Key Points
Approximately 20% to 25% of adults with B-ALL have Ph-like ALL with increased frequency of Ph-like ALL in adults with Hispanic ethnicity.
Adult patients with CRLF2+ ALL have poor long-term outcomes; novel strategies are needed to improve the outcomes.
Abstract
Philadelphia chromosome (Ph)-like acute lymphoblastic leukemia (ALL) is a high-risk subtype of ALL in children. There are conflicting data on the incidence and prognosis of Ph-like ALL in adults. Patients with newly diagnosed B-cell ALL (B-ALL) who received frontline chemotherapy at MD Anderson Cancer Center underwent gene expression profiling of leukemic cells. Of 148 patients, 33.1% had Ph-like, 31.1% had Ph+, and 35.8% had other B-ALL subtypes (B-other). Within the Ph-like ALL cohort, 61% had cytokine receptor-like factor 2 (CRLF2) overexpression. Patients with Ph-like ALL had significantly worse overall survival (OS), and event-free survival compared with B-other with a 5-year survival of 23% (vs 59% for B-other, P = .006). Sixty-eight percent of patients with Ph-like ALL were of Hispanic ethnicity. The following were associated with inferior OS on multivariable analysis: age (hazard ratio [HR], 3.299; P < .001), white blood cell count (HR, 1.910; P = .017), platelet count (HR, 7.437; P = .005), and Ph-like ALL (HR, 1.818; P = .03). Next-generation sequencing of the CRLF2+ group identified mutations in the JAK-STAT and Ras pathway in 85% of patients, and 20% had a CRLF2 mutation. Within the CRLF2+ group, JAK2 mutation was associated with inferior outcomes. Our findings show high frequency of Ph-like ALL in adults, an increased frequency of Ph-like ALL in adults of Hispanic ethnicity, significantly inferior outcomes of adult patients with Ph-like ALL, and significantly worse outcomes in the CRLF2+ subset of Ph-like ALL. Novel strategies are needed to improve the outcome of these patients.
Introduction
Treatment outcomes of adult patients with B-cell acute lymphoblastic leukemia (B-ALL) remain suboptimal with a long-term disease-free survival (DFS) of around 40% to 45%.1,2 This is in contrast to childhood B-ALL where DFS of >90% is routinely achieved.3 The inferior outcome of older patients has been linked to several factors, both disease-related (higher frequency of high-risk genomic subgroups such as Philadelphia chromosome [Ph+]) and patient-related (poor tolerance to chemotherapy). Recently, a high-risk subgroup of B-ALL called Ph-like ALL was identified in children and adolescents and young adults (AYAs).4-7 The leukemic cell gene expression profile of Ph-like ALL is similar to that of Ph+ ALL; however, instead of BCR-ABL1, such patients harbor a highly diverse range of genetic alterations activating tyrosine kinase signaling.6,7 These patients have frequent deletion of the transcription factor IKAROS family zinc finger 1 (IKZF1), also common in Ph+ ALL.4,5,8 Ph-like ALL comprises up to 15% of childhood B-ALL, and 20% to 25% in AYAs.7 These patients have a very high rate of disease relapse and poor overall survival (OS).7,9-11
There are conflicting data regarding the incidence and prognosis of Ph-like ALL in adults.12-15 In a preliminary report of 692 patients with B-ALL, Ph-like ALL comprised 26% of patients between 21 and 39 years of age and 20% of patients age ≥40 years.7,12 These patients were treated on varying pediatric and adult ALL treatment regimens. Significantly inferior outcomes were reported for patients with Ph-like ALL, with a 5-year event-free survival (EFS) and OS of 23.2% and 26.5%, respectively. In contrast, Herold et al analyzed outcomes of 207 patients across all age groups treated on German ALL trials, and reported a lower incidence (13%) of Ph-like ALL in adults.14,15 Similarly, Boer et al analyzed patients treated on various Dutch-Belgian HOVON trials and reported 17% incidence of Ph-like ALL in adults (11% in ≥40-year age group).13 Both EFS and OS were lower in the Ph-like ALL subgroup than in the other B-ALL subgroup, albeit not statistically significant.
