Key Points
At current prices, ibrutinib is not a cost-effective initial CLL therapy in older patients without 17p deletion.
The cost of ibrutinib would need to be <$6800 per month to be cost-effective.
Abstract
Ibrutinib is a novel oral therapy that has shown significant efficacy as initial treatment of chronic lymphocytic leukemia (CLL). It is a high-cost continuous therapy differing from other regimens that are given for much shorter courses. Our objective was to evaluate the cost-effectiveness of ibrutinib for first-line treatment of CLL in patients older than age 65 years without a 17p deletion. We developed a semi-Markov model to analyze the cost-effectiveness of ibrutinib vs a comparator therapy from a US Medicare perspective. No direct comparison between ibrutinib and the best available treatment alternative, obinutuzumab plus chlorambucil (chemoimmunotherapy), exists. Therefore, we compared ibrutinib to a theoretical treatment alternative, which was modeled to confer the effectiveness of an inferior treatment (chlorambucil alone) and the costs and adverse events of chemoimmunotherapy, which would provide ibrutinib with the best chance of being cost-effective. Even so, the incremental cost-effectiveness ratio of ibrutinib vs the modeled comparator was $189 000 per quality-adjusted life-year (QALY) gained. To reach a willingness-to-pay threshold (WTP) of $150 000 per QALY, the monthly cost of ibrutinib would have to be at most $6800, $1700 less than the modeled cost of $8500 per month (a reduction of $20 400 per year). When the comparator efficacy is increased to more closely match that seen in trials evaluating chemoimmunotherapy, ibrutinib costs more than $262 000 per QALY gained, and the monthly cost of ibrutinib would need to be lowered to less than $5000 per month to be cost-effective. Ibrutinib is not cost-effective as initial therapy at a WTP threshold of $150 000 per QALY gained.
Introduction
Chronic lymphocytic leukemia (CLL) accounts for 25% to 30% of leukemia cases in the United States1 with an estimated 21 110 new CLL cases in 2017.2 CLL treatment has advanced significantly in recent years as a result of the introduction of oral targeted therapies. Ibrutinib is a first-in-class covalent inhibitor of Bruton tyrosine kinase (BTK), which is a critical component of the B-cell receptor signaling pathway that regulates the proliferation and survival of B cells.3 It has shown efficacy in relapsed and untreated CLL.4,5 RESONATE-2,5 a randomized trial comparing ibrutinib with chlorambucil for first-line treatment of CLL, demonstrated significantly increased overall survival and progression-free survival for patients receiving ibrutinib. Published updates to RESONATE-2 show sustained efficacy through a median follow-up of 28.6 months.6,7
Treatment of CLL is usually initiated for patients with symptomatic or advanced-stage disease. Current National Comprehensive Cancer Network (NCCN) category 1 recommendations for first-line therapy in patients older than age 65 years are ibrutinib or combination therapy with obinutuzumab plus chlorambucil (a chemoimmunotherapy regimen).8 Unlike chemoimmunotherapy, which is typically given for 6 months, ibrutinib is an oral drug that is more convenient to administer but requires ongoing therapy. Its current estimated monthly average wholesale price (AWP) is $13 324,9 and its price is expected to add considerable expense to the treatment of CLL.10,11 One analysis predicted that use of ibrutinib as first-line therapy compared with ibrutinib as second-line therapy would add $297 214 in pharmaceutical costs per treated CLL patient and $348 445 compared with a modeled scenario before ibrutinib was approved.10 No direct comparison between ibrutinib and chemoimmunotherapy for initial treatment exists to inform a cost-effectiveness analysis. We aim to gain insight into the cost-effectiveness of ibrutinib while avoiding comparisons across clinical trials, which may produce a biased estimate. Trial population differences between the RESONATE-2 trial and chlorambucil with obinutuzumab12-14 trials and crossover to ibrutinib after progression of patients randomly assigned to chlorambucil in the RESONATE-2 trial makes a direct comparison across these 2 trials problematic.
The objective of this analysis is to evaluate the cost-effectiveness, in terms of an incremental cost-effectiveness ratio (ICER) expressed in cost per quality-adjusted life-year (QALY), of ibrutinib as initial therapy for CLL compared with a theoretical treatment alternative modeled to confer the effectiveness of chlorambucil alone and the costs and adverse events (AEs) of combination therapy with obinutuzumab and chlorambucil, which throughout this article will be referred to as the comparator. The population modeled is based on the population of patients who participated in the RESONATE-2 trial and consists of a cohort of patients older than age 65 years without the 17p deletion mutation. In the comparator arm, ibrutinib is used as second-line therapy.
