Abstract
Treatment of severe aplastic anemia has improved significantly over the past 4 decades. This review will summarize the key areas of progress in the use of allogeneic hematopoietic cell transplantation and nontransplant immunosuppressive therapy (IST) for the treatment of aplastic anemia and then summarize the recommendations for first-line treatment. Based on recent data, we argue that guidelines for the initial treatment of patients with newly diagnosed severe aplastic anemia require revision. At the time of diagnosis, before beginning treatment, HLA typing should be done to identify a marrow donor among family members or in the unrelated donor registries, and a marrow transplant should be considered first-line therapy. The priority order of donor source for bone marrow transplantation is: (1) HLA-identical sibling, (2) HLA-matched unrelated donor, and (3) HLA-haploidentical donor if an HLA-matched unrelated donor is not rapidly available. Each of these donor marrow sources may be preferable to nontransplant IST. We make this recommendation because of the long-term persistent risk for disease relapse and secondary myelodysplastic syndrome or acute myeloid leukemia with the use of nontransplant IST for patients with aplastic anemia. In contrast, marrow transplantation is associated with high cure rates of aplastic anemia and a relatively low risk for graft-versus-host disease, with many patients now living for decades without the risk for disease recurrence or the development of clonal disorders. Implementation of this first-line treatment strategy will provide patients with severe aplastic anemia the best chance of long-term disease-free survival.
History
The 2 major current competing treatment strategies for severe aplastic anemia, allogeneic bone marrow transplantation (BMT) and immunosuppressive therapy (IST) with anti-thymocyte globulin (ATG), date back to 1970. That year saw the first successful marrow transplant from an HLA-identical sibling donor.1 The transplant was carried out based on principles established during years of preclinical experimentation, including the cyclophosphamide (CY)-conditioning regimen, the importance of HLA matching between marrow donor and recipient, and the need for postgrafting immunosuppressive drugs to control graft-versus-host disease (GVHD). The conceptual framework of allogeneic BMT was straightforward: replace the aplastic marrow in the patient with a marrow graft from a healthy donor. This concept was validated by the demonstration of sustained donor-type hematopoiesis in surviving patients. The original concept underlying the use of immunosuppression with ATG was also that of substituting a healthy marrow for a diseased one.2 ATG was meant to serve as an alternative conditioning regimen for less well-matched marrow grafts and, owing to its long half-life in the circulation, exert control over donor lymphocytes, thereby minimizing the risk for GVHD. However, it soon became apparent that the hematopoietic recovery seen in some patients was derived from the host and not from the graft.3-5 Moreover, hematopoietic recovery in patients who responded to ATG was not always complete, which contrasted with the generally complete recovery seen with HLA-matched marrow grafts after CY conditioning. Subsequent prospective randomized trials in ATG-treated patients showed that an HLA-mismatched marrow graft was not required and that ATG on its own sufficed for inducing remission of aplastic anemia in some patients.6,7 Therefore, the HLA-mismatched marrow infusion after ATG was abandoned.
Early results
Early on, neither of the 2 approaches for treating aplastic anemia was an unqualified success. Although most patients conditioned with CY for HLA-identical marrow grafts showed initial engraftment, a large percentage (36% in the Seattle experience) rejected their grafts, and some remaining patients succumbed to GVHD.8-10 In the 1970s, long-term survival after allogeneic marrow transplantation was 45%. The problem of graft rejection was predicted from preclinical studies and attributed to sensitization of patients to minor non-HLA antigen disparities with their respective donors through prior blood transfusions.11,12 Consistent with the preclinical data, graft rejection in untransfused severe aplastic anemia patients was rare, and their long-term survival was ∼80%.13,14
Mortality from GVHD was a second major problem in the era of postgrafting immunosuppression with methotrexate (MTX) monotherapy.15 Nevertheless, long-term survivors showed stable polyclonal donor–derived hematopoiesis with normal immune function, and they were leading normal lives.16,17 Pediatric patients had normal growth and development.18 Fertility was unimpaired, and patients were able to have normal children.19 One early problem was the then unknown hepatitis C virus infection, which, based on retrospective analyses, affected 45% of transfused patients.20 Consequently, a number of transplanted patients eventually developed chronic active hepatitis, liver cirrhosis, and even hepatocellular carcinoma.21 With the identification of the hepatitis C virus and the development of screening tests, hepatitis C is no longer a significant clinical problem. The incidence of cancers, whether related or unrelated to transplantation, has remained low.22,23 Retrospective analyses among long-term surviving patients showed an incidence of 4% at 20 years.24
The problems seen after IST with ATG were of a different nature. Complete, partial, or only minimal responses were seen in 45% of patients, whereas a majority of patients remained aplastic.25,26 Moreover, clonal disorders, including paroxysmal nocturnal hemoglobinuria, myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML), were seen in surviving patients. Tichelli et al reported a 26% incidence of MDS/AML, and a larger European study reported a 20% incidence of cancer.27,28 Similar incidences were reported by other investigators.29
How have the problems encountered with the 2 treatment strategies (BMT and IST) for aplastic anemia been addressed and what has the progress been?