Two broad genetic subgroups of Ph-like ALL have been identified.7 Approximately 50% of patients with Ph-like ALL have overexpression of cytokine receptor-like factor 2 (CRLF2).6,7,16-18 Almost half of the patients with CRLF2 overexpression have concomitant JAK-STAT mutations, most commonly JAK2 R683G, which result in JAK-STAT activation amenable to JAK inhibition.7 In Ph-like ALL patients without CRLF2 overexpression, fusions involving JAK2, ABL1, ABL2, and many other tyrosine kinases are common, and many are amenable to ABL-type inhibitors (tyrosine kinase inhibitors [TKIs]) (fusions involving ABL1, ABL2, CSF1R, or PDGFRB) or JAK inhibitors (rearrangements of JAK2 and EPOR).6,7,19 Thus, genomic characterization of Ph-like ALL has significant therapeutic implications with the emerging use of kinase inhibitors in this patient population.6,7,16,20-23 Therefore, it is imperative to establish the incidence and clinical/genomic features of Ph-like ALL in adults. Here, we report the genomic characteristics and outcomes of adult patients with Ph-like ALL uniformly treated with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) or an augmented Berlin-Frankfurt-Münster (BFM) regimen at a single institution. We also report data on targeted next-generation sequencing of 303 recurrently mutated genes in 40 patients with CRLF2 overexpression. Our data demonstrate a high frequency and poor outcome of Ph-like ALL in adults.
Patients and methods
Study groups
A total of 173 samples from newly diagnosed patients with B-ALL underwent genomic testing (see next section). These samples were tested as part of a larger multicenter study defining the genomics of Ph-like ALL.24 Thirty-three of these patients (age, <40 years) were included in a previous report.7 All patients were newly diagnosed and received induction chemotherapy at MD Anderson Cancer Center (MDACC). Twenty-five samples were excluded due to suboptimal sample quality. An additional 7 previously untreated patients were found to have CRLF2 overexpression by flow cytometry, and were confirmed by CRLF2 fluorescence in situ hybridization (FISH; both flow cytometry and FISH were performed at MDACC). These patients received induction chemotherapy at MDACC, and were included in the outcome analysis (but not for subtype frequency calculation). A total of 155 patients were included in the final data analysis reported here. Patients were categorized as Ph-like ALL, Ph+ (BCR-ABL1) ALL, and the remaining patients (not Ph-like, not Ph+) were categorized as B-other ALL. Patient ethnicity was per patient self-report as documented in the medical record at the time of the initial visit. Patients received either hyper-CVAD–based therapies (all age groups) or an augmented BFM regimen (for patients <40 years of age). The details of these regimens have been published previously.1,25-29 The choice of the 2 regimens was based on a variety of factors: time period (hyper-CVAD, year 2000 onwards; augmented BFM, years 2006-2012); age (18 years and older for hyper-CVAD–based regimens; 40 years and younger for augmented BFM); clinical trial eligibility; insurance coverage of clinical trials; and patient or physician preference. In the Ph+ cohort, all but 3 patients received TKIs (imatinib, n = 12; dasatinib, n = 24; ponatinib, n = 7). Response assessment was performed with bone marrow examination including 6-color flow cytometry after induction and every 1 to 2 months thereafter (until the best response was achieved).30 Minimal residual disease (MRD) was assessed by flow cytometry, with a sensitivity of 0.01%. This study was approved by the MDACC, St. Jude Children’s Research Hospital, and University of New Mexico (UNM) Cancer Center Institutional Review Boards and carried out in accordance with the Declaration of Helsinki.
Genomic profiling
Gene expression profiling was performed on 148 RNA samples using either U133 Plus 2.0 microarrays (Affymetrix, Santa Clara, CA) as previously described (n = 30),7 or a customized Taqman low-density array (LDA) card (n = 118) to identify patients with the Ph-like ALL gene signature,31 and additional alterations including BCR-ABL1, ETV6-ABL1, TCF3-PBX1, P2RY8-CRLF2, and ERG-deregulated ALL. Given the tight correlation between the Ph-like prediction results of LDA and gene expression arrays,31 these were used as alternative approaches. Patients with a coefficient of 0.5 to 1 by LDA card were designated as Ph-like. High expression of CRLF2 was determined by LDA (Δ cycle threshold ≤6), and CRLF2 rearrangement (IGH-CRLF2 or P2RY8-CRLF2) was confirmed using FISH.
Single-nucleotide polymorphism (SNP) microarray analysis was performed for cases with available DNA using either SNP 6.0 microarrays (Affymetrix) or the Infinium Omni 2.5 Exome-8 BeadChip kit (Illumina) (details in supplemental Methods, available on the Blood Web site). Targeted next-generation gene sequencing of 303 recurrently mutated genes (L300 panel; MDACC) (see supplemental Methods for details; supplemental Table 1 for gene list) was performed in a cohort of patients with CRLF2+ Ph-like ALL.