Methods
Decision model
We developed a discrete-time semi-Markov model comprising pre- and postprogression health states based on efficacy and safety data from RESONATE-2 during the reported study period. The model then derives poststudy and postprogression mortality probabilities from the C9011 trial,15,16 which provides long-term follow-up data for patients initially treated with chlorambucil. We modeled a population of 65% male and 35% female patients age 73 years without the 17p deletion mutation to reflect the population of RESONATE-2. Calibration to the progression-free and overall survival curves was performed by using an optimization-based algorithm to provide transition probabilities for the semi-Markov model (supplemental Figure 5). The analysis was performed using TreeAge Pro.17
Model structure
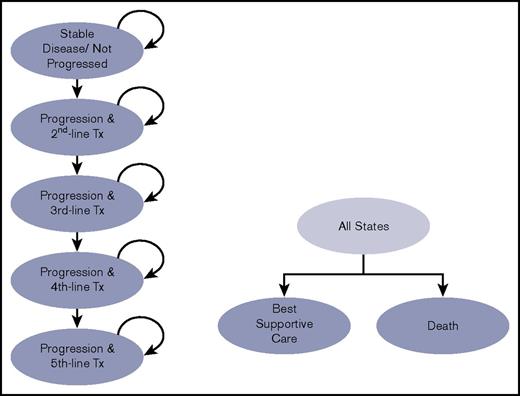
Figure 1 presents a diagram of the model. Patients eligible for first-line therapy begin in the “Not Progressed” state and may remain in this state, progress, or die each month. Once patients progress, they begin the next line of therapy after a period of 5 months, informed by the median time to next therapy after progression from Tam et al.18 If patients begin therapy in the comparator arm, they receive ibrutinib therapy as second-line therapy (also referred to as first-line salvage therapy). The subsequent therapy sequence we modeled is informed by the NCCN guidelines8 (Table 1). Costs, progression rates, and safety are modeled after trials that evaluate the relevant relapsed disease therapy (supplemental Tables 2 and 3). The model follows patients over their lifetime.
Diagram of the Markov model structure. Patients transition from the stable disease/not progressed disease state to progression or death according to transition probabilities derived by calibration to the published RESONATE-2 survival curves. Tx, therapy.
Diagram of the Markov model structure. Patients transition from the stable disease/not progressed disease state to progression or death according to transition probabilities derived by calibration to the published RESONATE-2 survival curves. Tx, therapy.
Sequence of therapies used in the model listed by initial therapy
| Therapy . | Ibrutinib . | Comparator . |
|---|---|---|
| Second line | Idelalisib + rituximab49 | Ibrutinib4 |
| Third line | Ofatumumab4 | Idelalisib + rituximab49 |
| Fourth line | Rituximab49 | Ofatumumab4 |
| Fifth line | Repeat of ofatumumab4 | Rituximab49 |
| Therapy . | Ibrutinib . | Comparator . |
|---|---|---|
| Second line | Idelalisib + rituximab49 | Ibrutinib4 |
| Third line | Ofatumumab4 | Idelalisib + rituximab49 |
| Fourth line | Rituximab49 | Ofatumumab4 |
| Fifth line | Repeat of ofatumumab4 | Rituximab49 |
The references in the table refer to the trials from which data regarding the progression probabilities while on therapy for relapsed disease were derived.
Efficacy and safety data
Efficacy data for ibrutinib is derived from RESONATE-2.5,7 The most recent reported data7 include overall survival and progression-free survival through 35 months, with a median follow-up of 29 months. Given the uncertainty of outcomes for the ibrutinib arm after the 35-month period reported, we conservatively assume that until progression, the mortality rate is equivalent to that of an age-matched general US population per the 2009 Centers for Disease Control and Prevention life tables.19
The comparator efficacy data were derived from the chlorambucil arm of the RESONATE-2 trial during the reported trial period. To model progression and mortality for the posttrial period, we performed a separate calibration using the overall survival and progression-free survival curves from the C9011 trial.15,16 For this analysis, the same postprogression mortality rate was used for both arms. This assumes that postprogression mortality is independent of initial therapy (explored in supplemental Figure 10).
Progression rates while receiving subsequent lines of therapy were modeled after the relevant relapsed disease therapy trials (supplemental Table 2); however, mortality rates used while relapsed patients received therapy were informed by the postprogression mortality rates derived from the calibration based on C9011 (see supplemental Table 1 and supplemental Figure 5). We assumed 11.6% of patients, once progressed, would not receive the next round of therapy after each therapy line and would instead initiate best supportive care; this assumption is based on the number of progressed patients not receiving salvage therapy in Else et al.20 Figure 2 illustrates calibrated survival curves used in the base case analysis.
