In this issue of Blood, van Vulpen et al present data that establish the pivotal role of interleukin (IL)-1β in blood-induced cartilage damage and show that blocking its activity can prevent these changes. This not only confirms what had been postulated regarding mechanisms of cartilage damage in hemophilia but also raises possibilities of alternative therapies for preserving joint integrity even after bleeding.1
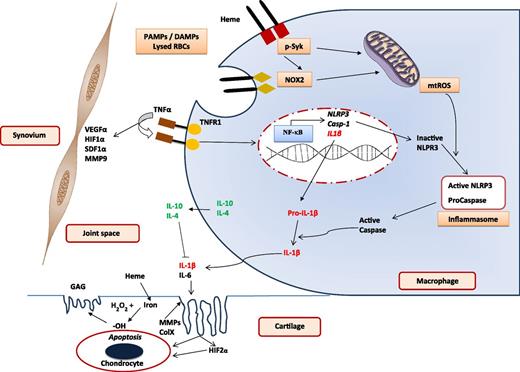
Blood-induced joint damage. Mechanisms involved in IL-1β–mediated inflammation, cartilage damage, and synovial reaction after exposure to blood. Various PAMPs or DAMPs prime macrophages to activate NF-κB and induce expression of NLRP3, caspase-1, and IL-1β. Heme induces Syk and NADPH oxidase 2 (NOX2) activation to generate mtROS required for activation of NLRP3 to create the inflammasome for generating active caspase-1. This in turn generates active IL-1β from the macrophage to initiate the inflammatory response leading to cartilage damage. Other cytokines promote (such as IL-6) and inhibit (such as IL-10 and IL-4) inflammation. A separate set of events, probably initiated by TNFα and mediated by VEGFα, HIFα, SDF1α, and MMP9, lead to synovial inflammation, hyperplasia, and neoangiogenesis. DAMP, distress-associated molecular pattern; GAG, glycosaminoglycan; HIF1α, hypoxia-inducible factor 1α; MMP9, matrix metalloprotease 9; mtROS, mitochondrial reactive oxygen species; NF-κB, nuclear factor-κB; PAMP, pathogen-associated molecular pattern; RBC, red blood cell; SDF1α, stromal cell-derived factor 1α; VEGFα, vascular endothelial growth factor α. The figure is based on Dutra et al,7 Schlesinger et al,9 Sen et al,11 and the article by van Vulpen et al beginning on page 2239.
Blood-induced joint damage. Mechanisms involved in IL-1β–mediated inflammation, cartilage damage, and synovial reaction after exposure to blood. Various PAMPs or DAMPs prime macrophages to activate NF-κB and induce expression of NLRP3, caspase-1, and IL-1β. Heme induces Syk and NADPH oxidase 2 (NOX2) activation to generate mtROS required for activation of NLRP3 to create the inflammasome for generating active caspase-1. This in turn generates active IL-1β from the macrophage to initiate the inflammatory response leading to cartilage damage. Other cytokines promote (such as IL-6) and inhibit (such as IL-10 and IL-4) inflammation. A separate set of events, probably initiated by TNFα and mediated by VEGFα, HIFα, SDF1α, and MMP9, lead to synovial inflammation, hyperplasia, and neoangiogenesis. DAMP, distress-associated molecular pattern; GAG, glycosaminoglycan; HIF1α, hypoxia-inducible factor 1α; MMP9, matrix metalloprotease 9; mtROS, mitochondrial reactive oxygen species; NF-κB, nuclear factor-κB; PAMP, pathogen-associated molecular pattern; RBC, red blood cell; SDF1α, stromal cell-derived factor 1α; VEGFα, vascular endothelial growth factor α. The figure is based on Dutra et al,7 Schlesinger et al,9 Sen et al,11 and the article by van Vulpen et al beginning on page 2239.