FISH
FISH for CRLF2 rearrangement was performed with sequential hybridization for CRLF2, P2RY8, and IGH using probes prepared from clones RP13-167H21, RP11-309M23, and RP13-76L22 (CRLF2 3′), WI2-3390E22, WI2-2865K18, WI2-0735M13, and WI2-1570L13 (region between CRLF2 and P2RY8), RP13-297E16 and RP11-449L4 (P2RY8 5′), and RP5-998D24 and RP11-1065N8 (IGH enhancer).
Statistical analysis
The Fisher exact test and the Mann-Whitney U test were used to assess categorical and continuous variables, respectively. The Kaplan-Meier method was used to asses OS and EFS using the log-rank (Mantel-Cox) test. The OS was calculated as the time from the date of diagnosis to the date of last follow-up or death of any cause. The EFS was calculated from the beginning of treatment until an event (relapse, treatment failure, death during induction, or death during complete remission). Patients who received allogeneic stem cell transplant (allo-SCT) were not censored at the time of transplant. Univariable analysis (UVA) and multivariable analysis (MVA) were performed to identify potential prognostic factors (age, sex, ethnicity, white blood cell [WBC] ≥30 × 109/L, hemoglobin <10 g/dL, platelet <100 × 109/L, central nervous system [CNS] involvement at diagnosis, Ph-like ALL, treatment received) associated with OS. For UVA/MVA, patients with Ph+ ALL were excluded. For MVA, we used the Cox proportional hazards model for OS. A P value <.05 (2-tailed) was considered statistically significant.
Results
Of the 148 patients whose samples were analyzed by gene expression profiling, 49 patients (33.1%) had Ph-like ALL, 46 patients (31.1%) had Ph+ ALL, and 53 patients (35.8%) had B-other ALL (Figure 1). A total of 14 patients within the B-other group had mixed-lineage leukemia (MLL) rearrangements. In the Ph-like ALL cohort, 30 patients (61%) had overexpression of CRLF2+, and 19 (39%) lacked CRLF2 overexpression. In the patients younger than 40 years of age, the incidence of Ph-like ALL was 42% compared with 24% in those 40 years or older (P = .02) (supplemental Figure 1). The incidence of Ph-like ALL was similar in the 40- to 59-year and ≥60 year age group (22% vs 26%, respectively). As expected, the incidence of Ph+ ALL was higher in the older patients (41% in patients ≥40 years compared with 22% in patients <40 years of age).
For further analysis, we included the 7 additional CRLF2+ patients (see “Patients and methods”). The median age for the entire cohort (N = 155) was 38 years (range, 15-84 years). Five patients were <18 years of age. The baseline characteristics of Ph-like ALL, Ph+ ALL, and B-other ALL are listed in Table 1. Patients with Ph-like ALL were younger (median age, 33.5 years) compared with B-other (median age, 38 years; P = .23) and Ph+ ALL (median age, 49 years; P = .001 for 3 group comparison). There were significantly more men in the Ph-like ALL group (66% in Ph-like vs 48% Ph+ vs 36% B-other; P = .006). Interestingly, 68% of the patients with Ph-like ALL were of Hispanic ethnicity. This was significantly higher compared with Ph+ ALL (35%) and B-other (30%) (P < .001). A total of 124 patients (80%) received hyper-CVAD–based induction chemotherapy. The remaining 20% were treated with an augmented BFM regimen. A total of 21 patients underwent allo-SCT in first remission (Ph-like [n = 2, both CRLF2+], Ph+ [n = 9], and B-others [n = 10]).