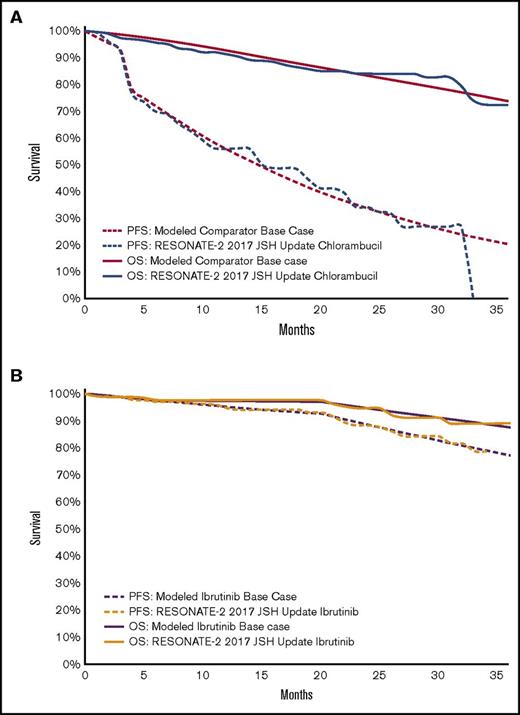
Comparator and ibrutinib survival curves. (A) Modeled comparator overall survival (OS) and progression-free survival (PFS) curves compared with the published data. Illustration of the modeled survival curves for the comparator arm (red) compared with digitized data from the chlorambucil arm of RESONATE-2 trial7 (blue). Long-term survival curves for the modeled base case are available in supplemental Figure 1. (B) Modeled ibrutinib OS and PFS survival curves compared with the published data. Illustration of the modeled survival curves for the ibrutinib arm (purple) compared with digitized data from the ibrutinib arm of RESONATE-2 trial7 (orange). JSH, Japanese Society of Hematology.
Comparator and ibrutinib survival curves. (A) Modeled comparator overall survival (OS) and progression-free survival (PFS) curves compared with the published data. Illustration of the modeled survival curves for the comparator arm (red) compared with digitized data from the chlorambucil arm of RESONATE-2 trial7 (blue). Long-term survival curves for the modeled base case are available in supplemental Figure 1. (B) Modeled ibrutinib OS and PFS survival curves compared with the published data. Illustration of the modeled survival curves for the ibrutinib arm (purple) compared with digitized data from the ibrutinib arm of RESONATE-2 trial7 (orange). JSH, Japanese Society of Hematology.
Adverse event data are derived from RESONATE-2 for ibrutinib and for the obinutuzumab-plus-chlorambucil arm from Goede et al12 for the comparator. AE data for each relapsed disease therapy reflect the incidence from the corresponding relapsed disease therapy trial cited in Table 1. Grade 3 and 4 AEs are included; each AE has a cost and a utility decrement associated with it. Atrial fibrillation is also modeled as a chronic condition at rates consistent with those in the RESONATE-2 trial7 for the ibrutinib arm and at the age-matched population rate21 for other therapies.
Costs
Perspective.
The evaluation is performed from a US Medicare health care perspective. Costs and health outcomes were discounted at 3% annually. When referenced costs are not in 2017 dollars, they were inflated to 2017 dollars using the Personal Health Care Expenditure index22 where available (through 2014) and the Personal Consumption Expenditure index23 otherwise, per the guidelines of the Second Panel on Cost-Effectiveness in Health and Medicine.24 The base case treatment start date was December 2017.
Drug costs.
It is expected that payment for ibrutinib in the modeled age group will be primarily through Medicare Part D, so we followed the recommendations of the Veterans Affairs Health Economics Resource Center25,26 and set drug costs to 64% of the AWP26 if on patent and 27% of the AWP if off patent. This methodology was also used for chlorambucil and oral therapies for relapsed disease. The comparator arm treatment costs were modeled on the basis of the Medicare Part B Payment Allowance Limit for obinutuzumab and other drugs paid for through Medicare Part B. For the comparator, patients received up to 6 months of chemoimmunotherapy. The course could be shortened if a patient progressed before completing 6 months of treatment. Dosages for relapsed disease therapy were consistent with those used in the trials that studied each respective drug for use in relapsed CLL (Table 1). Costs of all therapy lines were incurred only when a patient was receiving therapy. Drug costs were decreased at patent expiration (supplemental Table 3). The percentage of patients who continued to receive ibrutinib for initial and relapsed therapy was matched to the reported data for the appropriate trials (Table 2) to more accurately reflect the quantity of drug used to achieve the results of the respective trials.4,6 Drug administration costs are outlined in supplemental Table 5.
