Key Points
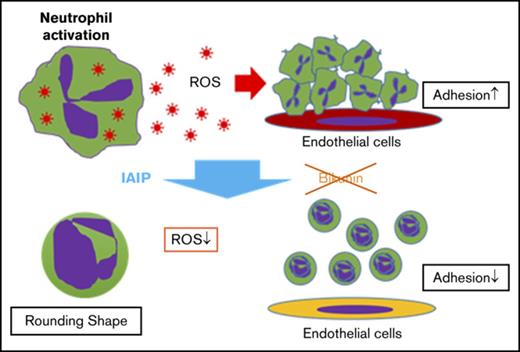
IAIP, but not bikunin, maintains spherical shape, small size, and smooth surface of human neutrophils and supports capillary passage.
IAIP reduced ROS production from neutrophils in a concentration-dependent manner probably through the p47phox phosphorylation on Ser328.
Abstract
The plasma levels of inter-α inhibitor proteins (IAIPs) are decreased in patients with sepsis and the reduced levels correlate with increased mortality. In the present study, we examined the effects of IAIPs on human neutrophils to better understand the beneficial effects of IAIPs in the treatment of sepsis. We demonstrated that IAIPs induced a spherical shape that was smaller in size with a smooth cellular surface in a concentration-dependent manner. These changes were inhibited by a specific antibody against IAIPs. In contrast, bikunin, light chain of IAIP, had no effect on neutrophil morphology. The neutrophils treated with IAIPs could easily pass through the artificial microcapillaries and were prevented from entrapment inside the capillaries. Coincubation of human blood neutrophils with a confluent human vascular endothelial monolayer showed that adhesion of neutrophils on endothelial cells was suppressed by treatment with IAIPs. IAIPs inhibited the spontaneous release of reactive oxygen species (ROS) in a concentration-dependent fashion. ROS inhibition was associated with reductions in p47phox phosphorylation on Ser328. These results suggest that IAIP-induced morphological changes that render neutrophils quiescent, facilitate passage through the microvasculature, and reduce adhesion to vascular endothelial cells and production of ROS. Thus, IAIP plays a key role in controlling neutrophil activation.
Introduction
Sepsis remains a critical problem with significant morbidity and mortality even in the modern era of critical care management. It is associated with life-threatening organ dysfunction caused by dysregulated host responses to infection.1 Neutrophils have long been considered as short-lived effector cells of the innate immune system, possessing limited capacity for biosynthetic activity. However, neutrophils play a primary role in resistance against extracellular pathogens and in acute inflammation. These cells are classically characterized as phagocytic cells, releasing lytic enzymes from their granules and producing reactive oxygen intermediates with antimicrobial potential.2 On the contrary, excessive reactive oxygen species (ROS) and nitric oxide (NO) are also linked to tissue damage, increased vascular permeability, and organ injury.3,4 Adult respiratory distress syndrome (ARDS) and organ failure may result from the plugging of neutrophils in the lungs and other organs.5
Experimental and clinical data have demonstrated that increased activity of neutrophil-derived serine proteases, such as leukocyte elastase and cathepsin G, play a prominent role in sepsis-related tissue damage.6 Therefore, administration of protease inhibitors has been proposed as a therapeutic strategy to restore the balance between proteases and protease inhibitors in sepsis.7 One such inhibitor is the inter-α inhibitor protein (IAIP) family.8-10 It is a group of serine protease inhibitors found in relatively high concentrations in human plasma (300-600 μg/mL). IAIPs are composed of heavy- and light-chain polypeptide subunits that are covalently linked by a glycosaminoglycan chain. The major forms found in human plasma are inter-α inhibitor (IAI) with molecular weight of 250 kDa, consisting of 2 heavy chains and a single light chain, and pre-α inhibitor (PAI) with molecular weight of 125 kDa, consisting of 1 heavy and 1 light chain. In this report, IAIPs refer to both major complex protein forms.11 The light chain, also called bikunin, contains the serine protease inhibitory activity.12-14 Bikunin has been widely used as a drug for patients with inflammatory disorders such as pancreatitis, shock, and disseminated intravascular coagulation (DIC).15,16
IAIPs are involved in many physiological and pathological processes. In 2004, Wu et al10 demonstrated that plasma concentrations of IAIPs were significantly decreased in experimental sepsis in rats. In vitro, IAIP inhibits several serine proteases that are involved in inflammation, including elastase, plasmin, and cathepsin G.17 Clinical studies of patients with severe sepsis have revealed significant reductions in circulating IAIPs caused by increased consumption and excretion accompanied by decreased hepatic synthesis.8,18 IAIP administration exerts potent immunomodulatory actions that result in improved survival from sepsis in neonatal mice.9 Moreover, IAIP neutralizes the cytotoxic effects of histones and inhibits histone-induced platelet aggregation by direct binding to histones.19 However, the effects of IAIPs on the circulating leukocytes remain to be determined.
The purpose of the present study was to examine the effects of IAIPs and bikunin on the function of neutrophils, specifically focusing on their morphology, capillary passage, adhesion on endothelial cells, and ROS production. We demonstrated that IAIP, but not bikunin, maintains the spherical shape, smooth cellular surface, and smaller size of neutrophils. These morphological changes render neutrophils quiescent with respect to the passage through the microvasculature, reduce cell adhesion to vascular endothelial cells, and diminish spontaneous release of ROS.
Methods
Materials
Isoluminol, horseradish peroxidase (HRP) type IV, and granulocyte-macrophage colony-stimulating factor (GM-CSF; human recombinant) were obtained from Sigma-Aldrich (St. Louis, MO). Phalloidin–Alexa 568, DNaseI–Alexa 488, 4′,6-diamidino-2-phenylindole (DAPI), Hoechst 33342, and calcein-AM were obtained from Life Technologies (Carlsbad, CA). IAIP and anti-IAIP mouse monoclonal antibody (mAb) 69.26 immunoglobulin G1 (IgG1) was produced as previously described.20 Bikunin (urinary trypsin inhibitor, Ulinastatin) was purchased from ProSpec (East Brunswick, NJ). Anti-p47phox or anti–neutrophil cytosolic factor 1 (NCF 1) rabbit mAb (EPR 13134) was obtained from Abcam (Cambridge, United Kingdom). Anti-p47phox (phosphor-S328) rabbit polyclonal antibody (Ab) was obtained from Bioworld (St. Louis, MO). Human serum albumin (HSA) and bovine serum albumin (BSA) were obtained from Sigma-Aldrich and were used as negative protein controls. Human histidine-rich glycoprotein (HRG) was purified from plasma and was used for comparison with IAIP.