Clinical outcomes
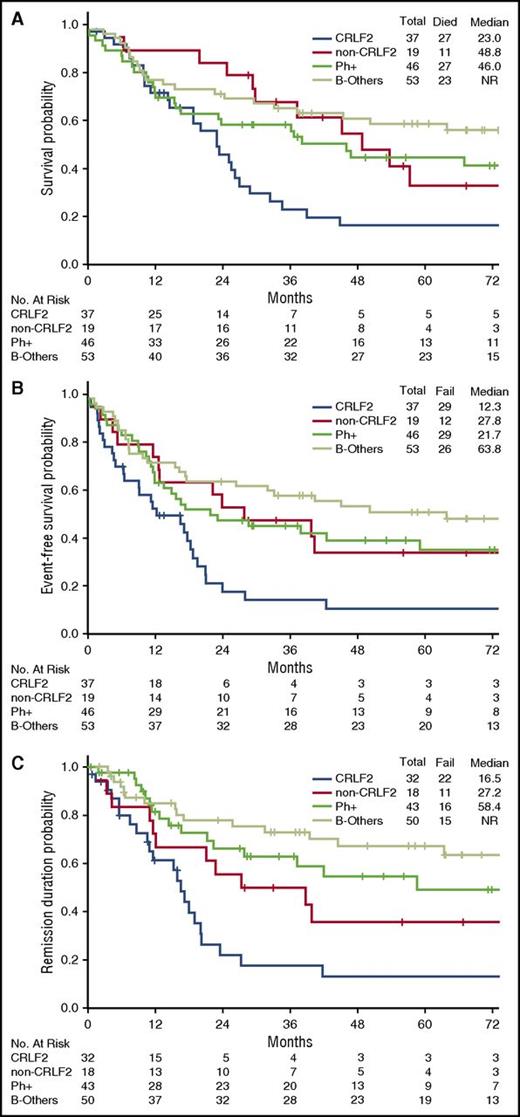
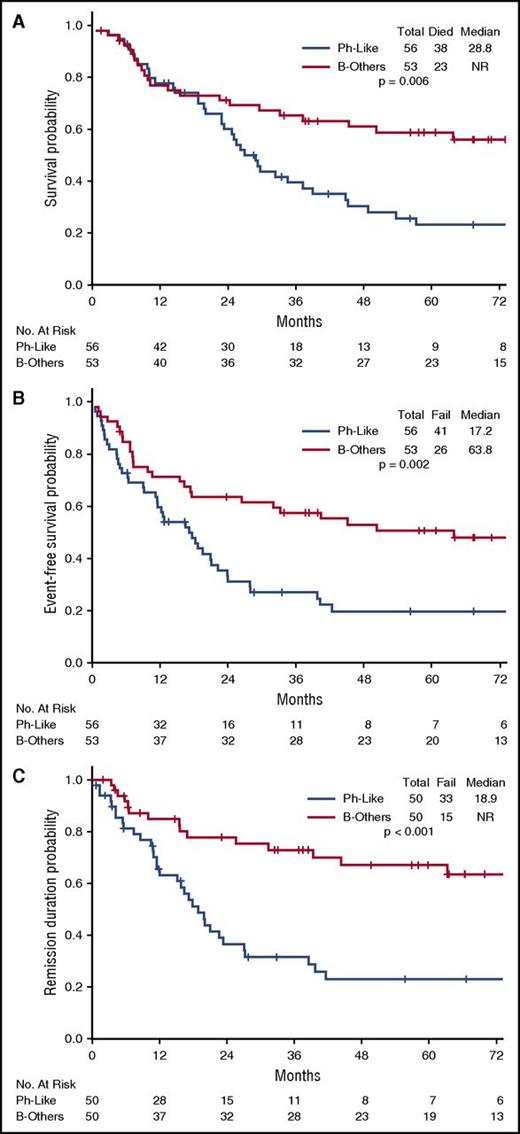
The rate of complete remission (CR)/CR with incomplete platelet recovery (CRp) was similar in the 3 disease subgroups (Ph-like ALL, 89%; Ph+ ALL, 93%; B-other, 94%; P = .57) (Table 2). However, patients with Ph-like ALL were significantly less likely to achieve MRD− remission as assessed by flow cytometry (30% for Ph-like ALL vs 56% for Ph+ ALL vs 87% for B-other; P < .001). Outcomes of the Ph-like ALL and B-other group based on MRD status are shown in supplemental Figure 2. Achievement of MRD negativity at the time of remission had no impact on inferior long-term outcomes of Ph-like ALL subset (median OS; MRD− group, 26.2 months vs MRD+ group, 23.0 months; P = .318). Patients with Ph-like ALL had significantly worse OS, EFS, and remission duration compared with B-other (Figure 2; supplemental Figure 3). The 5-year OS for Ph-like ALL and B-other were 23% and 59%, respectively (P = .006). Patients with MLL rearrangements had very poor outcomes with a median OS of 10.2 months (supplemental Figure 3).
Clinical outcomes of patients with Ph-like ALL and B-other ALL. (A) OS, (B) EFS, and (C) remission duration of Ph-like ALL and B-other ALL.