Parameters used for base case analysis
| Parameters . | Both . | Ibrutinib . | Comparator . | Value . | Reference . | Comments . |
|---|---|---|---|---|---|---|
| Health utilities (yearly), QALYs per life-year | ||||||
| PFS on initial therapy | 0.71 | 0.67 | 29 | |||
| PFS not on therapy after initial therapy | 0.82 | 29 | ||||
| Progressed, awaiting second-line therapy | 0.66 | 29 | ||||
| PFS on second-line therapy | 0.55 | 29 | ||||
| PFS completed second-line therapy | 0.71 | 29 | ||||
| Progressed, awaiting third-line therapy | 0.59 | 29 | ||||
| PFS on third- or greater-line therapy | 0.42 | 29 | ||||
| PFS completed third-line therapy | 0.59 | 29 | ||||
| Receiving best supportive care/hospice | 0.59 | 29 | ||||
| Drug cost-related | ||||||
| AWP for drugs on patent, % | 64 | 25,26 | VA HERC recommendations | |||
| AWP for drugs off patent, % | 27 | 25,26 | VA HERC recommendations | |||
| Ibrutinib per month, $ | 8 527.04 | 9 | 64% of AWP from Redbook Online; 420 mg/d | |||
| Comparator (obinutuzumab) month 1, $ | 17 227.50 | 50 | Medicare Part B maximum payment allowance | |||
| 12 | 3000 mg (see supplemental Appendix for further details) | |||||
| Comparator (obinutuzumab) months 2-6, $ | 5 742.50 | 50 | Medicare Part B maximum payment allowance | |||
| 12 | 1000 mg/mo | |||||
| Comparator (chlorambucil) per month, $ | 249.60 | 51 | 27% of AWP from RedBook Online | |||
| Administration costs comparator month 1, $ | 1 080.99 | See supplemental Appendix for further details | ||||
| Administration costs comparator months 2-6, $ | 263.07 | |||||
| Patients receiving ibrutinib as initial therapy at 29 months, % | 79 | 6 | Additional patients were modeled to come off therapy without progressing. At 28.5-mo median follow-up, 79% of patients were receiving ibrutinib. Seven of 22 go on to receive bendamustine plus rituximab (see supplemental Table 3 for further details). | |||
| Patients stopping oral salvage therapy in first 9 months, % | 5.6 | 4 | These patients were modeled to come off therapy without progressing; 11 of 195 patients stopped therapy for reasons other than progression or death at median follow-up of 9.4 months. | |||
| Patients experiencing grade 3 or 4 AEs during trial, % | ||||||
| Diarrhea | 7 | 0 | ||||
| Anemia | 6 | 4 | ||||
| Neutropenia | 10 | 37 | ||||
| Thrombocytopenia | 2 | 10 | ||||
| Infections (eg, pulmonary nodular amyloidosis) | 10 | 12 | ||||
| Infusion-related reaction | 0 | 20 | ||||
| Hyponatremia | 3 | 0 | ||||
| Rash | 3 | 0 | ||||
| Atrial fibrillation | ||||||
| Grade 3 or 4 | 4* | 1.6† | ||||
| Chronic | 107 | 421 |
| Parameters . | Both . | Ibrutinib . | Comparator . | Value . | Reference . | Comments . |
|---|---|---|---|---|---|---|
| Health utilities (yearly), QALYs per life-year | ||||||
| PFS on initial therapy | 0.71 | 0.67 | 29 | |||
| PFS not on therapy after initial therapy | 0.82 | 29 | ||||
| Progressed, awaiting second-line therapy | 0.66 | 29 | ||||
| PFS on second-line therapy | 0.55 | 29 | ||||
| PFS completed second-line therapy | 0.71 | 29 | ||||
| Progressed, awaiting third-line therapy | 0.59 | 29 | ||||
| PFS on third- or greater-line therapy | 0.42 | 29 | ||||
| PFS completed third-line therapy | 0.59 | 29 | ||||
| Receiving best supportive care/hospice | 0.59 | 29 | ||||
| Drug cost-related | ||||||
| AWP for drugs on patent, % | 64 | 25,26 | VA HERC recommendations | |||
| AWP for drugs off patent, % | 27 | 25,26 | VA HERC recommendations | |||
| Ibrutinib per month, $ | 8 527.04 | 9 | 64% of AWP from Redbook Online; 420 mg/d | |||
| Comparator (obinutuzumab) month 1, $ | 17 227.50 | 50 | Medicare Part B maximum payment allowance | |||
| 12 | 3000 mg (see supplemental Appendix for further details) | |||||
| Comparator (obinutuzumab) months 2-6, $ | 5 742.50 | 50 | Medicare Part B maximum payment allowance | |||
| 12 | 1000 mg/mo | |||||
| Comparator (chlorambucil) per month, $ | 249.60 | 51 | 27% of AWP from RedBook Online | |||
| Administration costs comparator month 1, $ | 1 080.99 | See supplemental Appendix for further details | ||||
| Administration costs comparator months 2-6, $ | 263.07 | |||||
| Patients receiving ibrutinib as initial therapy at 29 months, % | 79 | 6 | Additional patients were modeled to come off therapy without progressing. At 28.5-mo median follow-up, 79% of patients were receiving ibrutinib. Seven of 22 go on to receive bendamustine plus rituximab (see supplemental Table 3 for further details). | |||
| Patients stopping oral salvage therapy in first 9 months, % | 5.6 | 4 | These patients were modeled to come off therapy without progressing; 11 of 195 patients stopped therapy for reasons other than progression or death at median follow-up of 9.4 months. | |||
| Patients experiencing grade 3 or 4 AEs during trial, % | ||||||
| Diarrhea | 7 | 0 | ||||
| Anemia | 6 | 4 | ||||
| Neutropenia | 10 | 37 | ||||
| Thrombocytopenia | 2 | 10 | ||||
| Infections (eg, pulmonary nodular amyloidosis) | 10 | 12 | ||||
| Infusion-related reaction | 0 | 20 | ||||
| Hyponatremia | 3 | 0 | ||||
| Rash | 3 | 0 | ||||
| Atrial fibrillation | ||||||
| Grade 3 or 4 | 4* | 1.6† | ||||
| Chronic | 107 | 421 |
AEs were included for all drugs if the incidence was 3% or higher for any drug as reported in any trial used for this analysis. Ibrutinib AEs were modeled based on Burger et al,5 except atrial fibrillation, which was based on the incidence reported by Hillmen et al.7 For the comparator, atrial fibrillation was not reported as an AE in Goede et al,12 so rates based on the age-matched general population were used.21 Probabilities for AEs were adjusted for median time on therapy for respective trial (see supplemental Figure 7). See supplemental Appendix for costs and dysutilities associated with AEs. Median time receiving therapy from respective trials was 18.4 months for ibrutinib and 6 months for the comparator. “Both” refers to ibrutinib and comparator.