Isolation of neutrophils
Human neutrophils were isolated from peripheral blood obtained from healthy volunteers under a protocol approved by the institutional review board and in accordance with The Declaration of Helsinki. The blood was drawn from the antecubital vein and human polymorphonuclear neutrophils (PMNs) were isolated by density gradient centrifugation over polymorphprep (Axis-Shield, Oslo, Norway). Briefly, blood was layered over an equal volume of polymorphprep and centrifuged at 500g for 45 minutes at 22°C. The lower band containing neutrophils was subsequently collected and washed with phosphate-buffered saline (PBS) by centrifugation at 400g for 10 minutes. Cells were counted with a hemocytometer (EKDS, Tokyo, Japan) by trypan blue dye exclusion. After the centrifugation, the pellet was resuspended in Hanks balanced salt solution (HBSS).
Staining of neutrophils by calcein-AM and Hoechst 33342
Calcein-AM (1:1000) and Hoechst 33342 (0.5:1000) were added to the suspension of cells. The mixtures were incubated for 15 minutes at 37°C, and then washed once with PBS. Then, the cells were resuspended in HBSS to a final concentration of 2 × 106 cells/mL.
Cell culture
EA.hy 926 human vascular endothelial cell lines were purchased from American Type Culture Collection (ATCC; Manassas, VA) and maintained in high-glucose Dulbecco modified Eagle medium (DMEM) medium containing 10% fetal bovine serum (FBS), 1% l-glutamine, and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed every 3 days during cell culture until the cells reached 90% confluence. The cultured cells were preincubated for 24 hours in DMEM with FBS after reaching confluence and before any experimental interventions.
Neutrophil shape assay
Purified human neutrophils prestained with Hoechst 33342 (nuclei) and calcein-AM (cytosol) were aliquoted in a volume of 100 μL (5 × 104 cells per well) to a 96-well microtiter plate. The incubation started with BSA (1 μM), HSA (1 μM), different concentrations of IAIPs (25, 50, 100, 200, and 400 μg/mL), or bikunin (25, 50, 100, 200, and 400 μg/mL). The neutrophils were incubated for 1 hour at 37°C. The cell shape and cell size were analyzed by using an In Cell Analyzer 2000 (GE Healthcare/Life Sciences, Tokyo, Japan) and In Cell Analyzer Workstation software (GE Healthcare/Life Sciences). The form factor (maximum diameter/minimum diameter) and the cell size (area) were determined in each group.
Actin distribution and cell surface structure in neutrophils
Neutrophils (2 × 106 cells/mL) were seeded onto poly l-lysine–coated cover glass (Matsunami, Tokyo, Japan) and incubated with HBSS, HSA (1 μM), BSA (1 μM), HRG (1 μM), or IAIP (100 μg/mL) at 37°C for 1 hour. The neutrophils were stained for actin after fixation with 4% paraformaldehyde (PFA) and treatment with 0.1% Triton X-100 by staining with phalloidin–Alexa 568 (F-actin), DNaseI–Alexa 488 (G-actin), and DAPI (nuclei). The samples were observed using a confocal microscope (LSM 780; Carl Zeiss, Oberkochen, Germany). The relative proportion of G-actin and F-actin was determined in each cell by the relative intensities of their staining under constant conditions. The relative amount of the total 150 cells from 4 fields was evaluated in each group. The comparison of the effects of IAIP with those of HRG was performed because HRG induced a similar round shape in neutrophils.21 The samples were fixed in 4% paraformaldehyde, then postfixed using 1% osmium tetroxide for 1 hour at 4°C for scanning electron microscopy. OsO4 coating with an osmium coater HPC-1S (Vacuum Device, Mito, Japan) was used. Each sample was examined using an s-4800 scanning electron microscope (Hitachi, Tokyo, Japan).
Binding of IAIP on neutrophil surface
To examine the direct binding of IAIP on the surface of neutrophils, fluorescein-labeled IAIP was prepared according to the instruction by manufacturer (Dojindo, Japan). The neutrophils were incubated for 30 minutes at 37°C with 100 or 200 μg/mL of labeled IAIP in the absence or presence of 400 μg/mL of unlabeled IAIP. After washing with PBS once, the cells were fixed with 0.5% PFA and applied for fluorescence-activated cell sorter (FACS) analysis.
MC-FAN
The isolated human neutrophils were treated with HBSS, IAIP (100 and 200 μg/mL), HSA (1 μM), or bikunin (100 and 200 μg/mL) for 30 minutes at 37°C. The neutrophil samples were forced to flow through artificial silicon microchannels (width ,6.4 μm; depth, 4.5 μm; length, 30 μm) under a constant suction of −20 cm H2O (microchannel array flow analyzer [MC-FAN]; MC Laboratory, Tokyo, Japan). The time required for the passage of a 100 μL of the suspension of neutrophils through the MC-FAN was quantified.
Neutrophil adhesion assay
Purified human neutrophils were labeled with calcein-AM (green), Hoechst 33342 (blue) for 20 minutes as described in “Neutrophil shape assay.” After washing, the neutrophils were incubated with HBSS, IAIP (100 μg/mL), BSA (1 μM), or HSA (1 μM) for 1 hour at 37°C in a 96-well polystyrene microtiter plate or on the monolayer of EA.hy 926 cells. Neutrophil adhesion was evaluated by counting the residual cell numbers by In Cell Analyzer 2000 (GE Healthcare, Life Sciences,) before and after the wells were gently washed twice with PBS to remove any non-adherent cells.
Determination of ROS production
The release of ROS from neutrophils was determined by the addition of isoluminol (final concentration, 50 μM) and HRP type IV (final concentration 4 U/mL) to the incubation media containing HBSS or different concentrations of IAIP. Neutrophil extracellular ROS production was determined for 30 minutes after the start of incubation at 37°C by the measurement of chemiluminescence intensity using Flexstation 3 (Molecular Devices, Sunnyvale, CA).
Neutrophil preparation and p47phox immunoblotting
Neutrophils in HBSS (4 × 106 cells/mL) were treated with GM-CSF (25 ng/mL), HBSS, HSA (1 μM), or IAIP (100 μg/mL) at 37°C for 20 minutes. The reaction was stopped by adding ice-cold buffer and by centrifugation at 400g for 10 minutes at 4°C. The cells were lysed by resuspension in lysis buffer (20 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM Na2 EDTA, 1% Triton X-100, 1 mM EGTA, 1 mM Na3 vanadate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/mL leupeptin, pH 7.5), and then resuspended and sonicated for 30 seconds. Debris from the lysed cells was pelleted by centrifugation at 15 000 rpm for 10 minutes at room temperature. The supernatant was then removed and stored at −80°C before use. Protein concentration of each sample was assayed using the BCA protein assay kit (Pierce Chemical, Rockford, IL) standardized to BSA, according to the manufacturer’s protocol. For detection of Ser328 phosphorylation in neutrophils by a specific Ab, 50 μg of protein was analyzed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti-p47phox or anti-NCF 1 (phosphor-S328) Ab (1/1000 dilution) or anti-p47phox or anti-NCF 1 rabbit mAb (1/1000 dilution) and HRP-labeled goat anti-rabbit Ab. The reaction was detected using ECL reagents.
Statistical analysis
The statistical analysis across multiple treatment groups was determined with analysis of variance, followed by the post hoc Fisher test. All data are presented as the means plus or minus standard error (SEM). P < .05 was considered statistically significant.