Clinical outcomes of patients with Ph-like ALL and B-other ALL. (A) OS, (B) EFS, and (C) remission duration of Ph-like ALL and B-other ALL.
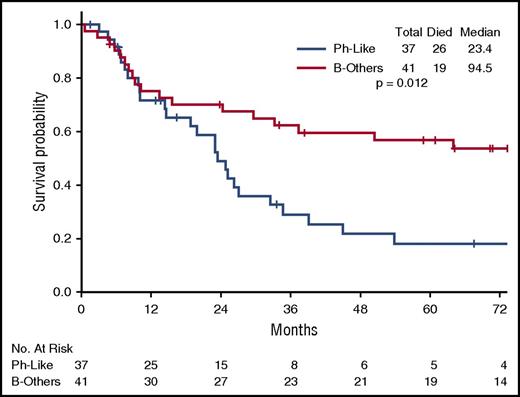
The poor outcome of Ph-like ALL was maintained when only hyper-CVAD–treated patients were considered (Figure 3; supplemental Figure 4). For the hyper-CVAD–treated patients, the median OS for Ph-like ALL and B-others was 23.4 months and 94.5 months, respectively (P = .012).
We did not find evidence that the outcome with the augmented BFM regimen (n = 19) was different from that of hyper-CVAD (n = 18) in Ph-like patients under 40 years of age (P = .505; supplemental Figure 5), but due to small patient numbers we do not have the power to address this question in this study.
Ph-like ALL characteristics, genomics, and outcomes
Table 3 lists the baseline characteristics of Ph-like ALL, divided by CRLF2 expression status. The patients with CRLF2 rearrangement tended to be older with higher WBC count at presentation than non-CRLF2 Ph-like ALL. Seventy-eight percent of patients with CRLF2 overexpression were of Hispanic ethnicity compared with 48% with non-CRLF2 Ph-like ALL and 30% with B-other ALL. From 37 patients with CRLF2 overexpression, 29 patients had FISH performed for CRLF2 rearrangement. The majority of patients had IGH-CRLF2 rearrangement (76%), followed by P2RY8-CRLF2 (17%). Two patients had an unknown fusion partner.
There was no difference in the CR/CRp rate and MRD remission rate between the Ph-like CRLF2+ and Ph-like non-CRLF2 group (supplemental Table 2). Patients with CRLF2 overexpression had significantly inferior OS, EFS, and remission duration when compared with other genomic subgroups, including Ph-like non-CRLF2 group (Figure 4). Notably, 5-year survival in the CRLF2+ group was <20%.
Clinical outcomes of patients with CRLF2+ Ph-like ALL, non-CRLF2 Ph-like ALL, Ph+ ALL, and B-other ALL. (A) OS, (B) EFS, and (C) remission duration of CRLF2+ Ph-like ALL, non-CRLF2 Ph-like ALL, Ph+ ALL, and B-other ALL. (A) For OS, P for comparison between Ph-like CRLF2+ vs B-other was .001; P for comparison between Ph-like CRLF2+ vs Ph-like non-CRLF2 was .01; all other comparisons were not significant. (B) For EFS, P for comparison between Ph-like CRLF2+ vs B-other was <.001; P for comparison between Ph-like CRLF2+ vs Ph-like non-CRLF2 was .01; P for comparison between Ph-like CRLF2+ vs Ph+ was .02; all other comparisons were not significant. (C) For remission duration, P for comparison between Ph-like CRLF2+ vs B-other was <.001; P for comparison between Ph-like CRLF2+ vs Ph+ was .001; P for comparison between Ph-like non-CRLF2 vs B-other was .03; all other comparisons were not significant.
Clinical outcomes of patients with CRLF2+ Ph-like ALL, non-CRLF2 Ph-like ALL, Ph+ ALL, and B-other ALL. (A) OS, (B) EFS, and (C) remission duration of CRLF2+ Ph-like ALL, non-CRLF2 Ph-like ALL, Ph+ ALL, and B-other ALL. (A) For OS, P for comparison between Ph-like CRLF2+ vs B-other was .001; P for comparison between Ph-like CRLF2+ vs Ph-like non-CRLF2 was .01; all other comparisons were not significant. (B) For EFS, P for comparison between Ph-like CRLF2+ vs B-other was <.001; P for comparison between Ph-like CRLF2+ vs Ph-like non-CRLF2 was .01; P for comparison between Ph-like CRLF2+ vs Ph+ was .02; all other comparisons were not significant. (C) For remission duration, P for comparison between Ph-like CRLF2+ vs B-other was <.001; P for comparison between Ph-like CRLF2+ vs Ph+ was .001; P for comparison between Ph-like non-CRLF2 vs B-other was .03; all other comparisons were not significant.