PFS, progression-free survival; VA HERC, Veterans Affairs Health Economics Resource Center.
At 29-month follow-up.7
Per 29 months.
AE costs.
Costs of AEs are based on the most common grade 3 and 4 toxicities reported in clinical trials as outlined in supplemental Table 8. Atrial fibrillation, an important AE observed in RESONATE-2, was also modeled as a chronic condition with patients modeled to receive anticoagulation and a beta blocker.
Supportive care costs.
Components of costs for best supportive care were based on expert opinion and are consistent with a prior cost-effectiveness analysis.27 Patients were assumed to have monthly clinic visits, laboratory tests, and a baseline risk of infectious complications equal to a 1% monthly risk of inpatient admission.
Additional health care costs.
Additional health care costs were age-based per data from Neuman et al.28 Additional monitoring costs were modeled (supplemental Table 4).
Utilities
Health state utilities.
Results
Base case analysis
In the modeled cohort, the projected median overall survival in the ibrutinib arm was 103 months, and the projected median survival in the comparator arm was 58 months. The projected median progression-free survival in the ibrutinib arm was 79 months, and the projected median progression-free survival in the comparator arm was 15 months. Supplemental Table 10 contains the percentage of patients who reached the various lines of therapy.
The combination of modeled survival and progression benefits led to 5.49 QALYs (9.60 undiscounted years; 8 discounted years) for the ibrutinib arm and 3.09 QALYs (5.45 undiscounted years; 4.89 discounted years) for the comparator, although the costs for the ibrutinib arm were substantially higher (Table 3). The incremental cost-effectiveness ratio for ibrutinib was $189 000 per QALY gained relative to the comparator.
Cost-effectiveness analysis results from the base case
| Strategy . | Discounted cost, $ . | Incremental cost, $ . | Discounted QALYs . | Incremental QALYs . | ICER, $/QALY . |
|---|---|---|---|---|---|
| Comparator with ibrutinib as second-line therapy | 336 418 | 3.09 | |||
| Ibrutinib as initial therapy | 791 670 | 455 252 | 5.49 | 2.40 | 189 326 |
| Strategy . | Discounted cost, $ . | Incremental cost, $ . | Discounted QALYs . | Incremental QALYs . | ICER, $/QALY . |
|---|---|---|---|---|---|
| Comparator with ibrutinib as second-line therapy | 336 418 | 3.09 | |||
| Ibrutinib as initial therapy | 791 670 | 455 252 | 5.49 | 2.40 | 189 326 |
Ibrutinib as first-line therapy was modeled to cost an additional $455 000 in health care costs and add an additional 2.40 QALYs compared with the modeled comparator, which assumes ibrutinib as second-line therapy. Costs are from a Medicare perspective and include additional health care costs.
Sensitivity analysis
In 1-way sensitivity analyses, the results of altering key model parameters are presented in a tornado diagram in Figure 3A. The general finding for these analyses as well as the other scenario analyses described below is that initial treatment with ibrutinib did not cost less than $160 000 per QALY gained at its modeled drug price.
One-way sensitivity analyses. (A) Tornado diagram of 1-way sensitivity analysis on multiple model parameters. Parameter values corresponding to the low and high ICER are highlighted. (B) Sensitivity to the cost of ibrutinib at initiation of therapy. Values on labeled points represent monthly costs of ibrutinib to reach the corresponding willingness-to-pay (WTP) thresholds. Notably, to be considered cost-effective for a health care system that is willing to pay $150 000 per QALY, the cost of ibrutinib would need to be less than $6800 per month when compared to the comparator, which is likely to be less effective than combination chemoimmunotherapy in the population analyzed. Supplemental Table 11 contains costs of ibrutinib that reach the same WTP thresholds presented above when examining more effective comparators. (C) A sensitivity analysis on a hazard ratio (HR) applied only to the progression rate of the comparator drug, holding all other variables constant. The PFS and OS curves are plotted, with the corresponding ICERs labeled on the right. The base case ICER of $189 000 is highlighted along with increasing ICERs as the efficacy of the comparator is improved, which illustrates worsened performance by ibrutinib from a cost-effectiveness perspective. GChl, obinutuzumab plus chlorambucil (survival curves from Goede et al14 ).
