No ADAMTS13 enzymatic activity could be detected in plasma samples of Adamts13–/– mice by either qualitative (Figure 3A) or quantitative (Figure 3B) methods using GST-mVWF73-H and FRETS-VWF73, respectively, as substrates. Enzymatic activity in Adamts13+/– mice was reduced to approximately 35% that seen in Adamts13+/+ mice (Figure 3B).
ADAMTS13 activity in plasma. (A) Qualitative assay using a recombinant substrate, GST-mVWF73-H. The substrate and product bands are indicated by arrows and arrowheads, respectively. (B) Quantitative assay using a fluorogenic substrate, FRETS-VWF73. Data are mean ± SD from 4 mice for each genotype. The average activity measured in wild-type mice was defined as 100%.
ADAMTS13 activity in plasma. (A) Qualitative assay using a recombinant substrate, GST-mVWF73-H. The substrate and product bands are indicated by arrows and arrowheads, respectively. (B) Quantitative assay using a fluorogenic substrate, FRETS-VWF73. Data are mean ± SD from 4 mice for each genotype. The average activity measured in wild-type mice was defined as 100%.
Analysis of plasma VWF multimers. (A) VWF multimer patterns. Plasma samples (1 μL/lane) from Adamts13+/+, Adamts13+/–, and Adamts13–/– mice were electrophoresed on SDS-agarose gels and transferred to nitrocellulose membranes. VWF multimers were detected with anti-VWF antibodies. Normal human plasma (NHP) and ADAMTS13-deficient TTP patient plasma (TTP) were analyzed in parallel (0.2 μL/lane). (B) Relative intensities of plasma VWF multimers. The chemiluminescent intensities of the VWF multimer patterns (A) were scanned using image analysis software. HMW indicates high molecular weight; LMW, low molecular weight.
Analysis of plasma VWF multimers. (A) VWF multimer patterns. Plasma samples (1 μL/lane) from Adamts13+/+, Adamts13+/–, and Adamts13–/– mice were electrophoresed on SDS-agarose gels and transferred to nitrocellulose membranes. VWF multimers were detected with anti-VWF antibodies. Normal human plasma (NHP) and ADAMTS13-deficient TTP patient plasma (TTP) were analyzed in parallel (0.2 μL/lane). (B) Relative intensities of plasma VWF multimers. The chemiluminescent intensities of the VWF multimer patterns (A) were scanned using image analysis software. HMW indicates high molecular weight; LMW, low molecular weight.
Accumulation of UL-VWF multimers in plasma
In humans, genetic defects in ADAMTS13 lead to the accumulation of UL-VWF multimers in plasma. Analysis of VWF-multimer patterns in plasma detected UL-VWF multimers in Adamts13–/– mice (Figure 4), suggesting ADAMTS13 deficiency supports the accumulation of plasma UL-VWF multimers. Because the laddering patterns of VWF multimers in Adamts13+/+ and Adamts13+/– mice were similar, less than half of the normal plasma ADAMTS13 activity (Figure 3B) was sufficient to regulate VWF multimer size. VWF multimers in mice were larger than those in humans (Figure 4B); the multimer sizes seen in Adamts13+/+ mice were similar to those observed in patients with TTP.
No TTP symptoms in ADAMTS13-deficient mice
Genotyping of 195 offspring of Adamts13+/– intercrosses showed the expected 1:2:1 mendelian distribution of Adamts13+/+ (52 of 195), Adamts13+/– (91 of 195), and Adamts13–/– (52 of 195). Thus, ADAMTS13 deficiency did not cause embryonic lethality. Adamts13–/– mice were viable and fertile. To date, 4 Adamts13–/– mice exhibited lateral flexion of upper body; one of them had a cloudy eye. Further study is required to uncover whether this rare phenotype is caused by ADAMTS13 deficiency. Although pregnancy is a triggering event for TTP,26 deficient females survived pregnancy, delivering viable offspring in normal-sized litters. No significant difference in blood cell counts (Table 1) or plasma haptoglobin levels (Table 2) was observed between Adamts13+/+ and Adamts13–/– mice. Peripheral blood smears from Adamts13–/– mice did not show erythrocyte fragmentation (data not shown), demonstrating a lack of spontaneous thrombocytopenia and hemolytic anemia in Adamts13–/– mice. The renal histology of Adamts13–/– mice during pregnancy did not exhibit microvascular thrombi deposition or excessive accumulation of VWF antigen (data not shown). Thus, Adamts13 disruption in mice did not cause TTP-like symptoms.
