In this issue of Blood, Banno et al generated and characterized a mouse line with this point mutation of the protein S (PS) gene that is found in the Japanese population.1
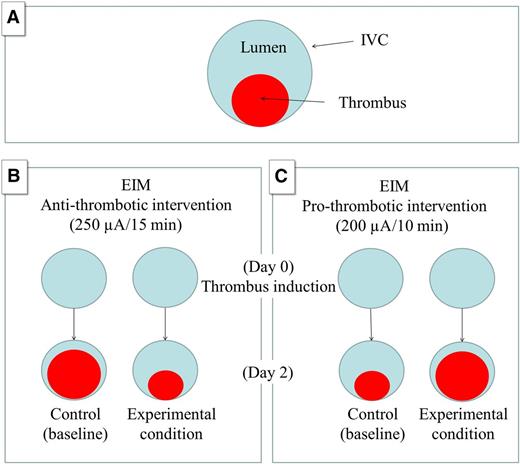
Schematic representation of the animal model of VT (EIM) to study antithrombotic and prothrombotic conditions. (A) IVC (large circle) and the thrombus (small circle). (B) The EIM generates 80% occlusion of the vein wall lumen in control animals by day 2 using 250 µA over 15 minutes. An antithrombotic intervention will reduce the thrombus size at the same time point (experimental condition). (C) The modification introduced by Banno et al (200 µA over 10 minutes) generates a smaller thrombus size in the control animals by day 2, allowing for prothrombotic interventions that will increase the thrombus size at the same time point (experimental condition).
Schematic representation of the animal model of VT (EIM) to study antithrombotic and prothrombotic conditions. (A) IVC (large circle) and the thrombus (small circle). (B) The EIM generates 80% occlusion of the vein wall lumen in control animals by day 2 using 250 µA over 15 minutes. An antithrombotic intervention will reduce the thrombus size at the same time point (experimental condition). (C) The modification introduced by Banno et al (200 µA over 10 minutes) generates a smaller thrombus size in the control animals by day 2, allowing for prothrombotic interventions that will increase the thrombus size at the same time point (experimental condition).
In recent years, there has been an increased interest in understanding the race-specific genetic risk factors for vein thrombosis (VT).2 For example, PS mutation (K196E) has been recognized as a risk factor for VT in the Japanese population. Approximately 1/12 000 individuals is homozygous for the 196E allele, which suggests that ∼10 000 inhabitants in Japan are currently affected.3 To put the magnitude of the problem in perspective, these numbers are larger than the AIDS cases reported by the Annual Report on AIDS Trends, AIDS Surveillance Committee, Ministry of Health, Labor and Welfare of the same country (7203 people).4 Although in vitro studies have shown that recombinant PS with the K196E mutation lose activated protein C (APC)-dependent anticoagulant activity,5 little is known about the pathogenic causality of this mutation.
Specifically, Banno et al generated PS-K196E knock-in mice and analyzed phenotypes comparing homozygous vs heterozygous PS-deficient mice, as well as mice carrying the factor V Leiden mutation, a race-specific genetic risk for VT in whites. Using 2 animal models to test their hypothesis, the findings by Banno et al support that (1) the murine PS-K196E mutation reduces its APC anticoagulant cofactor activity in plasma; (2) PS-K196E mice and heterozygous PS deficiency are more vulnerable to venous thrombosis than wild-type mice, proving pathogenic causality for the K196E mutation; and (3) PS-K196E mice may provide a novel murine resource for in vivo studies of thrombosis.
Finally, I would like to emphasize the critical role of using animal models in research. Banno et al used 2 different animal models to conduct their study: the pulmonary embolism model and the electrolytic inferior vena cava injury model (EIM). Although the EIM was originally developed to produce a nonocclusive and consistent inferior vena cava (IVC) thrombus in the presence of constant blood flow,6,7 Banno et al modified the original technique for the purpose of their study.5 Specifically, the original EIM description includes the activation of endothelial cells within the mouse IVC and thrombus formation using 250 µA of electric current over 15 minutes delivered by a copper wire.6,7 Typically, maximum thrombus burden is observed at day 2 after thrombus initiation with a mean thrombus:lumen ratio of 75%:25%, making this method very suitable to study “thrombus reduction” in a highly sensitive way,7 an attractive option when it comes to drug development and testing the potential role of pharmaceutical compounds for VT.7
Although the original EIM was designed to study reduction in thrombus size, Banno et al introduced modifications to the original method that allow them to study hypercoagulable states.1 They adapted the original EIM to study prothrombotic phenotype mice modifying the time (10 minutes instead of 15 minutes) and the current (200 µA instead of 250 µA), understanding that the thrombotic process in this model is time and current dependent. These modifications resulted in smaller initial thrombus size for control mice (compared with 250 µA/15 minutes in the original technique) allowing the use of procoagulant mice that, in theory, would produce larger thrombus size compared with controls. In my humble opinion, these modifications of the original EIM technique constitute a great contribution to the available EIM model that makes it not only useful to study a potential “reduction” in thrombus size, but also suitable to study potential “increases” in thrombus size (see figure). This is a clear example of how simple changes to an original technique may broaden the application of an animal model. Animal models represent an important tool in the field of VT that crossroads with innumerable disciplines including but not limited to medicine, medical engineering, and, like in this study, molecular epidemiology. This study demonstrates that using animal models may require an open mind (and some creativity) to apply modifications that may result in improvements to an existing model without compromising accuracy, and ultimately assist us in better defining and treating a disease.
Conflict-of-interest disclosure: J.A.D. is on the Board of Directors of the American Venous Forum, as a Research Council Chair.