One-way sensitivity analyses. (A) Tornado diagram of 1-way sensitivity analysis on multiple model parameters. Parameter values corresponding to the low and high ICER are highlighted. (B) Sensitivity to the cost of ibrutinib at initiation of therapy. Values on labeled points represent monthly costs of ibrutinib to reach the corresponding willingness-to-pay (WTP) thresholds. Notably, to be considered cost-effective for a health care system that is willing to pay $150 000 per QALY, the cost of ibrutinib would need to be less than $6800 per month when compared to the comparator, which is likely to be less effective than combination chemoimmunotherapy in the population analyzed. Supplemental Table 11 contains costs of ibrutinib that reach the same WTP thresholds presented above when examining more effective comparators. (C) A sensitivity analysis on a hazard ratio (HR) applied only to the progression rate of the comparator drug, holding all other variables constant. The PFS and OS curves are plotted, with the corresponding ICERs labeled on the right. The base case ICER of $189 000 is highlighted along with increasing ICERs as the efficacy of the comparator is improved, which illustrates worsened performance by ibrutinib from a cost-effectiveness perspective. GChl, obinutuzumab plus chlorambucil (survival curves from Goede et al14 ).
The cost of ibrutinib is an important driver of its cost-effectiveness in this analysis. Assuming that the efficacy of the comparator is equal to that of chlorambucil alone, with ibrutinib as second-line therapy, if a payer can negotiate prices for ibrutinib below $6800 per month, the incremental cost-effectiveness ratio would fall below $150 000 per QALY gained. This drug cost represents a $20 400-per-year decrease in comparison with the modeled base case in which the cost of ibrutinib was assumed to be $8500 per month. A price of $4600 per month would reduce the incremental cost-effectiveness ratio to below $100 000 per QALY. However, ibrutinib costs would likely need to be even lower than this because obinutuzumab plus chlorambucil likely has effectiveness that is better than the comparator modeled in this study, based on its relative efficacy compared with chlorambucil in Goede et al.12,14 This is examined in an exploratory sensitivity analysis shown in Figure 3C, which considers the effect of improved progression-free survival on the results by applying a hazard ratio to decrease the rate of progression in the comparator arm. Although first-line treatment costs increase in the comparator arm when improved comparator effectiveness is modeled, downstream costs decrease and QALYs increase for an overall increase in the ICER, making ibrutinib more expensive per QALY gained. For drugs with performance characteristics more similar to those of obinutuzumab plus chlorambucil in Goede et al, the incremental cost-effectiveness ratio for ibrutinib approaches $263 000 per QALY gained, again suggesting that substantial price reductions would be needed to establish the cost-effectiveness of ibrutinib, likely to below $5000 per month (further explored in supplemental Table 11). Given the relatively short time of the RESONATE-2 follow-up to this point, sensitivity analyses to poststudy period ibrutinib progression and mortality rates can be found in supplemental Figures 6 and 10.
Notably, changes in the cost of another expensive oral therapy used for relapsed disease (idelalisib) do not substantially affect these results. When the cost of obinutuzumab is varied ± 20%, the ICER does not vary significantly (Figure 3A). Because there are numerous choices available for therapy for relapsed disease, we also explored how changes in efficacy and cost of therapies for relapsed disease affected the results in a 2-way sensitivity analysis (supplemental Figure 3A). Even when therapy costs and efficacy for relapsed disease were increased or decreased by 30%, ibrutinib costs more than $186 000 per QALY gained.
Utilities for health states, when varied across the 95% confidence intervals from Kosmas et al29 in 1-way sensitivity analyses, did not reduce the ICER for ibrutinib to below $170 000 per QALY gained.
For both younger Medicare patients (age 65 years) and older Medicare patients (age 75 years), ibrutinib costs more than $165 000 per QALY gained. We did not vary age outside this range because the published survival curves from the RESONATE-2 population were not likely to be representative of other ages.
One mechanism by which the cost of ibrutinib may fall is through introduction of a generic version to the market. We performed analyses to examine the cost-effectiveness of ibrutinib for patients who initiated therapy at different starting dates, and thus were exposed to different costs of ibrutinib therapy throughout the course of treatment. Many factors influence when generic drugs will come to market.32-34 We modeled 28 December 2026, the date of the earliest expiration of an ibrutinib use patent,35 as the time when a generic competitor would come to market and the cost of ibrutinib would drop from 64% of AWP to 27% of AWP. The base case treatment start date was December 2017. As the modeled start date approaches April 2023, the ICER drops to $150 000 per QALY gained (supplemental Figure 2). If the comparator is modeled with improved effectiveness such that comparator survival curves more closely resemble the obinutuzumab-plus-chlorambucil arm of Goede et al, the ICER for ibrutinib drops to $150 000 per QALY gained in April 2026. For the base case comparator, if patent expiration is not modeled for any drug, the ICER becomes $221 000 per QALY gained. The relative cost of generic drugs is not a major driver of the results of this analysis; this is explored in Figure 3A. approval by the US Food and Drug Administration of a competitor drug is another mechanism by which drug prices may fall, although competitive pressure has not proven to be a major factor in pricing in the oral anticancer drug market. A study by Bennette et al36 found that US Food and Drug Administration approval of a competitor drug led to a decrease of less than 2% in cost of oral anticancer drugs. Nevertheless, the results of this analysis are not sensitive to introduction of a competitor within the next 1 to 5 years (supplemental Figure 9).