Blood cell counts
. | Adamts13+/+ . | Adamts13-/- . |
|---|---|---|
| Red blood cell count, × 1012/L | 8.19 ± 0.41 | 7.97 ± 0.25 |
| Hemoglobin level, g/L | 129 ± 5 | 126 ± 4 |
| Hematocrit concentration | .426 ± .021 | .422 ± .008 |
| Platelet count, × 109/L | 512 ± 42 | 532 ± 62 |
. | Adamts13+/+ . | Adamts13-/- . |
|---|---|---|
| Red blood cell count, × 1012/L | 8.19 ± 0.41 | 7.97 ± 0.25 |
| Hemoglobin level, g/L | 129 ± 5 | 126 ± 4 |
| Hematocrit concentration | .426 ± .021 | .422 ± .008 |
| Platelet count, × 109/L | 512 ± 42 | 532 ± 62 |
Values are mean ± SD of 7 mice in each genotype.
Hematologic and coagulation parameters
. | Adamts13+/+ . | Adamts13-/- . |
|---|---|---|
| Haptoglobin, % | 100 ± 67 | 103 ± 69 |
| VWF antigen, % | 100 ± 23 | 129 ± 31* |
| FVIII activity, % | 100 ± 10 | 146 ± 22† |
| Fibrinogen, % | 100 ± 5 | 98 ± 7 |
| PT, s | 16.1 ± 0.8 | 16.0 ± 1.0 |
| APTT, s | 44.2 ± 3.7 | 43.3 ± 2.5 |
| Blood loss, μL | 12.5 ± 8.4 | 9.5 ± 3.1 |
. | Adamts13+/+ . | Adamts13-/- . |
|---|---|---|
| Haptoglobin, % | 100 ± 67 | 103 ± 69 |
| VWF antigen, % | 100 ± 23 | 129 ± 31* |
| FVIII activity, % | 100 ± 10 | 146 ± 22† |
| Fibrinogen, % | 100 ± 5 | 98 ± 7 |
| PT, s | 16.1 ± 0.8 | 16.0 ± 1.0 |
| APTT, s | 44.2 ± 3.7 | 43.3 ± 2.5 |
| Blood loss, μL | 12.5 ± 8.4 | 9.5 ± 3.1 |
Values are mean ± SD of 12 mice in each genotype except for the blood loss, where it is mean ± SD of 18 mice.
P < .05 when compared with Adamts13+/+ mice
P < .001 when compared with Adamts 13+/+ mice.
Increased thrombogenesis in ADAMTS13-deficient mice
Plasma VWF antigen levels in Adamts13–/– mice were elevated in comparison with those from Adamts13+/+ mice (Table 2). The activity of plasma FVIII, which correlates with VWF levels, was also significantly increased in Adamts13–/– mice (Table 2). The plasma fibrinogen levels, however, were comparable between Adamts13+/+ and Adamts13–/– mice (Table 2). PT and APTT suggested the coagulant state in Adamts13–/– mice was normal (Table 2). To investigate the effects of ADAMTS13 deficiency on hemostasis in vivo, we measured blood loss after tail transection. There were no significant differences in blood loss between Adamts13+/+ and Adamts13–/– mice (Table 2), suggesting UL-VWF multimers did not impair hemostasis.
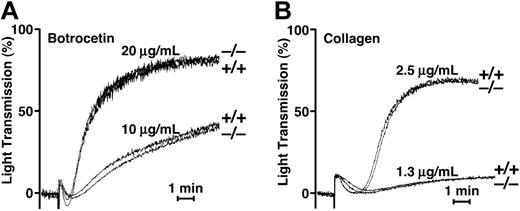
To uncover a latent prothrombotic state caused by the presence of UL-VWF multimers in Adamts13–/– mice, we investigated platelet aggregation under static or flow conditions. We examined agonist-induced platelet aggregation under static conditions. Aggregation responses to botrocetin and collagen in Adamts13–/– mice were indistinguishable from those seen in Adamts13+/+ mice (Figure 5). Thus, an UL-VWF–mediated prothrombotic state could not be detected in Adamts13–/– mice under static conditions.
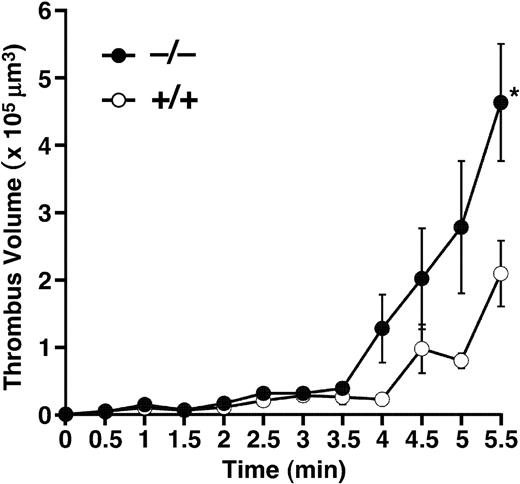
Focusing on thrombus formation under flow, whole blood was perfused over a collagen-coated surface in a parallel plate flow chamber. Even though mice have smaller platelets than humans, thrombus formation was more prominent in mice than in humans, under our flow chamber system. The maximum shear rate to follow up thrombus formation in mouse blood was 750 s–1 and we selected this rate for comparing thrombogenesis between the groups. Cumulative thrombus volume was recorded every 0.5 minute after beginning perfusion (Figure 6). Until 3.5 minutes of perfusion, thrombus formation progressed slowly; the thrombus volume did not differ between the Adamts13+/+ and Adamts13–/– groups. After 3.5 minutes, the thrombus grew rapidly in Adamts13–/– mice; the thrombus volume at 5.5 minutes was significantly higher in Adamts13–/– mice than in Adamts13+/+ mice. Thus, ADAMTS13 deficiency in mice does not affect the initial adhesion of platelets to collagen, but enhances thrombus growth under shear stress.