IKZF1 status was analyzed in a subset of Ph-like ALL patients with available material (n = 41 of 56, 73%). IKZF1 deletions were detected in 68% of patients (28 of 41) with Ph-like ALL and were more common in the CRLF2+ group (CRLF2+, 21 of 25 [84%]; non-CRLF2, 7 of 16 [44%], P = .014). Within the Ph-like ALL subgroup, the presence of IKZF1 deletions did not influence the OS (supplemental Figure 6).
We performed targeted sequencing of 303 genes (L300 panel; MDACC) in 40 CRLF2+ patients (32 treatment naive, 8 relapsed/refractory), 15 of whom had matched germ line control (DNA from remission bone marrow). The mutation spectrum is shown in Figure 5 and in supplemental Table 3. Overall, 85% of the patients had a gene mutation leading to activation of JAK-STAT or the Ras pathway. JAK2 mutation was detected in 18 of the patients (45%) (clonal, n = 8; subclonal, n = 10) (R683G, n = 6; T875N, n = 4; R683S, n = 3; R687Q, n = 2; I682F, n = 1; D873E, n = 1; L611S, n = 1), and JAK1 mutation in 4 patients (10%) (clonal, n = 3; subclonal, n = 1). KRAS was found to be mutated in 10 patients (25%) (clonal, n = 5; subclonal, n = 5) (G13D, n = 3; G12D, n = 2; G12V, n = 2; A146T, n = 2; G13GG, n = 1) and NRAS in 5 patients (12.5%) (clonal, n = 2; subclonal, n = 3) (G12S, G12C, G12D, Q61R, and Q61L, each n = 1). Other recurrent mutations included CRLF2 (n = 8, 20%) (clonal, n = 2; subclonal, n = 6), IKZF1 (n = 7, 17.5%) (clonal, n = 4; subclonal, n = 3), ASXL1 (n = 5, 12.5%), and PAX5 (n = 5, 12.5%). Four patients had polyclonal mutations in the signaling pathways.
Mutational landscape in CRLF2+ Ph-like ALL (n = 40), categorized by JAK2 mutation status. *Patients with paired tumor and germ line samples. #Relapsed samples.
Mutational landscape in CRLF2+ Ph-like ALL (n = 40), categorized by JAK2 mutation status. *Patients with paired tumor and germ line samples. #Relapsed samples.
Notably, the CRLF2 mutation (F232C, n = 7; W46C, n = 1) was identified in 20% of patients in this study. Of the 8 patients with a CRLF2 mutation, CRLF2 FISH was performed on 6 patients; all had CRLF2 translocation detected (IGH-CRLF2 [n = 5]; P2RY8-CRLF2 [n = 1]). CRLF2 mutation was mutually exclusive with K/NRAS mutations but 2 patients had concomitant JAK2 and CRLF2 mutations. Among the CRLF2+ group, both IKZF1 deletion and mutation data were available for 20 patients. Interestingly, all CRLF2+ patients had an IKZF1 aberration (13 deletion, 4 concomitant deletion and mutation, and 3 mutation only).
Among the previously untreated patients with CRLF2+ ALL (n = 32), JAK2 mutation was detected in 14 (44%). The median OS for JAK2-mutated patients was 18.8 months compared with 26.9 months for patients with wild-type JAK2 (P = .012) (supplemental Figure 7).
UVA and MVA for OS
By UVA, the following variables were significant for survival: age (<60 vs ≥60 years), WBC count (<30 vs ≥30 × 109/L), platelet count (<100 vs ≥100 × 109/L), and Ph-like ALL (Table 4). In MVA adjusting for the variables used in UVA, the same variables were significant for survival (age: hazard ratio [HR], 3.299 [P < .001]; WBC count: HR, 1.910 [P = .017]; platelet count: HR, 7.437 [P = .005]; Ph-like ALL: HR, 1.818 [P = .03]).
Discussion
We report here long-term clinical outcomes in one of the largest series of newly diagnosed adult patients with Ph-like ALL. All patients received induction chemotherapy at our institution with either hyper-CVAD or an augmented BFM regimen. Our findings show (1) high frequency of Ph-like ALL in adults, (2) increased frequency of Ph-like ALL in adult patients of Hispanic ethnicity, (3) significantly inferior outcomes of adult patients with Ph-like ALL in a previously untreated patient cohort, and (4) among the Ph-like ALL group, significantly worse outcomes in patients with CRLF2 overexpression.
