Skip Nav Destination
Development of a prognostically relevant cachexia index in primary myelofibrosis using serum albumin and cholesterol levels
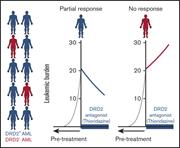
A phase 1 trial evaluating thioridazine in combination with cytarabine in patients with acute myeloid leukemia
Double-blind, randomized, multicenter phase 2 study of SC411 in children with sickle cell disease (SCOT trial)
Validation of the 2017 revision of the WHO chronic myelomonocytic leukemia categories
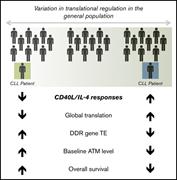
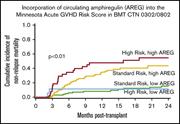
Amphiregulin modifies the Minnesota Acute Graft-versus-Host Disease Risk Score: results from BMT CTN 0302/0802
Issue Archive
August 14 2018
In this Issue
Table of Contents
DRUG ADVANCES
EXCEPTIONAL CASE REPORT
STIMULUS REPORTS
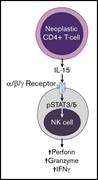
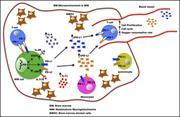
Highly cytotoxic natural killer cells are associated with poor prognosis in patients with cutaneous T-cell lymphoma
Bethany Mundy-Bosse,Nathan Denlinger,Eric McLaughlin,Nitin Chakravarti,Susan Hwang,Li Chen,Hsiaoyin Charlene Mao,David Kline,Youssef Youssef,Rebecca Kohnken,Dean Anthony Lee,Gerard Lozanski,Aharon G. Freud,Pierluigi Porcu,Basem William,Michael A. Caligiuri,Anjali Mishra
Development of a prognostically relevant cachexia index in primary myelofibrosis using serum albumin and cholesterol levels
Clinical Trials & Observations
Ayalew Tefferi,Maura Nicolosi,Domenico Penna,Mythri Mudireddy,Natasha Szuber,Terra L. Lasho,Curtis A. Hanson,Rhett P. Ketterling,Naseema Gangat,Animesh D. Pardanani
REVIEW ARTICLE
CLINICAL TRIALS AND OBSERVATIONS
A phase 1 trial evaluating thioridazine in combination with cytarabine in patients with acute myeloid leukemia
Clinical Trials & Observations
Lili Aslostovar,Allison L. Boyd,Mohammed Almakadi,Tony J. Collins,Darryl P. Leong,Rommel G. Tirona,Richard B. Kim,Jim A. Julian,Anargyros Xenocostas,Brian Leber,Mark N. Levine,Ronan Foley,Mickie Bhatia
Double-blind, randomized, multicenter phase 2 study of SC411 in children with sickle cell disease (SCOT trial)
Clinical Trials & Observations
Ahmed A. Daak,Carlton D. Dampier,Beng Fuh,Julie Kanter,Ofelia A. Alvarez,L. Vandy Black,Melissa A. McNaull,Michael U. Callaghan,Alex George,Lynne Neumayr,Lee M. Hilliard,Fredrick Sancilio,Adrian L. Rabinowicz,Matthew M. Heeney
IMMUNOBIOLOGY AND IMMUNOTHERAPY
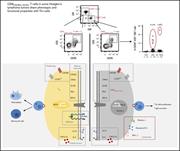
CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas
Kieu-Suong Le,Patricia Amé-Thomas,Karin Tarte,Françoise Gondois-Rey,Samuel Granjeaud,Florence Orlanducci,Etienne D. Foucher,Florence Broussais,Reda Bouabdallah,Thierry Fest,Dominique Leroux,Sapna Yadavilli,Patrick A. Mayes,Luc Xerri,Daniel Olive
LYMPHOID NEOPLASIA
MYELOID NEOPLASIA
Validation of the 2017 revision of the WHO chronic myelomonocytic leukemia categories
Clinical Trials & Observations
Sanam Loghavi,Dawen Sui,Peng Wei,Guillermo Garcia-Manero,Sherry Pierce,Mark J. Routbort,Elias J. Jabbour,Naveen Pemmaraju,Rashmi Kanagal-Shamanna,H. Deniz Gur,Shimin Hu,Zhuang Zuo,L. Jeffrey Medeiros,Hagop M. Kantarjian,Joseph D. Khoury
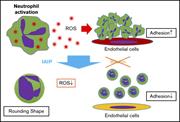
PHAGOCYTES, GRANULOCYTES, AND MYELOPOIESIS
RED CELLS, IRON, AND ERYTHROPOIESIS
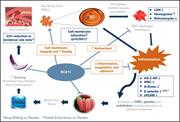
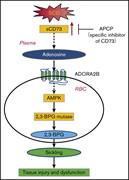