Results
Effects of IAIPs and bikunin on morphology of purified human neutrophils
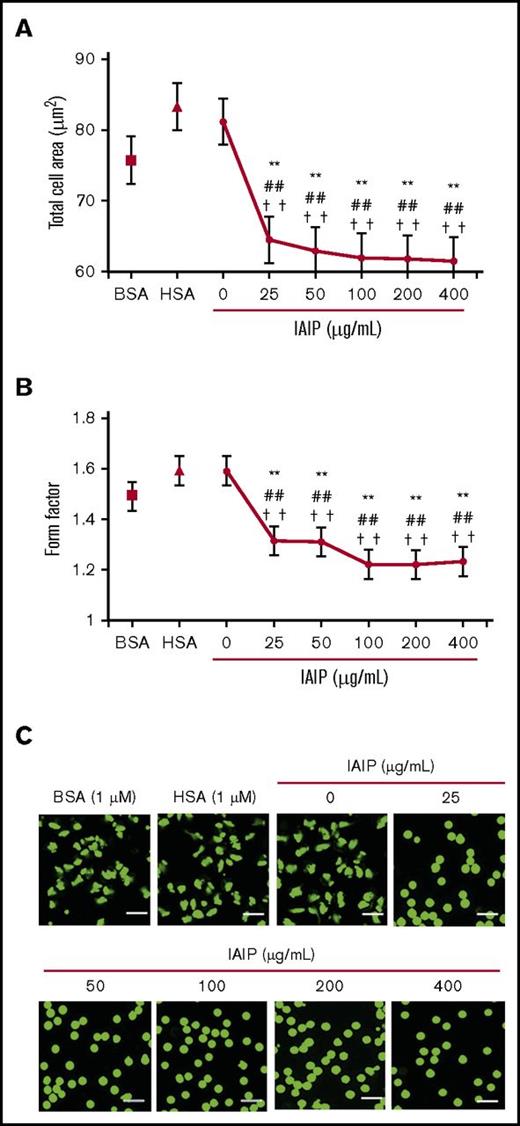
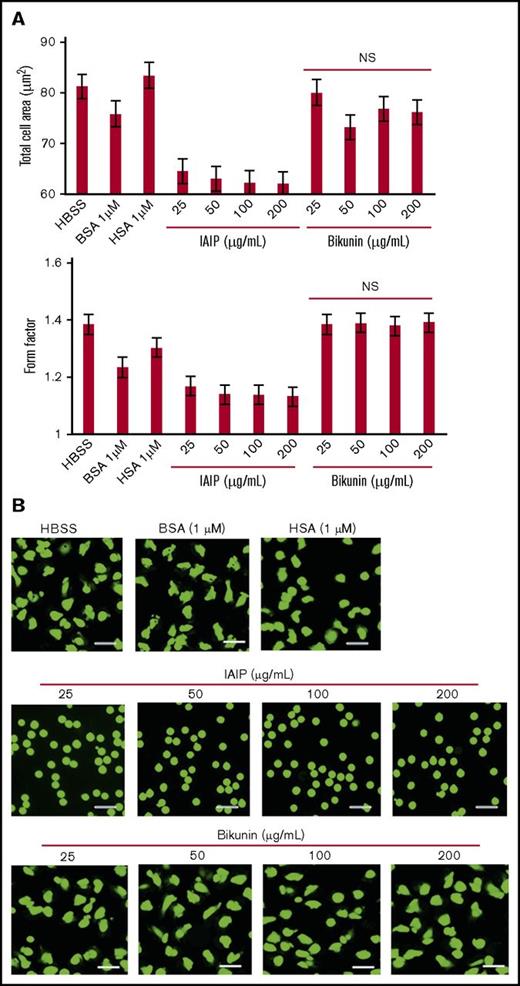
The effects of IAIPs and bikunin were determined on human neutrophils purified from peripheral blood that were labeled with calcein-AM (cytosol) and Hoechst 33342 (nuclei). IAIP-induced morphological changes were observed under a fluorescence microscope 1 hours after the start of incubation without a fixation procedure. IAIPs induced a rounded shape in the neutrophils in a concentration-dependent manner, which was evaluated by the average cell areas (Figure 1A) and form factor (Figure 1B). The morphological changes induced by IAIPs included the following: spherical shape changes, smaller in size and loss of irregularity of the shape (Figure 1C). The media containing HSA (1 μM) or BSA (1 μM) did not show the spherical shape-inducing effects of IAIPs.
Spherical shape-inducing effects of IAIPs on purified human neutrophils. The neutrophils were incubated with HSA (1 μM), BSA (1 μM), or IAIPs (0-400 μg/mL) for 1 hour and the neutrophil shape was observed under a fluorescence microscope. (A-B) The rounding and size of neutrophils were analyzed by the In Cell Analyzer 2000. Total cell area (A) (µm2) and form factor (B) were determined. The results shown are the means ± SEM of 7 experiments. **P < .001 vs 0 μg/mL of IAIPs; ##P < .001 vs HSA and ††P < .001 vs BSA. (C) Typical picture of fluorescence staining from each group is shown (calcein stain; scale bars, 20 μm).
Spherical shape-inducing effects of IAIPs on purified human neutrophils. The neutrophils were incubated with HSA (1 μM), BSA (1 μM), or IAIPs (0-400 μg/mL) for 1 hour and the neutrophil shape was observed under a fluorescence microscope. (A-B) The rounding and size of neutrophils were analyzed by the In Cell Analyzer 2000. Total cell area (A) (µm2) and form factor (B) were determined. The results shown are the means ± SEM of 7 experiments. **P < .001 vs 0 μg/mL of IAIPs; ##P < .001 vs HSA and ††P < .001 vs BSA. (C) Typical picture of fluorescence staining from each group is shown (calcein stain; scale bars, 20 μm).
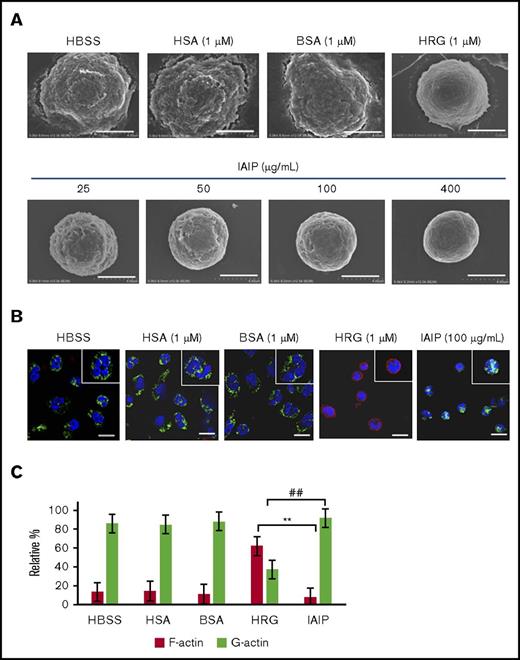
Scanning electron microscopic observation confirmed the loss of surface microvilli structures on neutrophils in HRG- and IAIP-treated groups in a concentration-dependent manner (Figure 2A). The neutrophils treated with buffer alone, HSA or BSA showed the polymorphic shapes with many microvilli or folds on their surface.