Because of the considerable uncertainty regarding outcomes in the poststudy periods, we performed a sensitivity analysis to examine the effect of varying the postprogression mortality rate through use of a modeled hazard ratio applied to the base case mortality rate. Findings in this study are not sensitive to assumptions made about the postprogression mortality rate when it is assumed to be the same between arms (Figure 3A). There is evidence that patients who progress on ibrutinib have poor outcomes.37 Therefore, we examined the effect of assuming the postprogression mortality rate is higher for patients who progress on ibrutinib. This led to an increased ICER, rendering ibrutinib more expensive per QALY gained (supplemental Figure 10).
Our assumption regarding how long after progression patients begin the next line of therapy is based on Tam et al,18 which was a study of younger patients who received fludarabine, cyclophosphamide, and rituximab. With variations of this parameter, there are slight changes in the ICER, but key findings do not change (supplemental Figure 11).
Discussion
This study evaluates the cost-effectiveness of ibrutinib as a first-line therapy versus a comparator characterized by the effectiveness of chlorambucil alone and the costs of obinutuzumab plus chlorambucil with ibrutinib reserved as second-line therapy. The modeled population was based on that in the RESONATE-2 trial, which included patients older than age 65 years without the 17p deletion mutation. The main finding was that ibrutinib as first-line therapy delivered substantial survival and progression-free survival benefits thus producing gains in quality-adjusted life expectancy, but given its high drug price, it costs more than $189 000 per QALY gained.
We modeled the comparator to have overall survival and progression-free survival equivalent to the chlorambucil arm of RESONATE-2. In other trials occurring concurrently with RESONATE-2, chlorambucil alone was shown to be inferior to chemoimmunotherapy.12,38 Chlorambucil alone is now considered an NCCN category 3 choice for initial therapy for patients older than age 65 years.8 Our analysis, therefore likely underestimates the effectiveness of the real-world best treatment alternative, obinutuzumab plus chlorambucil. Thus, we intentionally biased our analysis in favor of ibrutinib, given that simple comparisons across different clinical trials may produce bias. The cost-effectiveness of ibrutinib determined by our analysis is therefore more favorable (lower) than it would be if obinutuzumab plus chlorambucil could be directly compared with ibrutinib. Our results, even with a less effective comparator, indicate that the incremental cost-effectiveness ratio of ibrutinib as first-line therapy are likely significantly greater than $150 000 per QALY, which suggests that ibrutinib at its current price is unlikely to be cost-effective at what is an often-cited willingness-to-pay threshold for the US health care system of $150 000 per QALY.39
Ibrutinib represents a milestone in therapy for CLL and has demonstrated efficacy as both a salvage therapy and as initial therapy,4,5 yet at its current price and expected length of treatment, it is unlikely to meet accepted thresholds to be considered cost-effective as initial therapy, and our analysis illustrates that. As a continuous therapy, the considerable costs of prolonged use will likely have significant impact on the cost of CLL care in the US health care system. The high cost of ibrutinib has previously been analyzed by Shanafelt et al.10 They estimated that use of ibrutinib as first-line vs second-line therapy could add $297 214 per treated CLL patient to the 10-year pharmaceutical cost of treatment and that the increase in US CLL spending could be nearly $1.4 billion when applied to 16 000 yearly CLL diagnoses. Consistent with this, our analysis estimates an additional $379 000 in discounted health care costs per patient in the ibrutinib arm compared with the comparator arm at 10 years ($455 000 over a patient’s lifetime).
This analysis adds to a growing body of research examining the value of ibrutinib in CLL. Shanafelt et al10 have demonstrated the significant budget impact that is expected as a result of the adoption of ibrutinib as first-line therapy in CLL. An analysis published by Chen et al11 models the economic impact and cost-effectiveness of oral therapies in CLL and finds that when expected increases in prevalence of CLL and life expectancy of CLL patients are taken into account (factors they attribute to differences between their estimate and that of Shanafelt et al), oral therapies may add $20 billion in US CLL-related costs between 2011 and 2025 over expected costs if chemoimmunotherapies were standard of care. Recently, a cost-effectiveness analysis by Sinha et al40 examined ibrutinib as first-line therapy using a United Kingdom cost perspective. However, the modeled costs differ substantially from costs in the United States, and hence the results are not directly applicable to the United States. In addition, Sinha et al followed National Institute for Health and Care Excellence guidelines,41 which differ from those of the Second Panel on Cost-Effectiveness in Health and Medicine.42
There are important differences between our analysis and that of Chen et al that make a direct comparison difficult. Chen et al report an incremental cost-effectiveness ratio for an oral therapy strategy vs chemoimmunotherapy strategy for the years 2011 to 2025, which includes a period of time during which no patients were modeled to be receiving oral therapy (2011-2014). Patients without the 17p deletion mutation did not begin initial oral therapy until 2016. Because the Chen analysis was limited to the years 2011 to 2025, it does not capture a full lifetime time horizon for all patients. Their analysis aggregates QALYs and costs for a heterogeneous group of patients combining patients who are undergoing initial therapy or relapsed disease therapy and some who did not receive oral therapy. Chen et al did not evaluate a scenario in which ibrutinib as first-line therapy is compared with ibrutinib as second-line therapy after initial therapy with chemoimmunotherapy, which is the focus of our study. In adopting a comprehensive health care perspective, we have included future unrelated health care costs, which is a recommendation of the Second Panel on Cost-Effectiveness in Health and Medicine,42 whereas Chen et al focus on CLL-related costs only. Chen et al highlight the overall economic burden of shifting strategies in treatment of CLL at a population level, thus illustrating the economic impact that adoption of oral targeted therapies will have in the United States between 2011 and 2025, but our model focuses on cost-effectiveness going forward of ibrutinib for first-line therapy vs second-line therapy for the older adult population without a 17p deletion mutation. This analysis is thus complementary to the study by Chen et al, although methodological differences must be considered by those using our respective analyses.