In children, the incidence of Ph-like ALL is reported to be 10% to 15%, and is associated with poor outcomes.7,9-11 In adults, the data so far are limited and conflicting.12-14 We report here a 33% incidence of Ph-like ALL in adults with B-ALL. Analysis of 692 adult patients with B-ALL that included a subset of patients described in this manuscript reported 20% to 25% incidence of Ph-like ALL.12,24 In contrast, 2 other studies have reported a lower incidence of Ph-like ALL in adults at around 10%.13,14 Notably, both studies that reported lower incidence of Ph-like ALL are from Europe, and racial and ethnic differences in the patient population studied may have contributed to these differences (see next paragraph). Additionally, variance in the genetic signatures used to classify Ph-like ALL could have led to these differences.32
We also report a significantly higher rate of Ph-like ALL in adult patients of Hispanic ethnicity. This was striking for the CRLF2+ group where 78% of the patients were Hispanic. Harvey et al had reported similar preponderance of children and adolescents with Hispanic ethnicity in a Children’s Oncology Group study of patients with CRLF2+ ALL.9 Genome-wide association studies in children and AYAs with ALL have identified inherited genetic variants in GATA3 that are associated with Ph-like ALL, and show an increased frequency in Hispanics and individuals with Native American or indigenous genetic ancestry.33,34 Together, these data provide a plausible explanation for increased incidence and poor outcomes of Hispanic patients with B-ALL and may explain the higher incidence of Ph-like ALL seen in adults within the United States vs other non-Hispanic Northern European populations.35,36 The significantly high number of patients of Hispanic ethnicity in our study reflects the referral pattern for our institution.
The majority (148 of 155, 95.4%) of patients included in this series had Ph-like ALL status determined by gene expression profiling. We additionally included 7 patients in the Ph-like ALL cohort who had overexpression of CRLF2 by flow cytometry and had CRLF2 rearrangements by FISH but were not tested by gene expression. Recognizing this as a potential caveat, the presence of CRLF2+ overexpression without the Ph-like gene signature is reportedly rare (<5%) in young adults and older adults with B-ALL.7,12 Additionally, the clinical outcomes data remained unaffected after the exclusion of these 7 patients (supplemental Figure 8).
Children and adolescents with Ph-like ALL have poor clinical outcomes.7 We corroborate these findings in a large cohort of uniformly treated adult patients. The 5-year survival for Ph-like ALL was markedly inferior to B-other ALL (23% vs 59%, respectively; P = .006). This was despite the fact that the patients with Ph-like ALL were younger compared with those in the B-other group. At MDACC, hyper-CVAD is used as a standard induction regimen for AYAs with outcomes similar to augmented BFM in a nonrandomized study.29 The poor outcomes of Ph-like ALL were maintained when only hyper-CVAD–treated patients were considered. In a multivariable analysis, Ph-like ALL remained significant for inferior survival. Boer et al reported lower EFS and OS in the Ph-like ALL subgroup than in the other B-ALL subgroup in an analysis of patients treated on Dutch-Belgium HOVON trials.13 This was, however, not statistically significant likely due to small patient numbers. Again, the difference in the patient population studied including differences in genetic ancestry, treatment regimen used, and differences in the genetic signature and detailed genomic classification used to classify Ph-like ALL may have contributed to these inconsistent results. Only 2 of the 56 patients with Ph-like ALL underwent allo-SCT in first remission in our study. Given the limited data with transplant, we are unable to assess the role of transplant for these patients.
In children, MRD-directed therapy has been reported to abolish the prognostic significance of Ph-like ALL.37 We assessed the outcomes by MRD status. In our analysis, the outcomes of Ph-like ALL, even if they achieve MRD− remission, remained suboptimal with a median OS of 26.2 months compared with 23.0 months for MRD+ group (supplemental Figure 2, P = .318). Because the majority of patients with Ph-like ALL received hyper-CVAD (an intensive regimen) based treatment, we believe further intensification of chemotherapy treatment is unlikely to benefit adult patients with Ph-like ALL. It remains to be determined whether addition of novel monoclonal antibodies (such as inotuzumab ozogamicin) or bispecific antibodies (such as blinatumomab) could improve the outcome of this group of patients.