Effects of IAIPs on surface structure and cytoskeletal arrangement in neutrophils. The purified human neutrophils were incubated with HBSS, HSA (1 μM), BSA (1 μM), HRG (1 μM), or different concentrations of IAIPs (25-400 μg/mL) in a 96-well microtiter plate for 1 hour at 37°C. (A) After fixation as described in “Methods,” scanning electron microscopic pictures of neutrophils were obtained. Typical cell appearance is shown from each group. All images were acquired at 12 000× magnification. Scale bars, 4.0 μm. (B) After 1-hour incubation at 37°C, the neutrophils were fixed with 4% PFA, and monomer actin (green) and F-actin (red) were stained immunohistochemically. All images acquired at 40× original magnification by confocal laser microscope. Typical cells are shown from each group. Insets represent the higher magnification picture of a single cell from each group. Scale bars, 10 μm. (C) The numbers of F-actin and G-actin dominant cells in panel B were counted in each group and were expressed as relative percentage. The results shown are the means ± SEM of 3 experiments. **P < .001, HRG F-actin vs IAIP F-actin; ##P < .001, HRG G-actin vs IAIP G-actin.
Effects of IAIPs on surface structure and cytoskeletal arrangement in neutrophils. The purified human neutrophils were incubated with HBSS, HSA (1 μM), BSA (1 μM), HRG (1 μM), or different concentrations of IAIPs (25-400 μg/mL) in a 96-well microtiter plate for 1 hour at 37°C. (A) After fixation as described in “Methods,” scanning electron microscopic pictures of neutrophils were obtained. Typical cell appearance is shown from each group. All images were acquired at 12 000× magnification. Scale bars, 4.0 μm. (B) After 1-hour incubation at 37°C, the neutrophils were fixed with 4% PFA, and monomer actin (green) and F-actin (red) were stained immunohistochemically. All images acquired at 40× original magnification by confocal laser microscope. Typical cells are shown from each group. Insets represent the higher magnification picture of a single cell from each group. Scale bars, 10 μm. (C) The numbers of F-actin and G-actin dominant cells in panel B were counted in each group and were expressed as relative percentage. The results shown are the means ± SEM of 3 experiments. **P < .001, HRG F-actin vs IAIP F-actin; ##P < .001, HRG G-actin vs IAIP G-actin.
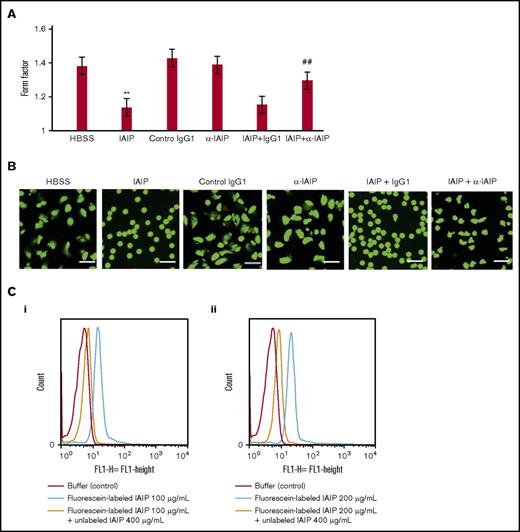
The cytochemical staining of F- and G-actin in neutrophils demonstrated that F-actin was dominant in HRG-treated neutrophils. Cytosolic G-actin was dominant in neutrophils treated with HBSS, HSA, BSA and IAIPs, (Figure 2B-C). A few neutrophils had an F-actin ring under these conditions. The spherical shape-inducing effects of IAIPs were inhibited by the addition of mouse monoclonal Ab against IAIPs, but not by control IgG, while anti-IAIP Ab alone had no effects on the morphology of neutrophils (Figure 3). These results confirmed the specificity of the IAIP effects on neutrophil morphology.
Effect of mAb against IAIPs on IAIP-induced induction of spherical shape in neutrophils. (A) Anti-IAIP mAb (69.26; 10 μg/mL) or control IgG1 was added simultaneously with IAIPs (100 μg/mL) and the incubation continued for 1 hour. The form factors were determined. The results shown are the means ± SEM of 7 experiments. **P < .001 vs HBSS; ##P < .001 compared with IAIPs + IgG1. (B) Typical picture from each group is shown (calcein stain; scale bars, 20 μm). (C) FACS analysis of IAIP binding on isolated human neutrophils. Purified human neutrophils were incubated with fluorescein-labeled IAIPs (100 and 200 μg/mL) in the presence or absence of unlabeled IAIPs (400 μg/mL) for 30 minutes at 37°C. After washing once, the fluorescein-labeled IAIPs were fixed by 0.5% PFA. The fluorescence intensity of the binding of IAIPs on neutrophils was analyzed by a FACS caliber. (i) Fluorescein-labeled IAIPs, 100 µg/mL; (ii) fluorescein-labeled IAIPs, 200 µg/mL. Buffer control is shown in red, fluorescein-labeled IAIPs is shown in blue, and fluorescein-labeled IAIPs in the presence of unlabeled IAIPs is shown in yellow. The data are representative of 2 independent experiments.
Effect of mAb against IAIPs on IAIP-induced induction of spherical shape in neutrophils. (A) Anti-IAIP mAb (69.26; 10 μg/mL) or control IgG1 was added simultaneously with IAIPs (100 μg/mL) and the incubation continued for 1 hour. The form factors were determined. The results shown are the means ± SEM of 7 experiments. **P < .001 vs HBSS; ##P < .001 compared with IAIPs + IgG1. (B) Typical picture from each group is shown (calcein stain; scale bars, 20 μm). (C) FACS analysis of IAIP binding on isolated human neutrophils. Purified human neutrophils were incubated with fluorescein-labeled IAIPs (100 and 200 μg/mL) in the presence or absence of unlabeled IAIPs (400 μg/mL) for 30 minutes at 37°C. After washing once, the fluorescein-labeled IAIPs were fixed by 0.5% PFA. The fluorescence intensity of the binding of IAIPs on neutrophils was analyzed by a FACS caliber. (i) Fluorescein-labeled IAIPs, 100 µg/mL; (ii) fluorescein-labeled IAIPs, 200 µg/mL. Buffer control is shown in red, fluorescein-labeled IAIPs is shown in blue, and fluorescein-labeled IAIPs in the presence of unlabeled IAIPs is shown in yellow. The data are representative of 2 independent experiments.
Using fluorescein-labeled IAIPs, we directly detected the binding of IAIPs on neutrophils that was antagonized by the presence of unlabeled IAIPs, indicating the specificity of the binding (Figure 3C).