Progress in BMT
HLA-matched related grafts
Preclinical studies drove the impressive improvement in survival of marrow-grafted patients, which, at our transplant center, is now at 100%.30,31 Animal experiments showed that the risk for graft rejection could be minimized by using leukocyte-poor blood products, by irradiating blood products before transfusion, and by introducing a conditioning regimen in which CY alternated every 12 hours with ATG, which resulted in immunosuppressive synergism.32-34 After these changes were adopted clinically, graft rejection rates declined to <4%.35 Other preclinical studies focused on GVHD prevention.36 Synergistic immunosuppression was discovered when MTX was combined with a calcineurin inhibitor, either cyclosporine (CSP) or tacrolimus.37,38 Subsequent prospective phase 3 randomized clinical trials confirmed superior GVHD prevention with the drug combination compared with either drug alone.39-42
Combined CY/ATG conditioning and MTX/calcineurin inhibitor immunosuppression postgrafting have become standard of care. The acute GVHD seen tends to be of only mild to moderate severity, and in almost all patients it responds to first-line therapy with prednisone. As a rule, grade 4 acute GVHD is no longer seen.24,30,31,35 Also, with ursodiol prophylaxis, liver GVHD has become very rare.43,44
Although acute GVHD can be controlled in almost all cases and, thus, is associated with a very low risk for death, chronic GVHD has remained a problem. Cumulative incidences ranging from 0% to 44% have been reported with marrow as the graft source.45-49 We observed that the incidence of chronic GVHD was 16% among patients in whom marrow grafts were limited to ≤2.5 × 108 nucleated cells per kilogram.31 Treatment duration for chronic GVHD is long, although that information is generally not provided in published reports. Among our patients, the median treatment duration was 17 months (range, 14 to 69 months). Encouragingly, in all but 1 patient, therapy could be discontinued without recurrence of chronic GVHD. The single patient still on therapy receives once-weekly low-dose MTX. The recommendation to limit the number of infused marrow cells is a promising approach to reduce the risk for chronic GVHD, and this approach awaits confirmation in future trials. Limiting the number of infused marrow cells is in stark contrast to the earlier recommendations made in the 1970s-1980s to infuse large doses of donor marrow cells (and buffy coat) to reduce the risk for graft rejection. It is now clear that the earlier recommendations are no longer valid. Since the 1970s-1980s, the risk for graft rejection has been dramatically reduced by minimizing sensitization to non-HLA antigens through the use of leukocyte-depleted and in vitro irradiation of blood products and by the use of the more immunosuppressive CY/ATG conditioning regimen.9-15,30-35
As a consequence of the reduced risks for graft rejection, serious acute GVHD and chronic GVHD, survival of patients given HLA-matched marrow grafts has steadily improved. Most studies now report survival rates ranging from 70% to 90%.49,50 In our experience, survival of patients younger than 20 years has been 100% for the past 28 years, and a more recent study that included pediatric and adult patients with a median follow-up of 4 years also showed 100% survival.30,31 For some older patients (>50 years), conditioning with reduced-dose CY plus fludarabine (FLU) has achieved improved survival, with less risk for transplant-related mortality.51-53 A retrospective combined European Group for Blood and Marrow Transplantation/Center for International Blood and Marrow Transplant Research study of transplants in older patients with aplastic anemia (median age, 58 years; range, 50-77 years) had a median time from diagnosis to transplant of 10 months and included 275 HLA-identical sibling grafts, 224 8/8 HLA-matched unrelated grafts, and 39 7/8 HLA-matched unrelated grafts.54 The patients’ overall 3-year survival was 56%. Two factors adversely affected outcome: grafts from unrelated donors and a performance score < 90%. Although the data support, in principle, the feasibility of allogeneic transplantation in older aplastic anemia patients, the data need to be interpreted with caution given the retrospective nature of the analysis and the possibility of selection bias.