Elevated ecto-5′-nucleotidase: a missing pathogenic factor and new therapeutic target for sickle cell disease
Hong Liu,Morayo Adebiyi,Rong Rong Liu,Anren Song,Jeanne Manalo,Yuan Edward Wen,Alexander Q. Wen,Tingting Weng,Junsuk Ko,Modupe Idowu,Rodney E. Kellems,Holger K. Eltzschig,Michael R. Blackburn,Harinder S. Juneja,Yang Xia
THROMBOSIS AND HEMOSTASIS
TRANSFUSION MEDICINE
TRANSPLANTATION
Amphiregulin modifies the Minnesota Acute Graft-versus-Host Disease Risk Score: results from BMT CTN 0302/0802
Clinical Trials & Observations
Shernan G. Holtan,Todd E. DeFor,Angela Panoskaltsis-Mortari,Nandita Khera,John E. Levine,Mary E. D. Flowers,Stephanie J. Lee,Yoshihiro Inamoto,George L. Chen,Sebastian Mayer,Mukta Arora,Jeanne Palmer,Corey S. Cutler,Sally Arai,Aleksandr Lazaryan,Laura F. Newell,Madan H. Jagasia,Iskra Pusic,William A. Wood,Anne S. Renteria,Gregory Yanik,William J. Hogan,Elizabeth Hexner,Francis Ayuk,Ernst Holler,Udomsak Bunworasate,Yvonne A. Efebera,James L. M. Ferrara,Joseph Pidala,Alan Howard,Juan Wu,Javier Bolaños-Meade,Vincent Ho,Amin Alousi,Bruce R. Blazar,Daniel J. Weisdorf,Margaret L. MacMillan
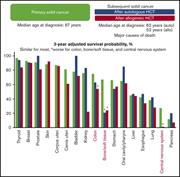
Outcomes of patients who developed subsequent solid cancer after hematopoietic cell transplantation
Yoshihiro Inamoto,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Tomohiro Matsuda,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Ken Tabuchi,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Saiko Kurosawa,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Hideki Nakasone,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Hisakazu Nishimori,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Satoshi Yamasaki,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Noriko Doki,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Koji Iwato,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Takehiko Mori,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Satoshi Takahashi,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Hiromasa Yabe,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Akio Kohno,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Hirohisa Nakamae,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Toru Sakura,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Hisako Hashimoto,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Junichi Sugita,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Hiroatsu Ago,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Takahiro Fukuda,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Tatsuo Ichinohe,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Yoshiko Atsuta,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group,Takuya Yamashita,on behalf of the Japan Society for Hematopoietic Cell Transplantation Late Effects and Quality of Life Working Group
ERRATA
-
Cover Image
Cover Image
![issue cover]()
COVER FIGURE
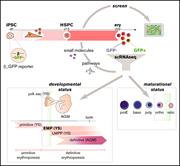
Plots illustrating changes in chromatin during differentiation from hematopoietic stem and progenitor cells (HSPCs) to erythroblasts. Promoters silenced in erythroblasts and promoters silent in both HSPCs and erythroblasts exhibit much more dramatic chromatin changes than promoters activated in erythroblasts. See the article by Bartholdy et al. - PDF Icon Front MatterFront Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Advertisement intended for health care professionals