Next, we investigated whether the light chain of IAIP, bikunin, induced similar changes in the morphology of the neutrophils. We observed that concentrations of bikunin similar to those of IAIPs did not affect the morphology of the neutrophils (Figure 4).
Comparison of the effects of IAIPs and bikunin on neutrophil shapes. The neutrophils were incubated with HBSS, HSA (1 μM), BSA (1 μM), IAIPs (25-200 μg/mL), or bikunin (25-200 μg/mL) for 1 hour and the neutrophil shapes were observed under a fluorescence microscope. (A) The rounding and size of neutrophils were analyzed by the In Cell Analyzer 2000. Cell area and form factor were determined on 5 × 104 cells on each group. Bikunin did not induce spherical shape even at high concentrations in neutrophils. The results shown are the means ± SEM of 7 experiments. (B) Typical picture from each group is shown (calcein stain; scale bars, 20 μm). NS, not significant compared with HBSS group.
Comparison of the effects of IAIPs and bikunin on neutrophil shapes. The neutrophils were incubated with HBSS, HSA (1 μM), BSA (1 μM), IAIPs (25-200 μg/mL), or bikunin (25-200 μg/mL) for 1 hour and the neutrophil shapes were observed under a fluorescence microscope. (A) The rounding and size of neutrophils were analyzed by the In Cell Analyzer 2000. Cell area and form factor were determined on 5 × 104 cells on each group. Bikunin did not induce spherical shape even at high concentrations in neutrophils. The results shown are the means ± SEM of 7 experiments. (B) Typical picture from each group is shown (calcein stain; scale bars, 20 μm). NS, not significant compared with HBSS group.
Effects of IAIPs on microcapillary passage of purified human neutrophils
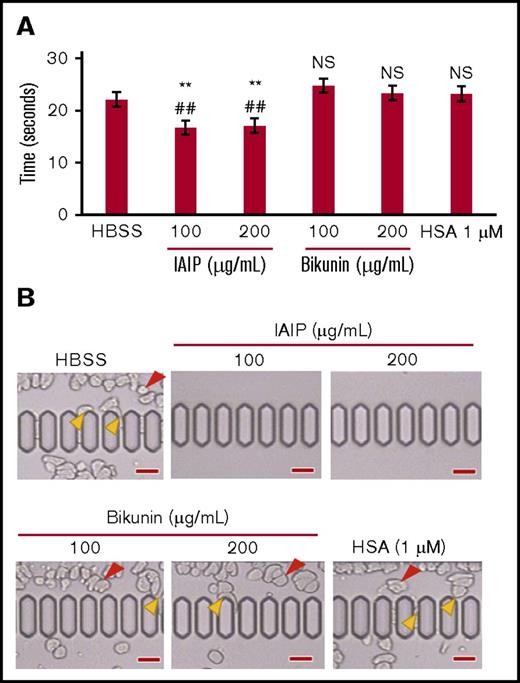
The passage of purified neutrophils through microcapillary slits (6.4 μm width, 4.5 μm depth) was evaluated by a Micro channel array flow analyzer (MC-FAN) under different conditions. Neutrophils treated with IAIPs (100 μg/mL) and (200 μg/mL) could pass microcapillaries more smoothly and easily compared with the other treatment groups such as HBSS, HSA (1 μM), bikunin (100 μg/mL) and (200 μg/mL). The passage time of 100 μL of neutrophils treated with bikunin (100 μg/mL) and (200 μg/mL) were significantly longer than those treated with IAIPs (100 μg/mL) and (200 μg/mL) and was similar to buffer control (Figure 5A). There were trapping of irregular shaped neutrophils observed before or on the microcapillary slits in HBSS, HSA and bikunin groups, but not in the IAIP-treated groups (Figure 5B; supplemental Video 1).
Neutrophil passage through microcapillaries. The purified human neutrophils were incubated with HBSS, HSA (1 μM), IAIPs (100 and 200 μg/mL), or bikunin (100 and 200 μg/mL) for 30 minutes and then applied to an MC-FAN. (A) The time required for the passage of 100-μL neutrophil suspension through the MC-FAN was determined (supplemental Video 1). The results shown are the means ± SEM of 5 experiments. **P < .01 vs HBSS; ##P < .01 vs HSA. (B) Red arrowheads indicate the leukocytes attached to the upper chamber. Yellow arrowheads indicate the leukocytes attached to the microcapillary entrance or slit (scale bars, 30 μm). NS, not significant compared with both HBSS and HSA groups.
Neutrophil passage through microcapillaries. The purified human neutrophils were incubated with HBSS, HSA (1 μM), IAIPs (100 and 200 μg/mL), or bikunin (100 and 200 μg/mL) for 30 minutes and then applied to an MC-FAN. (A) The time required for the passage of 100-μL neutrophil suspension through the MC-FAN was determined (supplemental Video 1). The results shown are the means ± SEM of 5 experiments. **P < .01 vs HBSS; ##P < .01 vs HSA. (B) Red arrowheads indicate the leukocytes attached to the upper chamber. Yellow arrowheads indicate the leukocytes attached to the microcapillary entrance or slit (scale bars, 30 μm). NS, not significant compared with both HBSS and HSA groups.
Effects of IAIPs on adhesion of purified human neutrophils on microtiter plate and vascular endothelial cells
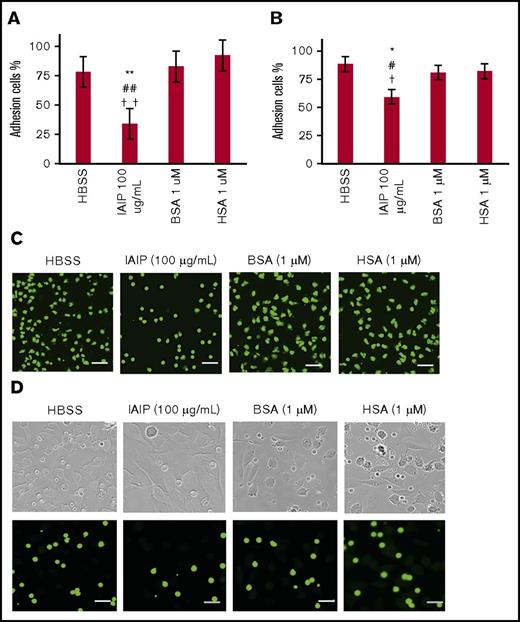
The adhesion of neutrophils to the plastic well and monolayer of EA.hy 926 were examined by counting the residual neutrophils after the wells were washed twice. The number of remaining neutrophils in microtiter plate treated with 100 μg/mL of IAIP decreased significantly compared with the other treatment groups HBSS or HSA (1 μM) or BSA (1 μM). This was also true for the adhesion to monolayer of vascular endothelial cells. These results showed that IAIP-induced rounding of the neutrophils were associated with reductions in the adhesion of neutrophils to the plastic well (Figure 6A,C) as well as the monolayer of vascular endothelial cells (Figure 6B,D).