Of note, these improvements have been accomplished with marrow as the stem cell source. Studies using granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs) have consistently shown worse outcomes, in large part due to higher rates and greater severity of acute and chronic GVHD. Thus, marrow remains the transplant product of choice.49,50,55 Recent published data for HLA-identical sibling transplants are summarized in Table 1.
Select recent reports of HLA-matched sibling and unrelated donor hematopoietic cell transplantation for severe aplastic anemia
| Reference (year) . | Year of transplant . | Patients, n . | Age range (median), y . | Conditioning program . | Hematopoietic source (n) . | Prevention of GVHD . | Graft rejection/failure, % . | GVHD, % . | Survival, % . | Follow-up, range (median), y . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute . | Chronic . | ||||||||||
| HLA-identical sibling donor transplantation | |||||||||||
| 30 (2012) | 1971-1984 1981-1988 1989-2010 | 98 19 31 | 1.8-19 (12.8) | CY CY CY+ATG | BM | MTX CSP+MTX CSP+MTX | 22 32 7 | 21 11 39 | 21 21 10 | 66 95 100 | 11-37.2 (31.1) 15.2-27.1 (23) 0.3-21.5 (6.1) |
| 49 (2012) | 1999-2009 | 1886 | 1-68 (18) | CY+ATG (41%) and various | BM (1163) | CSP+MTX (41%) or other | 9 | 11 | 11 | Age ≤20 y: 90 Age >20 y: 74 | (2.1) |
| 1-69 (24) | PBSCs (723) | 10 | 17 | 22 | Age ≤20 y: 76 Age >20 y: 64 | (2.0) | |||||
| 52 (2009) | 1998-2007 | 30 | 31-66 (46) | FLU+CY+ATG | BM (20) PBSCs (10) | CSP+MTX or other | 3 | 10 | 13 | 77 | 1.1-6.8 (4.1) |
| 82 (2016) | 1999-2014 | BMT: 1732 IST: 802 | 0-20 21-40 >40 | BMT various | Compare BMT vs IST | Various for BMT | N/A | N/A | N/A | Age 0-20 y: 86 (BMT); 84 (IST) Age 21-40 y: 76 (BMT); 65 (IST) Age >40 y: 56 (BMT); 58 (IST) | 0.2-10 |
| IST various | |||||||||||
| 50 (2015) | 2005-2009 | 940 | Age >20 y: 50% | Various | BM (566) PBSCs (374) | Various | 9 | 13 | 14 | Low risk: 93 Int. risk: 78 High risk: 67 | 0.1-9.1 (3.1) |
| 31 (2016) | 2006-2015 | 21 | 3-52 (15) | CY+ATG | BM <2.5 × 108 TNC/kg | CSP+MTX | 0 | 47 | 16 (24) | 100 | 1-8 (4.0) |
| Unrelated donor transplantation | |||||||||||
| 57 (2012) | 2000-2010 | 44 | 3-19 (8) | FLU+CY+Alem various doses | BM (26) PBSCs (18) | CSP or CSP+MMF or MTX | 0 | 38 | 12 | 95 | 1-6.3 (2.9) |
| 50 (2015) | 2005-2009 | 508 | Age >20 y: 53% | Various | BM (264) PBSCs (244) | Various | 9 | 25 | 26 | Low risk: 83 Int. risk: 77 High risk: 64 | 0.1-9.1 (3.1) |
| 58 (2015) | 2005-2014 | 29 Upfront MUD HCT (no prior IST) | 1.7-19.1 (8.4) | FLU+CY+Alem (+2-3-Gy TBI for 1 Ag-MM) | BM (21) PBSCs (8) | CSP or CSP/MMF | 4 | 10 | 19 | 96 OS 92 2-y EFS | 0.2-8.5 (1.7) |
| 56 (2015) | 2006-2013 | 79 | 0.5-66 (24) | ATG+2-Gy TBI+CY-100 (n = 41) vs CY-50 (n = 38) | BM | CSP/MTX | CY-100: 15 CY-50: 12 | 27 24 | 32 23 | 81 at 1 y 97 at 1 y | 1.0-4.2 (2.0) 0.3-2.2 (1.4) |