Platelet aggregation under static condition. (A) Botrocetin-induced aggregation. Pooled PRP samples from Adamts13+/+ or Adamts13–/– mice were treated with botrocetin at a final concentration of 10 or 20 μg/mL. Aggregation was measured using an aggregometer at 37°C with constant stirring. (B) Collagen-induced aggregation. Pooled PRP samples were treated with acid-insoluble type I collagen at a final concentration of 1.3 or 2.5 μg/mL. Bars indicate 1 minute. The results of 3 typical experiments are shown.
Platelet aggregation under static condition. (A) Botrocetin-induced aggregation. Pooled PRP samples from Adamts13+/+ or Adamts13–/– mice were treated with botrocetin at a final concentration of 10 or 20 μg/mL. Aggregation was measured using an aggregometer at 37°C with constant stirring. (B) Collagen-induced aggregation. Pooled PRP samples were treated with acid-insoluble type I collagen at a final concentration of 1.3 or 2.5 μg/mL. Bars indicate 1 minute. The results of 3 typical experiments are shown.
To evaluate in vivo consequence of a lack of ADAMTS13, we examined a model of collagen-induced thrombosis. Under the conditions we examined, the mortality was not different between Adamts13+/+ and Adamts13–/– mice (1 of 12 and 1 of 15 died, respectively, P = .87 by χ2 test). However, platelet counts of treated mice were significantly lower in Adamts13–/– mice than in Adamts13+/+ mice (Figure 7), whereas platelet counts of untreated mice were not different between groups. These results indicate that ADAMTS13 deficiency generates prothrombotic state in vivo as well as in vitro.
Discussion
This study suggests 2 perspectives on the etiology of TTP. First, deficiency in ADAMTS13 alone is sufficient to generate UL-VWF multimers in plasma, leading to a prothrombotic state. Second, ADAMTS13 deficiency is insufficient to produce the typical symptoms of TTP in mice. ADAMTS13 deficiency may induce TTP only when combined with other triggering factors.
Under static conditions, platelet aggregation responses to collagen and botrocetin were indistinguishable in ADAMTS13-deficient mice from those seen in wild-type mice, although the plasma VWF multimer size was larger in ADAMTS13-deficient mice. This result is consistent with the previous report that botrocetin is active on rodent platelets, reacting to a broad spectrum of high to low molecular weight VWF multimers.27 Under flow conditions, however, thrombus formation on a collagen surface was enhanced in ADAMTS13-deficient mice. Although initial platelet adhesion to immobilized collagen was not affected, the growth rate of thrombus was significantly faster in ADAMTS13-deficient mice. In an in vivo thrombosis model, ADAMTS13-deficient mice were more sensitive to collagen-induced thrombocytopenia than wild-type mice, confirming in vitro observation in the flow chamber study. Thus, it was concluded that ADAMTS13 deficiency produces the prothrombotic state. Further study will be necessary to elucidate whether this prothrombotic state is ascribable to hyperreactivity of UL-VWF multimers in ADAMTS13-deficient mice.
Thrombogenesis on collagen surface under flow. Whole blood from Adamts13+/+ or Adamts13–/– mice containing mepacrine-labeled platelets was perfused over an acid-insoluble type I collagen-coated surface at a wall shear rate of 750 s–1. The cumulative thrombus volume, analyzed using a multidimensional imaging system, was measured every 0.5 minute until 5.5 minutes. Data are the mean ± SEM of 5 mice for each genotype. *Significant differences at P < .05 in comparison with Adamts13+/+ mice.
Thrombogenesis on collagen surface under flow. Whole blood from Adamts13+/+ or Adamts13–/– mice containing mepacrine-labeled platelets was perfused over an acid-insoluble type I collagen-coated surface at a wall shear rate of 750 s–1. The cumulative thrombus volume, analyzed using a multidimensional imaging system, was measured every 0.5 minute until 5.5 minutes. Data are the mean ± SEM of 5 mice for each genotype. *Significant differences at P < .05 in comparison with Adamts13+/+ mice.