Neutrophil adhesion assay. The purified human neutrophils were incubated with HBSS, IAIPs (100 μg/mL), BSA (1 μM), or HSA (1 μM) for 1 hour at 37°C in a 96-well polystyrene microtiter plate (A,C) or on a monolayer of EA.hy 926 (B,D). After gentle washing of the microtiter plate or EA.hy 926 monolayer with PBS twice, the residual cell numbers were counted and expressed as percentages of the initial cell numbers. (A-B) The results shown are the means ± SEM of 10 experiments. **P < .01 vs HBSS, ## P < .01 vs HSA, and ††P < .001 vs BSA on a 96-well microtiter plate (A) and *P < .05 vs HBSS, #P < .05 vs HSA, and †P < .05 vs BSA on a monolayer of EA.hy 926 (B). (C-D) Typical pictures are shown from each group (calcein stain; scale bars, 20 μm). Top panels in panel D show the phase contrast images of corresponding lower fluorescence microscopic pictures.
Neutrophil adhesion assay. The purified human neutrophils were incubated with HBSS, IAIPs (100 μg/mL), BSA (1 μM), or HSA (1 μM) for 1 hour at 37°C in a 96-well polystyrene microtiter plate (A,C) or on a monolayer of EA.hy 926 (B,D). After gentle washing of the microtiter plate or EA.hy 926 monolayer with PBS twice, the residual cell numbers were counted and expressed as percentages of the initial cell numbers. (A-B) The results shown are the means ± SEM of 10 experiments. **P < .01 vs HBSS, ## P < .01 vs HSA, and ††P < .001 vs BSA on a 96-well microtiter plate (A) and *P < .05 vs HBSS, #P < .05 vs HSA, and †P < .05 vs BSA on a monolayer of EA.hy 926 (B). (C-D) Typical pictures are shown from each group (calcein stain; scale bars, 20 μm). Top panels in panel D show the phase contrast images of corresponding lower fluorescence microscopic pictures.
Effects of IAIPs on the release of ROS from neutrophils
The release of ROS from neutrophils was determined for 30 minutes after the start of incubation. The ROS was measured by the detection of isoluminol chemiluminescence under the treatment with HBSS or different concentrations of IAIPs (12.5, 25 and 100 μg/mL) (Figure 7A). The extracellular ROS production was inhibited in a concentration-dependent manner by IAIPs when determined at 15 minutes after the start of incubation (Figure 7B). A significant effect was even detected at 1.5 μg/mL.
Effects of IAIPs on extracellular ROS production and p47phoxphosphorylation in neutrophils. (A) The purified human neutrophils were incubated with different concentrations of IAIPs (12.5, 25, and 100 μg/mL) or HBSS for 30 minutes and the ROS produced extracellularly was determined using isoluminol as a substrate. The chemiluminescence in the medium was determined by FlexStation 3. The time-course changes are shown. Results are expressed as means ± SEM of 6 experiments. (B) The ROS produced extracellularly was determined at 15 minutes in the presence of different concentrations of IAIPs. The results are expressed as means ± SEM of 6 experiments. **P < .01 vs HBSS. (C) The purified human neutrophils were incubated with 25 ng/mL GM-CSF (positive control), HBSS, HSA (1 μM), or IAIPs (100 μg/mL) for 20 minutes at 37°C. Cells were then lysed, and proteins from 4 × 106 cells were analyzed with SDS-PAGE. Western immunoblotting was performed with anti-p47phox and anti-phospho-Ser328 antibodies. (D) Western immunoblots from 3 different experiments were scanned; phosphorylated and total p47phox were quantified by densitometry. Results are expressed as means ± SEM (n = 3). **P < .01 vs HBSS. (E) IAIPs 6.25 μg/mL or 12.5 µg/mL were preincubated with control IgG1 20 µg/mL or IAIP Ab 20 µg/mL for 30 minutes at 37°C. Purified human neutrophils were then incubated with IAIPs for 30 minutes and the ROS produced extracellularly was determined using isoluminol as a substrate. The chemiluminescence in the medium was determined by FlexStation 3. Percentage suppression of ROS can be calculated using the following formula: ((chemiluminescence intensity of cells treated with HBSS or control IgG1 or IAIPs Ab alone [control] − chemiluminescence intensity of cells treated with IAIPs or IAIPs with IgG1 or IAIPs with IAIP Ab)/chemiluminescence intensity of control) × 100. The results are the means ± SEM of 3 experiments. ☥P < .05 IAIPs vs IAIPs + α-IAIP; &P < .05 IAIPs + control IgG vs IAIPs + α-IAIP.
Effects of IAIPs on extracellular ROS production and p47phoxphosphorylation in neutrophils. (A) The purified human neutrophils were incubated with different concentrations of IAIPs (12.5, 25, and 100 μg/mL) or HBSS for 30 minutes and the ROS produced extracellularly was determined using isoluminol as a substrate. The chemiluminescence in the medium was determined by FlexStation 3. The time-course changes are shown. Results are expressed as means ± SEM of 6 experiments. (B) The ROS produced extracellularly was determined at 15 minutes in the presence of different concentrations of IAIPs. The results are expressed as means ± SEM of 6 experiments. **P < .01 vs HBSS. (C) The purified human neutrophils were incubated with 25 ng/mL GM-CSF (positive control), HBSS, HSA (1 μM), or IAIPs (100 μg/mL) for 20 minutes at 37°C. Cells were then lysed, and proteins from 4 × 106 cells were analyzed with SDS-PAGE. Western immunoblotting was performed with anti-p47phox and anti-phospho-Ser328 antibodies. (D) Western immunoblots from 3 different experiments were scanned; phosphorylated and total p47phox were quantified by densitometry. Results are expressed as means ± SEM (n = 3). **P < .01 vs HBSS. (E) IAIPs 6.25 μg/mL or 12.5 µg/mL were preincubated with control IgG1 20 µg/mL or IAIP Ab 20 µg/mL for 30 minutes at 37°C. Purified human neutrophils were then incubated with IAIPs for 30 minutes and the ROS produced extracellularly was determined using isoluminol as a substrate. The chemiluminescence in the medium was determined by FlexStation 3. Percentage suppression of ROS can be calculated using the following formula: ((chemiluminescence intensity of cells treated with HBSS or control IgG1 or IAIPs Ab alone [control] − chemiluminescence intensity of cells treated with IAIPs or IAIPs with IgG1 or IAIPs with IAIP Ab)/chemiluminescence intensity of control) × 100. The results are the means ± SEM of 3 experiments. ☥P < .05 IAIPs vs IAIPs + α-IAIP; &P < .05 IAIPs + control IgG vs IAIPs + α-IAIP.