| 53 (2014) | 1999-2009 | 55 URD | 1-67 (18) | FLU+CY+Alem | BM (36) PBSCs (19) | Tacrolimus/MMF | 9 | 38 | 13 | 88 97 BM 70 PBSCs | 0.5-10 |
| Reference (year) . | Year of transplant . | Patients, n . | Age range (median), y . | Conditioning program . | Hematopoietic source (n) . | Prevention of GVHD . | Graft rejection/failure, % . | GVHD, % . | Survival, % . | Follow-up, range (median), y . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute . | Chronic . | ||||||||||
| HLA-identical sibling donor transplantation | |||||||||||
| 30 (2012) | 1971-1984 1981-1988 1989-2010 | 98 19 31 | 1.8-19 (12.8) | CY CY CY+ATG | BM | MTX CSP+MTX CSP+MTX | 22 32 7 | 21 11 39 | 21 21 10 | 66 95 100 | 11-37.2 (31.1) 15.2-27.1 (23) 0.3-21.5 (6.1) |
| 49 (2012) | 1999-2009 | 1886 | 1-68 (18) | CY+ATG (41%) and various | BM (1163) | CSP+MTX (41%) or other | 9 | 11 | 11 | Age ≤20 y: 90 Age >20 y: 74 | (2.1) |
| 1-69 (24) | PBSCs (723) | 10 | 17 | 22 | Age ≤20 y: 76 Age >20 y: 64 | (2.0) | |||||
| 52 (2009) | 1998-2007 | 30 | 31-66 (46) | FLU+CY+ATG | BM (20) PBSCs (10) | CSP+MTX or other | 3 | 10 | 13 | 77 | 1.1-6.8 (4.1) |
| 82 (2016) | 1999-2014 | BMT: 1732 IST: 802 | 0-20 21-40 >40 | BMT various | Compare BMT vs IST | Various for BMT | N/A | N/A | N/A | Age 0-20 y: 86 (BMT); 84 (IST) Age 21-40 y: 76 (BMT); 65 (IST) Age >40 y: 56 (BMT); 58 (IST) | 0.2-10 |
| IST various | |||||||||||
| 50 (2015) | 2005-2009 | 940 | Age >20 y: 50% | Various | BM (566) PBSCs (374) | Various | 9 | 13 | 14 | Low risk: 93 Int. risk: 78 High risk: 67 | 0.1-9.1 (3.1) |
| 31 (2016) | 2006-2015 | 21 | 3-52 (15) | CY+ATG | BM <2.5 × 108 TNC/kg | CSP+MTX | 0 | 47 | 16 (24) | 100 | 1-8 (4.0) |
| Unrelated donor transplantation | |||||||||||
| 57 (2012) | 2000-2010 | 44 | 3-19 (8) | FLU+CY+Alem various doses | BM (26) PBSCs (18) | CSP or CSP+MMF or MTX | 0 | 38 | 12 | 95 | 1-6.3 (2.9) |
| 50 (2015) | 2005-2009 | 508 | Age >20 y: 53% | Various | BM (264) PBSCs (244) | Various | 9 | 25 | 26 | Low risk: 83 Int. risk: 77 High risk: 64 | 0.1-9.1 (3.1) |
| 58 (2015) | 2005-2014 | 29 Upfront MUD HCT (no prior IST) | 1.7-19.1 (8.4) | FLU+CY+Alem (+2-3-Gy TBI for 1 Ag-MM) | BM (21) PBSCs (8) | CSP or CSP/MMF | 4 | 10 | 19 | 96 OS 92 2-y EFS | 0.2-8.5 (1.7) |
| 56 (2015) | 2006-2013 | 79 | 0.5-66 (24) | ATG+2-Gy TBI+CY-100 (n = 41) vs CY-50 (n = 38) | BM | CSP/MTX | CY-100: 15 CY-50: 12 | 27 24 | 32 23 | 81 at 1 y 97 at 1 y | 1.0-4.2 (2.0) 0.3-2.2 (1.4) |
| 53 (2014) | 1999-2009 | 55 URD | 1-67 (18) | FLU+CY+Alem | BM (36) PBSCs (19) | Tacrolimus/MMF | 9 | 38 | 13 | 88 97 BM 70 PBSCs | 0.5-10 |
Alem, alemtuzumab; BM, bone marrow; CY-50, 50 mg/kg CY; CY-100, 100 mg/kg CY; EFS, event-free survival; HCT, hematopoietic cell transplantation; Int., intermediate; MMF, mycophenolate mofetil; MUD, matched unrelated donor; N/A, not available; OS, overall survival; TBI, total body irradiation; TNC, total nucleated cell; URD, unrelated donor; 1 Ag-MM, 1 HLA-antigen mismatch.