It has long been recognized that joint bleeding is the trigger for changes that lead to the development of chronic hemophilic arthropathy. However, the mechanisms involved have been inadequately investigated and are poorly understood. As a consequence, the management of acute hemarthrosis has been confined to early replacement of clotting factor concentrate (CFC) to arrest and prevent bleeding combined with rest and pain relief until symptoms subside.2 No specific therapies have been directed at preventing damage resulting from blood that is already in the joint. Therefore, significant joint changes have been reported even among patients who receive high doses of CFC under monitored conditions.3
Over the last decade, reports from the Lafeber group and others on human cartilage (ex vivo) and work in animal models of joint bleeding have shown that the combination of lysed red cells (heme iron) and monocyte/macrophage leads to generation of reactive oxygen species (ROS) which then led to caspase-mediated chondrocyte damage resulting in decreased proteoglycan synthesis and apoptosis.4 It was demonstrated that these damaging effects, which were probably mediated by IL-1β and tumor necrosis factor (TNF) α, were enhanced by IL-6 but protected by IL-10, IL-4, and anti–IL-6.5,6 These experimental data were backed by clinical observations in patients with severe hemophilia that even among those who bled frequently into their joints, not all sustained cartilage injury whereas others had damaged cartilage with very few bleeds. This suggested that additional mediators were involved in the response to hemarthrosis.3
As an important follow-up to the previous studies, van Vulpen and colleagues have now confirmed that IL-1β is indeed the predominant mediator of cartilage damage (not TNFα) and, even more significantly, that blocking IL-1β with a variety of agents could almost completely prevent chondrocyte apoptosis and consequent damage to joint cartilage. Other recent work has also shown that heme-initiated inflammation through the NLRP3 inflammasome in macrophages leads to activation of IL-1β by caspase 1.7 It is quite likely, therefore, that similar mechanisms work in hemophilia to cause damage to joint cartilage (see figure). The development of a synovial reaction and hyperplasia as well as cartilage damage has often been considered to be a continuum of the same process that results in joint damage in hemophilia. However, clinical observation in minimally treated patients has shown that there can be significant dissociation between these 2 phenomena with some patients developing massive synovial reactions, very often with preserved cartilage and joint space, whereas others may have minimal synovial response but severe damage to articular cartilage. It is likely, therefore, that the mediators in their pathogenesis are different. There is limited data on the mechanisms of synovial changes in hemophilia but synovial proliferation and neoangiogenesis are considered to be significant components with the major mediators being TNFα, VEGFα, SDF1α, HIF1α, and MMP9 among others, all triggered by heme iron.8
Though limited by the ex vivo nature of their model, this work by van Vulpen et al is the first to demonstrate near complete prevention of cartilage damage by blocking IL-1β even after exposure to blood. Although further work is needed to confirm these findings, these data clearly pave the path for interventions which could impact clinical management of hemophilia after acute joint bleeding. With several IL-1 blockers already in clinical use: anakinra (a recombinant IL-1 receptor antagonist), rilonacept (an anti–IL-1β dimeric glycoprotein), and canakinumab (a recombinant human anti-IL-1β monoclonal antibody),9 there are new possibilities for preventing blood-induced joint damage in hemophilia. The therapeutic profile of these parenteral agents may not be best suited for this indication but they could certainly be used to evaluate proof of principle. It would be ideal if there were oral drugs which could be taken soon after a joint bleed for a short period of time during the period associated with damaging inflammatory responses along with CFC replacement to prevent further bleeding.
In a perfect world, we would have CFC replacement products and protocols which would ensure that no spontaneous bleeding occurred into joints. But as this is not likely to be the reality in the near future, we need options for countering the effects of such bleeds. Based on the intensity of CFC replacement, joint bleeds vary from 2 to 10 over 5 years at intermediate to high doses of CFC replacement.10 The cost of prevention of each additional joint bleed in this model of escalation of doses of CFC is estimated to be around $90 000 US dollars and even then success cannot be assured. Specific interventions which can prevent cartilage damage after a bleed would therefore be very desirable. While continuing to work on better CFC replacement protocols which can achieve “zero” bleeds, what the current study shows is that all may not be lost if a few unavoidable joint bleeds do occur as long as we can block the effects of the damaging cytokines that follow. This could be particularly significant for the vast majority of patients in the world who do not have access to prophylaxis with CFC.
Conflict-of-interest disclosure: The author declares no competing financial interests.