We also report significant poor outcomes for adult patients with CRLF2 rearrangement with a 5-year survival of <20%. The reason for the inferior outcomes of patients with Ph-like ALL and CRLF2 overexpression compared with non-CRLF2 Ph-like ALL is unclear, but could be associated with high frequency of IKZF1 aberrations found in this study. Roberts et al reported poor outcomes in young adults with IKZF1 aberrations, irrespective of the Ph-like ALL status.7 Patients with CRLF2+ and concomitant JAK2 mutation had significantly inferior outcomes compared with the CRLF2+ JAK2 wild-type group, consistent with previously reported findings in a cohort of children and young adults.7 CRLF2, also known as thymic stromal lymphopoietin receptor (TSLPR), is part of the heterodimeric receptor complex that includes interleukin-7 receptor α chain and signals in response to thymic stromal lymphopoietin (TSLP). TSLP is a cytokine that upon binding to the TSLPR induces JAK/STAT pathway signaling. Aberrant TSLP/TSLPR signaling has been shown to activate multiple signaling transduction pathways including JAK/STAT, PI3K/AKT/mTOR, STAT5, and others,38 many of which have been shown biologically important for ALL blast survival.39,40
We also report mutation profiling of adult CRLF2+ Ph-like ALL. A majority of patients (85%) harbored a mutation in 1 or more genes involved in activation of the JAK-STAT or RAS pathways. Notably, we found a higher frequency of CRLF2 mutation, seen in 20% patients in this study. This appears more frequent than in pediatric patients (3 of 134, 2.2%)41 and in range with a smaller adult series (3 of 14, 21.4%).42 CRLF2 F232C mutation is a gain-of-function mutation that promotes constitutive dimerization, cytokine-independent growth, and activation of JAK/STAT signaling.42
Constitutive or ligand-dependent activation of JAK/STAT signaling occurs in a large fraction of patients with Ph-like ALL (CRLF2 overexpression, JAK2/EPOR rearrangements, IL7R mutations).7,16,40 Preclinical data indicate antileukemia efficacy of JAK2 inhibitors such as ruxolitinib or novel type II JAK2 kinase inhibitors in the in vivo patient-derived xenograft models or genetically engineered mouse models,7,19,43 and combination of ruxolitinib and standard chemotherapy will be tested in ongoing or planned clinical trials.23 Patients with ABL1, ABL2, and PDGFRB rearrangements could be targeted by ABL-type kinase inhibitors such as dasatinib.6,12,20,21,23 Allo-SCT remains a viable option for this group of patients, though data in adult patients is lacking. Preclinical data with TSLPR-targeted CAR T cells is encouraging,44 and represents a potential therapy option.
In summary, Ph-like ALL represents a high-risk disease subtype of adult B-ALL. In a group of patients uniformly treated with frontline hyper-CVAD–based chemotherapy, the outcomes of Ph-like ALL were significantly inferior to the B-other subgroup. Novel strategies are needed to improve the outcome of this group of patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Cancer Prevention and Research Institute of Texas Grant “Defining and Treating Targetable Lesions in AYA Acute Lymphoblastic Leukemia” (N.J. and M.K.), the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital, Stand Up to Cancer Innovative Research Grant and St. Baldrick’s Foundation Scholar Award (C.G.M.), American Society of Hematology Scholar Award (K.G.R.), and National Institutes of Health, National Cancer Institute Grants CA21765 (St. Jude Cancer Center Support Grant), HHSN261200800001E (C.G.M.), U01 CA157937 (C.L.W.) and CA157937 and CA118100 (UNM Comprehensive Cancer Center), and a Leukemia & Lymphoma Society Specialized Center of Research Grant (St. Jude and UNM Comprehensive Cancer Centers).
Authorship
Contribution: N.J., K.G.R., C.G.M., H.K., and M.K. contributed to the conception of the study and wrote the manuscript; N.J., K.G.R., E.J., K.P., A.K.E., K.C., P.Z.-M., X.L., G.F., S.A.W., S. Konoplev, R.C.H., I.-M.C., D.P.-T., M.V., D.T., G.G.-M., F.R., J.C., S. Kornblau, S.O., S.P., J.J., K.R.M.S., C.L.W., C.G.M., H.K., and M.K. contributed to the provision of study materials, patient recruitment, acquisition of data, or data analysis and interpretation; and all authors participated in the reviewing of the manuscript and gave final approval to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Konopleva, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: mkonople@mdanderson.org.
References
Author notes
N.J. and K.G.R. contributed equally to this study.