HLA-matched unrelated marrow transplantation
The outcomes after unrelated marrow grafts for aplastic anemia have steadily improved over the past 3 decades. The most recent publications are summarized in Table 1. A recent analysis of European registry results from 508 unrelated and 940 HLA-identical sibling transplant recipients between 2005 and 2009 reported that the incidence of acute and chronic GVHD was greater for unrelated recipients than for HLA-identical recipients: 25% and 26% vs 13% and 14%, respectively.50 Although the overall survival after unrelated grafts was less than after HLA-identical sibling transplantation, the difference was not significant. The strongest negative predictor of survival was the use of PBSCs, followed by an interval of diagnosis to transplant ≥180 days, patient age ≥20 years, no ATG in the conditioning regimen, and positive donor/recipient cytomegalovirus status.50
Improvement in the outcome of unrelated donor transplantation has focused on reducing toxicity of the conditioning regimen, with a reduced dose of CY combined with FLU and low-dose total body irradiation (TBI). A recent multicenter trial determined the optimal conditioning regimen for unrelated grafts to include horse/rabbit ATG (30 and 3 mg/kg per day, respectively, on days −4 to −2), CY (50 mg/kg on day −4), FLU (30 mg/m2 per day on days −5 to −2), and 2- Gy TBI (on day −1).56 The incidence of acute and chronic GVHD was 23.7% and 22.5%, respectively. With a median follow-up of 17 months, the 1-year overall survival was 97.4%. With longer follow-up, there were 3 deaths (8%): 1 from graft failure and 2 from chronic GVHD. In summary, the combination of ATG, CY (50 mg/kg), FLU (120 mg/m2), and 2-Gy TBI, followed by a 10/10 HLA-allele matched unrelated marrow graft, appears to be the optimal regimen and graft source.56 An alternative regimen to consider includes conditioning with CY/FLU/alemtuzumab (Table 1); however, alemtuzumab is associated with a high risk for viral infections.53,57
Dufour et al reported on the outcome of 29 children with acquired severe aplastic anemia treated with unrelated grafts as first-line treatment (no IST prior to the transplant). The 2-year overall survival and event-free survival were 96% and 92%, respectively. These results appeared equal to a historical matched group of HLA-identical sibling BMT (group a) but superior to 2 other historical matched groups (b and c). In group a, for HLA-identical sibling marrow transplants (n = 87), the 2-year overall survival and event-free survival were 91% and 87%, respectively; in group b, for patients who received first-line IST with horse ATG and CSP (n = 58), 2-year overall survival and event-free survival were 94% and 40%, respectively; and in group c, for recipients of second-line therapy with unrelated donor transplantation after failure of IST (n = 24), 2-year overall survival and event-free survival were both 74%.58
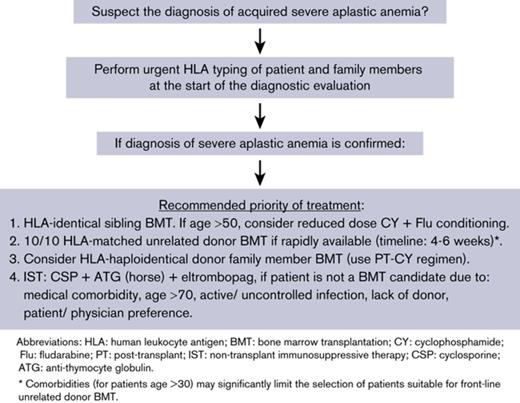
The recent data support the recommendation for first-line treatment with an HLA-matched unrelated marrow transplant for children with acquired severe aplastic anemia who lack an HLA-identical sibling donor.58-60 Given the improvement in outcomes with FLU-based conditioning regimens with low-dose TBI and CY and ATG or alemtuzumab53,56 and the increased risk for relapse and late complications after nontransplant IST,26,28,61 strong consideration should be given to first-line marrow transplantation for patients who are younger than 30 years old with aplastic anemia if a 10/10 HLA-matched unrelated donor is rapidly identified. An urgent unrelated donor search should identify and procure a marrow graft within 4-6 weeks from diagnosis; waiting longer than this will incur