Inhibition of p47phox phosphorylation on Ser328 by IAIPs in intact neutrophils
ROS production in neutrophils is dependent on the formation of the NADPH oxidase machinery complex and p47phox phosphorylation occurs during the formation of ROS. Therefore, we identified the effects of IAIPs on the p47phox site that is phosphorylated during oxidative bursts. We performed Western immunoblot analysis of whole-cell lysates from neutrophils treated with HBSS or HSA (1 μM) or IAIPs (100 μg/mL) or GM-CSF (25 ng/mL) (positive control) using the anti-p47phox Ab and anti-p47phox phospho-Ser328 Ab. The results showed that serine 328 phosphorylation occurred during the incubation with HBSS or HAS and that IAIPs clearly inhibited p47phox phosphorylation on Ser328 in neutrophils (Figure 7C-D). The extracellular ROS production was inhibited by IAIPs which was reversed by mAb against IAIPs, but not by control IgG. (Figure 7E).
FACS analysis of the expression of adhesion molecules on human neutrophils
IAIPs (100 μg/mL) did not induce any changes in CD 11b, CD 18 and CD 62L expression in the presence or absence of agonists (C5a, IL-8 and fMLP). On the other hand, the same concentration of IAIPs inhibited the expression of CD 162 on neutrophils both in the presence and absence of 3 agonists (C5a, IL-8 and fMLP) (supplemental Figure 1).
Effect of IAIPs on NET formation
Treatment of neutrophils with IAIPs (100 μg/mL) inhibited LPS (10 ng/mL)- or calcium inophore (A 23187) (1 µM and 5 µM)-induced NET formation compared with HBSS- and HSA-treated groups (supplemental Figure 2).
Effects of IAIPs on spontaneous death of isolated neutrophils
IAIPs (100 μg/mL) significantly inhibited the staining of DNA by Sytox suggesting the loss of integrity of plasma membrane and prevention of spontaneous death of isolated neutrophils (supplemental Figure 3).
Discussion
PMNs are the primary line of defense against invading microorganisms and are recruited as the first cells to inflammatory sites. There are abnormalities of systemic and microcirculatory blood flow resulting in tissue hypoperfusion in both sepsis and septic shock.22,23 The rheological properties of neutrophils appear to be defective during sepsis and septic shock.21 The deformability of neutrophils has previously been shown to be decreased in response to bacterial toxins and cytokines in vitro.24,25 Neutrophils have diameters of 9 to 12 μm. Therefore, they must be able to deform to pass through the systemic and pulmonary capillaries, which have average diameters of 5 to 6 μm. Consequently, the shape, deformability, and surface properties of neutrophils may directly affect the passage of neutrophils through microcapillaries.5,26
Many studies have reported that IAIPs has a significant role in regulation of systemic inflammation.27,28 Intraperitoneal administration of IAIPs improved survival to nearly 90% in both LPS- and live bacteria-induced sepsis in neonatal mice.9 IAIPs reduced the proinflammatory cytokine TNF-α and increased IL-10 in the mice with sepsis.9 Yang et al28 demonstrated that the administration of IAIPs in the early stages of hemodynamic instability attenuated the elevated concentrations of liver enzymes and lactate in rats with sepsis. In addition, repeated administration of IAIPs after delayed treatment improved survival rate in rats exposed to the cecal ligation and puncture (CLP) procedure.10 Furthermore, IAIP concentrations were decreased in patients with sepsis and the reduced levels predicted mortality rates.8,29
In the present study, we demonstrated that exposure to IAIP-induced changes in neutrophils such that the shape was more spherical, the size was smaller and there was a loss of microvilli on the surface of the neutrophils. Moreover, these changes were accentuated by increasing concentrations of IAIPs. These morphological changes in the neutrophils appeared to be similar to those induced by another plasma protein, HRG.21 However, the cytoskeletal rearrangements relating to morphological changes induced by IAIP and HRG21 differed with regard to the predominance and subcellular distribution of F-actin formation (Figure 2B-C). These findings suggest the potential that the signaling pathways that control the morphological features of neutrophils differ when triggered by IAIPs and HRG. The round shape with smooth surface in the presence of IAIPs facilitated the prompt passage of neutrophils through the microcapillary system and prevented neutrophil entrapment in the in vitro capillary system. The effects of IAIPs on the morphological features of the neutrophils and resultant rapid passage through the in vitro capillary system were saturable at 100 μg/mL in the present study. The normal concentration of IAIPs in human plasma is 300 to 600 μg/mL. Therefore, the salutary effects of IAIPs could be considerably reduced when endogenous IAIP levels decrease below 100 μg/mL during significant episodes of infection.
IAIP is composed of heavy and light chains and the free light chain is also termed bikunin.16 Previous work has suggested that bikunin could inhibit the infiltration of neutrophils and reduce the release of elastase and chemical mediators from neutrophils.16 In contrast to the previous findings,16 the results of our study demonstrated that a wide range concentrations of bikunin did not affect neutrophil morphology or function compared with those induced by similar concentrations of the complex molecules of IAIPs. Based upon the findings of our study, we postulate that heavy chains of IAIPs or heavy chains in combination with the light chain could be responsible for inducing the morphological changes, facilitating the passage through the microcapillary system and suppressing the tenacity of the attachment of neutrophils to the vascular endothelial cells. Previous work has also suggested that IAIPs and its anionic carbohydrate moieties directly bind to and inhibit histone-related cytotoxicity by preventing the charge-based interactions. Moreover, the neutralization of histones by IAIPs was not dependent upon the bikunin related protease inhibitory activity of IAIPs.19 The exact mechanism by which IAIPs affect neutrophils remains to be determined. However, the beneficial effects of IAIPs on neutrophils could improve systemic and microcirculatory blood flow and, thereby, prevent potential immunothrombosis during sepsis and septic shock like conditions.
The NADPH oxidase in neutrophils plays major role in the generating superoxide anion, which can destroy invading pathogens. However, uncontrolled and exaggerated production of ROS by phagocytes may produce deleterious effects in many inflammatory disorders especially in the blood stream.6 Therefore, tight control of the NADPH oxidase complex is necessary to avoid injury to normal tissues. The protein p47phox can be phosphorylated in several different serine sites, during the formation of the active enzyme complex of NADPH oxidase on the plasma membrane. Thus, p47phox is the phagocyte NADPH oxidase organizer.30
IAIPs inhibited extracellular ROS production in neutrophils. The inhibition was dependent upon the concentration of IAIPs and even very low levels of IAIPs were capable of inhibiting the release of ROS. Furthermore, IAIPs inhibited the p47phox phosphorylation on Ser328 among the different sites of phosphorylation on p47phox. Consequently, these results suggest that IAIPs suppressed ROS production under resting conditions in intact neutrophils and p47phox phosphorylation on a specific serine site. In our present study, we demonstrated that the monoclonal Ab against IAIPs inhibited the spherical shape-inducing effects of IAIPs on neutrophil morphology as well as the suppression of ROS production. Our findings confirmed the specificity of the effects of IAIPs on neutrophil morphology and ROS production. The findings of our study are important because the concentrations of IAIPs used in the present study were similar to normal plasma concentrations (300-600 μg/mL) observed in heathy humans. However, additional work is required to examine the roles of other serine target sites involved in the regulation of NADPH oxidase activity.