It has been noted that as drug costs increase, newer therapies may seem to be deceptively cost-effective because they are compared with expensive standards of care whose costs are increasing over time.43 This analysis examines the cost-effectiveness of ibrutinib at a critical point at which a continuous therapy is poised to overtake a therapy that is administered for a shorter period of time with significantly lower costs. This is especially important in light of ongoing trials that are evaluating a second-generation BTK inhibitor, acalabrutinib44 as well as ibrutinib in combination with another expensive therapy, venetoclax.45,46
There are some important limitations to our study. We limited our analysis to examining the use of ibrutinib as initial therapy compared with its use as second-line therapy. We did not examine scenarios in which ibrutinib is not considered for therapy or scenarios in which ibrutinib is used as third- or greater-line therapy. Another important limitation to our study is that data on ibrutinib efficacy are available only up to approximately 3 years of treatment.6 Informed assumptions were made regarding efficacy beyond this time period. In addition, a limitation of our study is that because of the lack of availability of randomized control data, we were not able to directly compare ibrutinib to a current standard of care (obinutuzumab plus chlorambucil), which is also an NCCN category 1 therapy for patients older than age 65 years with symptomatic or advanced-stage CLL; thus, we have attempted to examine how the value of ibrutinib might be altered if this data were available.
Our analysis acknowledges the important clinical benefit of ibrutinib. However, at current prices, ibrutinib is not likely to meet accepted thresholds for cost-effectiveness. The challenges of incorporating value considerations into clinical decision making are highlighted in the discussion by Garrison47 and by the American Heart Association/American College of Cardiology Task Force,48 which has recommended incorporating cost-effectiveness analysis into clinical guidelines. These challenges include lack of transparency regarding the actual cost of medications to patients and health care systems, which makes value assessment by the clinician difficult. Therefore, we recommend that this analysis be used to inform clinicians, medical professional organizations, payers, and health care systems about the broad financial impact of ibrutinib relative to other first-line therapeutic options, and thus it may influence clinical practice indirectly through updated practice guidelines. An understanding of the cost-effectiveness of therapeutic alternatives is important because clinicians, professional organizations, and policymakers aim to obtain the best outcomes for patients while delivering high-value care.
In conclusion, we demonstrate in this study that ibrutinib as first-line therapy does not reach commonly accepted thresholds for cost-effectiveness when compared with chlorambucil or obinutuzumab plus chlorambucil with ibrutinib as second-line therapy. It is likely that ibrutinib as first-line therapy would cost even more per QALY gained, and thus be less cost-effective, if compared with treatments such as obinutuzumab plus chlorambucil that have better progression-free survival than the comparator we used.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Health Services Research and Development (J.I.B.), by the Department of Veterans Affairs (D.K.O.), and by a Career Development Award (K01AG037593-01A1) from the National Institutes of Health, National Institute on Aging (J.D.G.-F.).
Authorship
Contribution: J.I.B., V.D., R.W., A.B., S.C., D.K.O., and J.D.G.-F. designed the study; J.I.B., V.D., R.W., and A.B. were responsible for data abstraction; J.I.B. was responsible for economic modelling; J.I.B., V.D., R.W., and A.B. wrote the first draft of the manuscript; and J.I.B., V.D., R.W., A.B., S.C., D.K.O., and J.D.G.-F. reviewed the manuscript for scientific content.
Conflict-of-interest disclosure: S.C. received consulting fees and travel, accommodations, and expenses from Genentech/Roche, Celgene, AbbVie, Pharmacyclics, Gilead Sciences, Novartis, and Janssen Oncology and has an immediate family member who holds equity in Pharmacyclics; his institution has received research funding from Celgene, Novartis, Pharmacyclics, Gilead Sciences, AbbVie, and Janssen Oncology. D.K.O. received consulting fees from Zoll Inc. The remaining authors declare no competing financial interests.
Correspondence: James I. Barnes, Center for Primary Care and Outcomes Research, Stanford University School of Medicine, 117 Encina Commons, Stanford, CA 94305; e-mail: jibarnes@stanford.edu.