an increased risk for infection for the patient. Patients from ethnic minorities who are underrepresented in the unrelated donor registries will probably not have a suitable unrelated donor identified rapidly. Unrelated donors with 9/10 high-resolution HLA matching may also be considered, but there are insufficient data to propose such donors for first-line treatment. Clinical trials prospectively comparing unrelated donor BMT with IST (eg, NCT02845596) are open to enrollment, and the results will help clarify the appropriate first-line treatment. After careful clinical evaluation of an informed patient, it may even be appropriate for some older adults to proceed with first-line unrelated donor hematopoietic cell transplantation (HCT). Although unrelated grafts can be successfully performed after failure of immunosuppression or after evolution to MDS/AML, overall survival is reduced when unrelated transplantation is used as second-line treatment.58 Our recommendation for considering first-line unrelated donor HCT over IST for patients older than 20 years differs from other published opinions,62 but the recent evidence for improved long-term survival after unrelated donor HCT supports the broader role of unrelated donor HCT as first-line therapy.
HLA-haploidentical related marrow transplantation
The successful application of posttransplant CY (PT-CY) for HLA-haploidentical grafts was recently reported for aplastic anemia patients. Between July 2011 and August 2016, 13 patients with refractory aplastic anemia received HLA-haploidentical–related marrow grafts.63 The nonmyeloablative conditioning regimen consisted of rabbit ATG (4.5 mg/kg total dose) from days −9 to −7, CY (14.5 mg/kg CY per day) on days −6 and −5, FLU (30 mg/kg per day) from days −6 to −2, and 2-Gy TBI on day −1. PT-CY (50 mg/kg per day, intravenously) on days +3 and +4, mycophenolate mofetil on days +5 through +35, and tacrolimus on day +5 through 1 year were administered for GVHD prophylaxis. The median age of the patients was 30 years (range, 11-69 years). All patients had sustained engraftment, 2 patients had mild acute and chronic GVHD that responded to treatment, and each patient surviving for >12 months was off immunosuppression. With a median follow-up of 21 months (range, 3 to 64 months), all patients were alive. Other groups have reported similar results with PT-CY and HLA-haploidentical marrow transplants, albeit with equally small numbers of patients.64
The use of PT-CY to achieve in vivo T-cell depletion has simplified the clinical use of HLA-haploidentical grafts and has achieved very encouraging early results. Again, it appears that bone marrow is the preferred graft source from HLA-haploidentical donors. The results from the Johns Hopkins group challenge the earlier consensus that HLA-haploidentical grafts should be used as second-line therapy for aplastic anemia once immunosuppression has failed and if an HLA-matched unrelated donor is unavailable.63 Prospective clinical trials evaluating front-line HLA-haploidentical BMT vs IST are needed to settle the issue of the appropriate course of treatment more definitively. Cord blood transplantation is probably inferior to HLA-haploidentical marrow grafts because of the low cell dose infused, the high risk for graft rejection, and delayed engraftment/immune recovery.65,66
Nontransplant IST
Progress has also been made with IST for aplastic anemia. The first step forward was to combine ATG with maintenance CSP. A randomized trial conducted in the late 1980s and with long-term follow-up showed ∼41% failure-free survival with ATG/CSP compared with only 25% with ATG alone.25,26 In part, based on that study, current patients receive the combination of ATG and CSP.
In an attempt to improve outcomes, a prospective randomized study from the European Group for Blood and Marrow Transplantation evaluated adding G-CSF to immunosuppression and found no benefit in survival.67 Moreover, another study identified G-CSF to be a risk factor for the development of MDS/AML with monosomy 7, especially in children.68 As previously discussed, acquired clonal disorders were identified in several studies, even in the absence of G-CSF, with rates ranging from 13% to 26%.27-29,69,70
Rabbit ATG was demonstrated to be inferior to horse ATG as first-line immunosuppressive treatment.71 A total of 120 consecutive patients with aplastic anemia, ages 2 to 77 years, who were not considered candidates for marrow grafts as first-line treatment, was randomly assigned to horse ATG or rabbit ATG (60 in each group). Horse ATG (40 mg/kg per day for 4 days) was compared with rabbit ATG (3.5 mg/kg per day for 5 days). CSP was given twice daily from day 1 and continued for ≥6 months in both groups. The 2 groups were matched in patient characteristics, and the median follow-up was 28 months. The hematologic response rate at 6 months was 68% with horse ATG and 37% with rabbit ATG (P < .001). The incidence of clonal evolution at 3 years was 21% in the horse ATG group and 14% in the rabbit ATG group. The overall survival at 3 years was 96% in the horse ATG group and 76% in the rabbit ATG group when data were censored at the time of marrow transplantation, and it was 94% and 70%, respectively, when not censored.71
Eltrombopag (Promacta), an oral thrombopoietin receptor agonist, was used in 43 patients who had remained pancytopenic after initial treatment with ATG + CSP immunosuppression.72 Although there was an early response rate of 40% at 4 months after starting eltrombopag, only 28% of the patients had a prolonged improvement in blood counts. In this salvage therapy study, response was defined as a prespecified improvement in at least one of the following: platelet, hematocrit, or neutrophil count.72 However, 19% of treated patients developed cytogenetic abnormalities, including monosomy 7, a poor prognostic finding. More recently, eltrombopag was added to standard immunosuppression (ATG + CSP) as front-line therapy in 92 patents with acquired aplastic anemia.61 The dose of eltrombopag was evaluated in 3 consecutive cohorts. Cohort 1 received eltrombopag from day 14 to 6 months, cohort 2 received it from day 14 to 3 months, and cohort 3 received it from day 1 to 6 months. The cohorts were analyzed separately. The duration of CSP treatment was increased from 6 to 24 months midway through the trial to reduce disease relapse. The rates of complete response at 6 months were 33% in cohort 1, 26% in cohort 2, and 58% in cohort 3. The overall response rates at 6 months were 80%, 87%, and 94%, respectively. In this front-line therapy study, complete response was defined as recovery of all 3 of the following blood counts at 6 months after the start of treatment: absolute neutrophil count >1 × 109/L, hemoglobin >10 g/dL, and platelet count >100 × 109/L; overall response included recovery of at least one of these blood counts. The overall survival rate at 2 years was 97% in all cohorts. Marrow transplantation was indicated in 12 patients; 6 patients had not had a response or remained dependent on blood cell transfusions, 3 had a relapse, and 3 had clonal evolution. Clonal cytogenetic evolution occurred in 7 patients (8%); however, the median follow-up was only 2 years. Five patients had loss of chromosome 7, 1 patient had a complex karyotype, and 1 patient had AML. Clonal evolution occurred 3 to 6 months after treatment in 5 patients and at 30 months in 2 other patients. The prolonged duration of IST (24 months) to achieve hematopoietic reconstitution may be significantly longer than after BMT. In summary, the addition of eltrombopag to immunosuppression was associated with higher rates of hematologic response among patients with severe aplastic anemia compared with a historical cohort, but there remained a high incidence of clonal evolution.61 Eltrombopag is approved by the US Food and Drug Administration for patients with severe aplastic anemia who have had an insufficient response to IST. Recently, it also granted breakthrough therapy designation to eltrombopag for use in combination with nontransplant IST for the treatment of severe aplastic anemia as a first-line therapy.
As described above, it has been recognized that a subset of patients with aplastic anemia treated with immunosuppression develop clonal hematopoiesis and some eventually develop cytogenetic abnormalities that lead to MDS or AML.27-29 With the development of high-throughput genomic sequencing, the molecular basis of clonal hematopoiesis in aplastic anemia has been comprehensively assessed.73 Using next-generation sequencing in a study of 439 patients with aplastic anemia, genetic abnormalities were detected in ∼50% of patients at ≥6 months after initiation of immunosuppression, most frequently as acquired mutations.74 One third of these patients had acquired somatic mutations in myeloid cancer candidate genes, including DNMT3A, ASXL1, and BCOR/BCORL1. Severe aplastic anemia patients with ASXL1, DNMT3A, TP53, RUNX1, JAK2, JAK3, and CSMD1 mutations had a significantly lower response to IST and worse overall survival.
A similar study from King’s College London found acquired somatic mutations in 19% of aplastic anemia patients, and most involved ASXL1, DNMT3A, and BCOR. Patients with somatic mutations had longer disease duration, shorter telomere lengths, and a 40% risk for subsequent transformation to MDS.75 In another study, accelerated telomere attrition was seen in aplastic anemia patients with somatic myeloid cancer gene mutations that progressed to MDS or AML with monosomy 7.76 Yet another study found that mutations in ASXL1 were associated with progression to MDS.77 Using whole-exome sequencing, one group reported somatic mutations in 73% of aplastic anemia patients involving 51 genes, including PIGA and 6pUPD, and signaling pathways STAT5B and CAMK2G.78
In summary, deep-genomic sequencing for commonly mutated MDS/AML genes has revealed a high incidence of mutations that lead to the emergence of clonal evolution and MDS/AML after immunosuppressive treatment of aplastic anemia. However, it is important to note that evidence of clonal hematopoiesis in the setting of bone marrow failure does not reliably predict evolution to leukemia. In one study, only a small minority of aplastic anemia patients with ASXL1 or DNMT3A mutations progressed to AML/MDS during the limited period of follow-up.79 In the future, genomic sequencing of the common MDS/AML genes may be useful to determine whether patients with poor-prognosis somatic mutations should be referred for allogeneic marrow transplantation either prior to failure of immunosuppression or to prevent the subsequent progression to MDS/AML. Although patients who evolve to MDS/AML after immunosuppression can be successfully treated with marrow grafts, outcomes are inferior to marrow transplantation early after the diagnosis of severe aplastic anemia.80
It is also useful to assess for minor paroxysmal nocturnal hemoglobinuria clones and telomere length by flow cytometry of the peripheral blood at the time of aplastic anemia diagnosis. Children with lymphocytes containing short telomeres and the absence of a paroxysmal nocturnal hemoglobinuria clone at diagnosis had a 19% response rate to IST at 6 months.81 These patients may benefit from first-line BMT, including from matched unrelated and HLA-haploidentical donors.
Taken together, immunosuppressive treatment is effective at ameliorating pancytopenia in a number of patients, but the lack of uniform success, the risk for somatic mutations resulting in evolution of MDS/AML, and the frequent recurrence of aplastic anemia make this treatment strategy a second choice behind marrow transplantation from an HLA-matched family member. Long-term (>10-year) survival with immunosuppression may be no better than 40% to 50%. The recent improvement in outcomes for marrow transplantation using HLA-identical sibling donors, HLA-matched unrelated donors, or HLA-haploidentical related donors strongly supports the decision to proceed directly to transplantation for the majority of patients with newly diagnosed severe aplastic anemia because of the long-term benefit of transplantation over immunosuppression.
Conclusions
Treatment outcomes with allogeneic marrow transplantation and with immunosuppression have improved since 1970. Complete and sustained donor-derived hematopoietic recovery, excellent quality of life, and high rates of survival are now the rule with marrow-transplantation approaches. In contrast, late complications after nontransplant immunosuppression continue to reduce survival. Therefore, faced with the choice of an HLA-identical sibling marrow graft or even an alternative donor graft vs immunosuppression, many patients should be counseled in favor of marrow transplantation as first-line therapy.
Acknowledgments
The work was supported in part by grants from the National Institutes of Health (P01 HL122173 and R01 HL125183 from the National Heart, Lung, and Blood Institute, and P30 CA015704 from the National Cancer Institute).
Authorship
Contribution: G.E.G. conducted the literature review and wrote and edited the manuscript; K.D. edited the manuscript; and R.S. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George E. Georges, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109-1024; e-mail: ggeorges@fredhutch.org.