Endothelial barrier function is also impaired in septic shock.31 Uncontrolled and excessively activated neutrophils attach firmly to vascular endothelial cells in septic disorders21 and subsequently release large quantities of proteolytic enzymes and ROS that can disrupt endothelial of barrier function, resulting in tissue injury. In our current study, coincubation of neutrophils with a confluent inactivated vascular endothelial monolayer showed that IAIPs suppressed the adhesion of neutrophils on the vascular endothelial cells. One possible explanation could be that the spherical shape of neutrophils probably minimized the surface contact area and physical contact between the neutrophils and endothelial cells of the microvasculature. The ability of IAIPs to suppress ROS production could also be beneficial to the protection from endothelial injury. Consequently, the ability of IAIPs to inhibit neutrophil adhesion to endothelial cells could represent an important component in the prevention of endothelial injury.
The transmigration process of neutrophils is mediated by cell adhesion molecules of the selectin and β2 integrins (CD11/CD18). CD162 (PSGL-1) is the major counterreceptor of P-selectin for neutrophil initial attachment and rolling on activated vascular endothelium.32 The inhibitory regulation of CD162 expression by IAIPs demonstrated in the present study suggests that the suppressive effects of IAIPs on the interaction between neutrophils and other effector cells attenuates the inflammatory response.
NETosis is 1 form of cell death pathway of neutrophils and was observed in the microcirculation in sepsis.21 Netosis participates not only in host defense but also contributes to the development of tissue damage due to immunothrombosis. Because it was suggested that NET formation requires the production of ROS,33 the finding that IAIPs inhibited the NETosis is consistent with the concentration-dependent inhibitory effects of IAIPs on ROS production. Taken together, IAIPs may inhibit immunothrombosis during polymicrobial infections.10
Neutrophils survive for <24 hours in normal circulation. The production of neutrophils and their removal must be equivalent to maintain homeostasis.34 Therefore, the turn over and lifespan of neutrophils must be finely regulated by many factors. The inhibitory effects of IAIPs on spontaneous neutrophil death suggests that IAIPs might be an important plasma factor in the regulation of neutrophil survival.
In conclusion, we have demonstrated that IAIPs, but not bikunin, strongly regulates the human neutrophil shape, facilitating easy and rapid passage through capillaries and inhibiting the adhesion to the vascular endothelial cells. IAIPs also renders neutrophils quiescent by suppressing the release of ROS. These effects observed in vitro could represent a component of the beneficial effects of systemically administered IAIPs in vivo in animal models of sepsis.9,10 Additional work is required to identify other mechanisms fundamental to the immunomodulatory and anti-inflammatory effects of IAIPs.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was in part supported by grants from Scientific Research from the Ministry of Health, Labour, and Welfare of Japan (WA2F2547, WA2F2601), the Japan Agency for Medical Research and Development, AMED (15lk0201014h0003), and from the Secom Science and Technology Foundation (M.N.), and grants from the National Institutes of Health under the following award numbers: National Institute of Neurological Disorders and Stroke 1R21NS095130, 1R21NS096525 (B.S.S.), and 2 R44 NS084575 (Y.-P.L. and B.S.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.S.H. performed the experiments, analyzed the data, and wrote the manuscript; M.N. supervised the study, wrote and edited the manuscript, and served as principal investigator; H.W. set up the experiments, analyzed the data, and wrote the manuscript; B.S.S edited the manuscript; Y.-P.L. produced IAIPs and anti-IAIP Ab, and edited the manuscript; K.L. and K.T. contributed to setting up the in vitro experiments using neutrophils; and all authors approved the final manuscript.
Conflict-of-interest disclosure: Y.-P.L. is employed by ProThera Biologics and has financial stakes in the company. The remaining authors declare no competing financial interests.
Correspondence: Masahiro Nishibori, Department of Pharmacology, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8558, Japan; e-mail: mbori@md.okayama-u.ac.jp.
References
Author notes
S.S.H. and H.W. contributed equally to this work.

![Figure 7. Effects of IAIPs on extracellular ROS production and p47phox phosphorylation in neutrophils. (A) The purified human neutrophils were incubated with different concentrations of IAIPs (12.5, 25, and 100 μg/mL) or HBSS for 30 minutes and the ROS produced extracellularly was determined using isoluminol as a substrate. The chemiluminescence in the medium was determined by FlexStation 3. The time-course changes are shown. Results are expressed as means ± SEM of 6 experiments. (B) The ROS produced extracellularly was determined at 15 minutes in the presence of different concentrations of IAIPs. The results are expressed as means ± SEM of 6 experiments. **P < .01 vs HBSS. (C) The purified human neutrophils were incubated with 25 ng/mL GM-CSF (positive control), HBSS, HSA (1 μM), or IAIPs (100 μg/mL) for 20 minutes at 37°C. Cells were then lysed, and proteins from 4 × 106 cells were analyzed with SDS-PAGE. Western immunoblotting was performed with anti-p47phox and anti-phospho-Ser328 antibodies. (D) Western immunoblots from 3 different experiments were scanned; phosphorylated and total p47phox were quantified by densitometry. Results are expressed as means ± SEM (n = 3). **P < .01 vs HBSS. (E) IAIPs 6.25 μg/mL or 12.5 µg/mL were preincubated with control IgG1 20 µg/mL or IAIP Ab 20 µg/mL for 30 minutes at 37°C. Purified human neutrophils were then incubated with IAIPs for 30 minutes and the ROS produced extracellularly was determined using isoluminol as a substrate. The chemiluminescence in the medium was determined by FlexStation 3. Percentage suppression of ROS can be calculated using the following formula: ((chemiluminescence intensity of cells treated with HBSS or control IgG1 or IAIPs Ab alone [control] − chemiluminescence intensity of cells treated with IAIPs or IAIPs with IgG1 or IAIPs with IAIP Ab)/chemiluminescence intensity of control) × 100. The results are the means ± SEM of 3 experiments. ☥P < .05 IAIPs vs IAIPs + α-IAIP; &P < .05 IAIPs + control IgG vs IAIPs + α-IAIP.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/15/10.1182_bloodadvances.2018018986/3/m_advances018986f7.jpeg?Expires=1767769474&Signature=eKKIlsUyMqe6Pc8v1S~Jb3eU~4vQVZ7vkuSShFnwmLUn73tj1z7dY4LpGT00yQMszT6SJ3SqY4tyjdovwpqhT7xqFtBBSTLCwqg6x9yK1f16Lk4D578PhGlD-W6o7STdwURizTlQckz84xomJ4Z24zomFzUydX04g~XGrEPg99ngnZOK~O5BjLdqVJnJTCCL5L07Y0lKa8ebWJdB55OvpS1dvbinxXwjW5~WDexLnh4Qru9qO-533tXnfVAUqPqXL0kQBQWunXFTx4KMyj7TqsQyy4P3JNmTIuWe2EbuHog8dhCpFykg8VtZuUW69GeBtryDyRWdjrTB24qZtEcx0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)