Platelet counts following collagen plus epinephrine challenge. Mice were given injections of 600 ng/g collagen plus 60 ng/g epinephrine via tail vein and platelet counts were measured 15 minutes after injection. Symbols represent platelet counts of a single mouse. Bars represent the mean values of groups. Platelet counts after the challenge were significantly lower in Adamts13–/– mice (n = 14) than Adamts13+/+ mice (n = 11) at 7.7 ± 2.9 × 104/μL and 16.4 ± 6.2 × 104/μL, respectively (mean ± SD; P < .001), whereas platelet counts without challenge were not different between groups (Adamts13–/–, 86.2 ± 13.2 × 104/μL; Adamts13+/+, 83.7 ± 3.3 × 104/μL; mean ± SD of 4 mice).
Platelet counts following collagen plus epinephrine challenge. Mice were given injections of 600 ng/g collagen plus 60 ng/g epinephrine via tail vein and platelet counts were measured 15 minutes after injection. Symbols represent platelet counts of a single mouse. Bars represent the mean values of groups. Platelet counts after the challenge were significantly lower in Adamts13–/– mice (n = 14) than Adamts13+/+ mice (n = 11) at 7.7 ± 2.9 × 104/μL and 16.4 ± 6.2 × 104/μL, respectively (mean ± SD; P < .001), whereas platelet counts without challenge were not different between groups (Adamts13–/–, 86.2 ± 13.2 × 104/μL; Adamts13+/+, 83.7 ± 3.3 × 104/μL; mean ± SD of 4 mice).
Although prolonged coagulation time was not observed, plasma levels of VWF antigen and FVIII activity were elevated in ADAMTS13-deficient mice, potentially reflecting endothelial damage induced by undetectable platelet aggregates. Alternatively, the plasma clearance rate of VWF multimers without cleavage by ADAMTS13 might be slower than cleaved VWF multimers. High levels of VWF antigen are also seen in the plasma of patients with low ADAMTS13 activity.28
ADAMTS13 deficiency in mice did not cause a major defect in hemostasis that would lead spontaneously to typical TTP symptoms. ADAMTS13 deficiency may cause a milder prothrombotic state in mice than in humans. The plasma VWF multimer sizes in wild-type mice were larger than those seen in humans, comparable to those in human TTP patients (Figure 4B). Mice lacking VWF exhibit milder tendencies to bleed than patients with type 3 von Willebrand disease.29 Thus, the dependence of platelet aggregation on VWF might differ in laboratory mice from humans.
Alternatively, ADAMTS13 deficiency may not be sufficient for the development of TTP, even in humans. There is a large variation in the phenotypes of TTP patients with ADAMTS13 deficiency. Most TTP patients with congenital ADAMTS13 deficiency had their first acute episode in the newborn period or early infancy. Only a number of exceptional cases remain asymptomatic until adulthood.30 Patients with identical ADAMTS13 genotypes, but different symptoms, have also been described,31,32 suggesting that the etiology of TTP cannot be explained by a single defect in ADAMTS13. Secondary triggering factors may promote the pathogenic platelet thrombus formation that results in TTP. Indeed, Motto et al32 independently reported generation of ADAMTS13-deficient mice and revealed that the injection of shigatoxin, a substance toxic to endothelium, provoked TTP-like symptoms in the ADAMTS13-deficient mice. In the present study, we observed enhanced thrombus formation on collagen surface under flow and promoted thrombocytopenia induced by the injection of a mixture of collagen and epinephrine in ADAMTS13-deficient mice. Genetic defects or environmental factors may stimulate endothelial activation or damage via TTP triggers, such as oxidative stress,33 infection,34 antiendothelial cell antibodies,35 or complement dysfunction.36,37 ADAMTS13-deficient mice may be useful to identify TTP triggers.
Prepublished online as Blood First Edition Paper, December 20, 2005; DOI 10.1182/blood-2005-07-2765.
Supported in part by grants-in-aid from the Ministry of Health, Labor, and Welfare of Japan; the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Japan Society for the Promotion of Science; and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biochemical Innovation of Japan.
F.B. designed research, performed research, analyzed data, and wrote the paper; K.K. designed research, performed research, and wrote the paper; T.O. contributed vital analytical tools and interpreted the data; S.H. contributed vital analytical tools and interpreted the data; S.M. contributed vital analytical tools and interpreted the data; H.K. performed research, contributed vital analytical tools, and interpreted the data; Y.T. contributed vital analytical tools and interpreted the data; and T.M. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Yoshihiro Fujimura (Nara Medical University) for providing the botrocetin, Dr Yuji Arai (National Cardiovascular Center Research Institute) for providing the R-CMTI-1A embryonic stem cells, and Ms Yuko Nobe (National Cardiovascular Center Research Institute) for her technical assistance.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal