Key Points
AUTO3 ± pembrolizumab for r/r LBCL was safe and, therefore, used in outpatient administration.
AUTO3 ± pembrolizumab showed durable remissions beyond 12 months in 54.4% of complete responders and was associated with robust expansion.
Abstract
Relapse after CD19-directed chimeric antigen receptor T-cell (CAR-T) therapy for large B-cell lymphoma (LBCL) is commonly ascribed to antigen loss or CAR-T exhaustion. Multiantigen targeting and programmed cell death protein-1 blockade are rational approaches to prevent relapse. Here, we test CD19/22 dual-targeting CAR-T (AUTO3) plus pembrolizumab in relapsed/refractory LBCL (NCT03289455). End points include toxicity (primary) and response rates (secondary). Fifty-two patients received AUTO3 and 48/52 received pembrolizumab. Median age was 59 years (range, 27-83), 46/52 had stage III/ IV disease and median follow-up was 21.6 months. AUTO3 was safe; grade 1-2 and grade 3 cytokine release syndrome affected 18/52 (34.6%) and 1/52 (1.9%) patients, neurotoxicity arose in 4 patients (2/4, grade 3-4), and hemophagocytic lymphohistiocytosis affected 2 patients. Outpatient administration was tested in 20 patients, saving a median of 14 hospital days per patient. Overall response rates were 66% (48.9%, complete response [CR]; 17%, partial response). Median duration of remission (DOR) for CR patients was not reached and for all responding patients was 8.3 months (95% confidence interval [CI]: 3.0-not evaluable). 54.4% (CI: 32.8-71.7) of CR patients and 42.6% of all responding patients were projected to remain progression-free at ≥12 months. AUTO3 ± pembrolizumab for relapsed/refractory LBCL was safe and delivered durable remissions in 54.4% of complete responders, associated with robust CAR-T expansion. Neither dual-targeting CAR-T nor pembrolizumab prevented relapse in a significant proportion of patients, and future developments include next-generation–AUTO3, engineered for superior expansion in vivo, and selection of CAR binders active at low antigen densities.
Introduction
Failure to achieve sustained responses is observed in ∼60% to 70% of adult patients with relapsed/refractory (r/r) large B-cell lymphoma (LBCL) receiving CD19-directed chimeric antigen receptor T-cell (CAR-T) therapy.1,2 CD19– relapse, likely because of antigen downregulation or loss due to CAR-T selection pressure favoring low antigen density clones,3 is associated with treatment failure in one-third of patients experiencing relapse.4,5 Impaired CAR-T expansion in vivo and T-cell exhaustion6,7 (including overexpression of programmed cell death protein 1 [PD-1]) is associated with treatment failure in the remaining patients.8,9 Gene expression profiles from LBCL tissue biopsies obtained before CAR-T, at day 14 after infusion, and at relapse in a subset of patients receiving commercially available CD19CAR-T show PD-L1 upregulation at day 1410 and high level of expression in 62% of patients with disease progression.5
Potential strategies to overcome these modes of relapse and to improve CAR-T therapy for LBCL include dual antigen targeting and modulation of the PD-1 or PD-L1 axis with checkpoint inhibitors.
Dual antigen targeting may be achieved using several methods, such as coadministration of 2 CAR-T products targeting CD19 and CD22 separately; cotransduction of T cells with 2 vectors encoding separate CD19 and CD22 CARs; bicistronic vectors permitting the coexpression of 2 independent receptors in parallel on the cell surface at a 1:1 ratio; and tandem CARs, with CD19 and CD22 binders being expressed on a single spacer or endodomain.11-13 Currently, there is limited data to select the approach that offers the best clinical outcomes for patients.
We developed AUTO3, a dual-targeting, humanized, second-generation autologous CD19/22CAR-T product, using a bicistronic vector encoding CD19CAR and CD22CAR within a single construct. Previously, AUTO3 demonstrated high efficacy and tolerable safety in pediatric B-cell acute lymphoblastic leukemia (B-ALL) (NCT NCT03289455).14 In the phase 1 ALEXANDER trial (NCT03289455), we tested AUTO3 plus PD-1 blockade with pembrolizumab in adults with r/r LBCL in the inpatient and outpatient (OPD) setting.
Methods
AUTO3 structure and manufacture
As described previously,14 AUTO3 is an autologous CAR-T product coexpressing 2 humanized second-generation CARs that recognize CD19 and CD22 by transduction with a single bicistronic γ-retroviral vector (Figure 1A). AUTO3 products were generated via the transduction and expansion of autologous leukapheresate, using a semiautomated closed–culture system. In vitro assays and manufacture methodology are described in the supplemental Appendix, available on the Blood website.
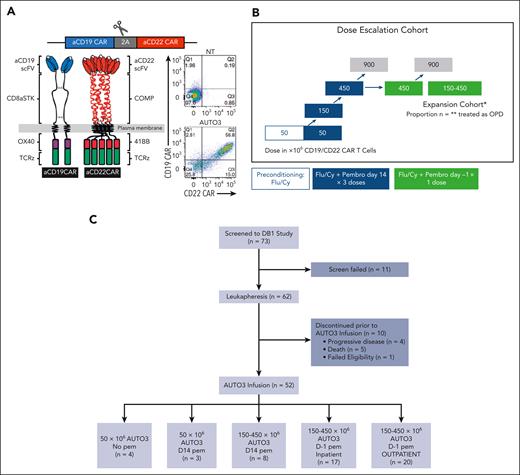
AUTO3 study design and recruitment. (A) AUTO3 CAR, CD19 CAR, and CD22 CAR are type I transmembrane proteins. Human HD37 recognizes CD19, is located at the extreme amino terminus and, in turn, connected to the CD8α stalk, transmembrane, and anchor. The intracellular endodomains are composed of costimulatory OX40 and CD3 ζ (TCRζ). Humanized LT22 recognizes CD22, is located at the extreme amino terminus and is connected to the pentameric α-helical coiled coil multimer forming domain of cartilage oligomeric matrix protein (COMP) and transmembrane anchor. The intracellular endodomains are composed from 41BB ζ and CD3 ζ (TCRζ).14 (B) AUTO3 trial schema. (C) AUTO3 consort diagram. Cy, cyclophosphamide; Flu, fludarabine; NT, nontransduced; scFV, single chain variable fragment; TCRz, T-cell receptor zeta.
AUTO3 study design and recruitment. (A) AUTO3 CAR, CD19 CAR, and CD22 CAR are type I transmembrane proteins. Human HD37 recognizes CD19, is located at the extreme amino terminus and, in turn, connected to the CD8α stalk, transmembrane, and anchor. The intracellular endodomains are composed of costimulatory OX40 and CD3 ζ (TCRζ). Humanized LT22 recognizes CD22, is located at the extreme amino terminus and is connected to the pentameric α-helical coiled coil multimer forming domain of cartilage oligomeric matrix protein (COMP) and transmembrane anchor. The intracellular endodomains are composed from 41BB ζ and CD3 ζ (TCRζ).14 (B) AUTO3 trial schema. (C) AUTO3 consort diagram. Cy, cyclophosphamide; Flu, fludarabine; NT, nontransduced; scFV, single chain variable fragment; TCRz, T-cell receptor zeta.
Study design
The AUTO3 ALEXANDER study was designed to determine toxicity and efficacy of AUTO3 in patients with LBCL when administered in combination with pembrolizumab. This multicenter, single-arm, nonrandomized, open-label dose-escalation phase 1 study enrolled adult patients aged ≥18 years with histologically confirmed LBCL15 after failure of ≥2 lines of therapy or autologous stem cell transplant. Inclusion and exclusion criteria, along with the clinical trial protocol, are listed in the supplemental Appendix (supplemental Table 1). Notably, prior CAR-T therapy was an exclusion on the study. Additional eligibility requirements for OPD or ambulatory administration of AUTO3 included the availability of 24 × 7 hour caregiver support for 6 weeks after AUTO3 infusion and residence of patients to be within 60 minutes of the clinical trial site at the time of treatment.
After nonmobilized leukapheresis and successful AUTO3 manufacture, patients received lymphodepletion (LD) with fludarabine (30 mg/m2 per day) and cyclophosphamide (300 mg/m2 per day) for 3 days before AUTO3 infusion. Cohort 1 (n = 4 patients) received 50 × 106 AUTO3 T cells without pembrolizumab. Cohort 2 (n = 3 patients) and cohort 3 (n = 8 patients) received 50 × 106 and 150 to 450 × 106 AUTO3 T cells, respectively, with three 200 mg doses of pembrolizumab at 3-week intervals from day 14. Modeling of pembrolizumab pharmacokinetics (PK) showed that a single dose of 200 mg is sufficient to achieve maximal blood and tumor receptor occupancy. Cohort 4 (n = 17 patients) and cohort 5 (n = 20 patients) received LD, day-1 pembrolizumab, and 150 × 106 and 450 × 106 AUTO3 T cells in the inpatient and OPD setting, respectively. The trial schema is illustrated in Figure 1B.
Primary end points were grade 3 to 5 toxicity within 75 days of AUTO3 infusion, and frequency of dose limiting toxicities.16 Secondary end points were engraftment, expansion, and persistence of AUTO3; safety; manufacture feasibility; clinical efficacy (complete response [CR] and overall response rate [ORR] as assessed based on the Lugano 2014 classification duration of remission [DOR], progression-free survival [PFS], and overall survival [OS]), and other exploratory end points are given in supplemental Table 2.
The study was approved in the United Kingdom by the UK Medicines and Healthcare Products Regulatory Agency (clinical trial authorization no. CTA 46113/0003/001-0015), the London/West London GTAC research ethics committee (REC ref no. 17/LO/0812), and the research and development departments of all participating National Health Service trusts. The study was approved in the United States by the Food and Drug Administration with investigational new drug application application number 18431.
The study was managed by Autolus Ltd. Written informed consent was obtained from patients before study entry in accordance with the Declaration of Helsinki. This report incorporates data from all participants who received AUTO3 during the study period. Data were locked on 28 February 2022.
Toxicity assessment
Adverse events for the first 28 days after CAR infusion were graded based on common terminology criteria for adverse events (version 4.03). Cytokine release syndrome (CRS) and neurotoxicity were graded based on the American Society for Transplantation and Cellular Therapy criteria16 and common terminology criteria for adverse events version 4.03. CRS was defined as the period from first onset of fever until the patient was afebrile for ≥24 hours. Hemophagocytic lymphohistiocytosis (HLH) was graded based on the study by Neelapu et al.17
Response assessment and translational analysis
Disease response assessments were performed at protocol-defined time points (pre-LD, months, 1, 3, 6, 9, 12, 18, and 24) using positron emission tomography–computed tomography (PET-CT) (or CT alone from month 6 in patients with complete metabolic response) per the Lugano Classification.15 Details of translational assays are described in the supplemental Appendix.
Statistical analysis
Details of statistical analysis are described in the supplemental Appendix.
Results
AUTO3 preclinical evaluation
AUTO3 (Figure 1A) has been described previously.14 In a T-cell exhaustion model, repeated antigenic stimulation of AUTO3 T cells induced PD-1 (>70%) and PD-L1 (>20%) overexpression (supplemental Figure 1A-B) with a concomitant reduction in cytotoxicity and cytokine secretion in PD-L1 expressing tumors is evaluated.
AUTO3 manufacture
AUTO3 was successfully manufactured using the Miltenyi CliniMACS Prodigy from 62 of 62 (100%) leukapheresates (supplemental Table 4; supplemental Appendix). All AUTO3 products on trial were manufactured from cryopreserved leukapheresis. AUTO3 product phenotyping for all infused products is illustrated in supplemental Figure 2A-C.
Patient demographics, disease characteristics, and bridging
A total of 73 patients were screened. Of these, 62 were registered, enrolled, and underwent leukapheresis and AUTO3 manufacture. Ten patients did not receive AUTO3 infusion, owing to death in 5 patients (3 from progressive disease [PD], 1 from COVID-19, and 1 from sepsis), PD (without death) in 4 patients, and failed eligibility (absence of PET-positive disease) in 1 patient (Figure 1C).
AUTO3 cells for the first 10 patients were manufactured at an academic facility. For the subsequent 42 patients, the median interval between apheresis and product release was 38 days (range, 30-80 days) and the median vein to vein time for patients was 52.5 days (range, 42-95 days). Patient or hospital-associated factors affected the median vein to vein time for patients.
Demographics of patients who received infusion and their disease features are summarized in Table 1. At screening, the median age of patients was 59 years (range, 27-83 years); 89% of them had stage III or IV disease, 36.5% had double-hit or triple-hit lymphoma, 15.4% had double-expressor lymphoma, 71% had refractory disease, and 61.5% had extranodal disease.18 Patients received a median of 3 prior lines of therapy (range, 1-10) including autologous stem cell transplant in 16 patients (31%). No patient received prior CD19CAR-T therapy or prior CD19- or CD22-targeted therapies.
Patient demographics for the ALEXANDER study
| Baseline characteristics . | N = 52 (%) . |
|---|---|
| Sex | |
| Female | 20 (38.5) |
| Male | 32 (61.5) |
| Median age in y (range) | 59.0 (27-83) |
| ECOG 0, n (%) | 26 (50) |
| ECOG 1, n (%) | 26 (50) |
| Disease characteristics, n (%) | |
| DLBCL, GCB | 24 (46.2) |
| DLBCL, non-GCB | 12 (23) |
| Transformed follicular lymphoma (tFL) | 10 (19.2) |
| Primary mediastinal LBCL | 1 (2) |
| Transformed nodal marginal zone lymphoma | 1 (2) |
| High grade B-cell lymphoma | 3 (5.8) |
| Molecular subtype, n (%) | |
| No high risk molecular features | 15 (28.8) |
| Double-hit | 14 (26.9) |
| Triple-hit | 5 (9.6) |
| Double-expressor | 8 (15.4) |
| Not done/unknown | 10 (19.2) |
| Disease stage, n (%) | |
| II | 6 (11.5) |
| III | 11 (21.2) |
| IV | 35 (67.3) |
| Baseline IPI, n (%) | |
| Low risk | 8 (15.4) |
| Low-intermediate risk | 16 (30.8) |
| High-intermediate risk | 13 (25) |
| High risk | 10 (19.2) |
| Unknown | 5 (9.6) |
| Prior lines of treatment, n | |
| Median (range) | 3 (1-10) |
| Prior autologous HSCT, n (%) | 16 (30.8) |
| Risk factors | |
| Baseline LDH, median (range) (IU/L) | 243.5 (132-1348) |
| SPD, median (min-max) (cm2) | 18.20 (2.1-260.8) |
| Baseline characteristics . | N = 52 (%) . |
|---|---|
| Sex | |
| Female | 20 (38.5) |
| Male | 32 (61.5) |
| Median age in y (range) | 59.0 (27-83) |
| ECOG 0, n (%) | 26 (50) |
| ECOG 1, n (%) | 26 (50) |
| Disease characteristics, n (%) | |
| DLBCL, GCB | 24 (46.2) |
| DLBCL, non-GCB | 12 (23) |
| Transformed follicular lymphoma (tFL) | 10 (19.2) |
| Primary mediastinal LBCL | 1 (2) |
| Transformed nodal marginal zone lymphoma | 1 (2) |
| High grade B-cell lymphoma | 3 (5.8) |
| Molecular subtype, n (%) | |
| No high risk molecular features | 15 (28.8) |
| Double-hit | 14 (26.9) |
| Triple-hit | 5 (9.6) |
| Double-expressor | 8 (15.4) |
| Not done/unknown | 10 (19.2) |
| Disease stage, n (%) | |
| II | 6 (11.5) |
| III | 11 (21.2) |
| IV | 35 (67.3) |
| Baseline IPI, n (%) | |
| Low risk | 8 (15.4) |
| Low-intermediate risk | 16 (30.8) |
| High-intermediate risk | 13 (25) |
| High risk | 10 (19.2) |
| Unknown | 5 (9.6) |
| Prior lines of treatment, n | |
| Median (range) | 3 (1-10) |
| Prior autologous HSCT, n (%) | 16 (30.8) |
| Risk factors | |
| Baseline LDH, median (range) (IU/L) | 243.5 (132-1348) |
| SPD, median (min-max) (cm2) | 18.20 (2.1-260.8) |
ECOG, Eastern Cooperative Oncology Group; GCB, germinal center B-cell like; HSCT, hematopoietic stem cell transplantation; IPI, international prognostic index.
Bridging therapy was administered in 73.1% of patients, and details are presented in supplemental Table 3. Before LD, the median level of lactate dehydrogenase (LDH) was 243.5 U/L (range, 132-1348), and the median estimate of tumor burden, as determined using the sum of the products of diameters (SPD), was 18.20 cm2 (range, 2.1-260.8).
Forty-eight of 52 patients received pembrolizumab: 37 of 52 received 1 dose on day 1, and 11 of 52 received 1 or 3 doses between day +14 and day 56 (1 dose, n = 3; 2 doses, n = 3; and 3 doses, n = 5), 4 of 52 patients received AUTO3 alone, without pembrolizumab.
AUTO3 toxicity
Details of AUTO3 toxicity are summarized in Table 2. Details on treatment emergent adverse events occurring any time after AUTO3 infusion in at least 10% patients are summarized using preferred term in Table 3. One death due to AUTO3 sepsis was observed among the completed cohort. This patient developed HLH with cytopenias and was treated with immunosuppressive therapy. The patient developed multiple infections leading to grade 5 septicemia.
Summary of immunotoxicity based on AUTO3 dose received
| . | 50 × 106 AUTO3 No Pembro. (N = 4) . | 50 × 106 AUTO3 D14 Pembro. (N = 3) . | 150 × 106-450 × 106 AUTO3 D14 Pembro. (N = 8) . | 150 × 106-450 × 106 AUTO3 D1 Pembro. inpatient (N = 17) . | 150 × 106-450 × 106 AUTO3 D1 Pembro. OPD (N = 20) . | Total (N = 52) . |
|---|---|---|---|---|---|---|
| Maximum grade CRS (ASTCT criteria) | ||||||
| G1 | 1 (25%) | 0 | 2 (25%) | 4 (23.5%) | 4 (20%) | 11 (21.2%) |
| G2 | 0 | 0 | 2 (25%) | 1 (5.9%) | 4 (20%) | 7 (13.5%) |
| G3 | 0 | 0 | 0 | 0 | 1 (5%)∗ | 1 (1.9%) |
| G4/5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum G neurotoxicity† | ||||||
| All grades | 1 (25%) | 0 | 0 | 1 (5.9%) | 2 (10%) | 4 (7.7%) |
| ≥G 3 | 1 (25%) | 0 | 0 | 0 | 1 (5%) | 2 (3.8%) |
| HLH | ||||||
| All grades | 0 | 0 | 1 (12.5%) | 1 (5.9%) | 0 | 2 |
| ≥G3 | 0 | 0 | 1 (12.5%) | 1 (5.9%) | 0 | 2 |
| Cytopenias at D30 | ||||||
| ≥G3 Neutropenia | 2 (50%) | 1 (33.3%) | 2 (25%) | 5 (29.4%) | 7 (35%) | 17 (32.7%) |
| ≥G3 thrombocytopenia | 3 (75%) | 3 (100%) | 3 (37.5%) | 5 (29.4%) | 7 (35%) | 21 (40.4%) |
| Maximum grade infections | ||||||
| All grades | 0 | 1 (33.3%) | 7 (87.5%) | 6 (35.3%) | 7 (35%) | 21 (40.4%) |
| ≥G3 | 0 | 0 | 6 (75%) | 3 (17.6%) | 3 (15%) | 12 (23.1%) |
| Hypogammaglobuliemia (≤4 g/L IgG) | ||||||
| All grades | 0 | 0 | 1(12.5%) | 1 (5.9%) | 0 | 2 (3.8%) |
| ≥G3 | 0 | 0 | 0 | 0 | 0 | 0 |
| . | 50 × 106 AUTO3 No Pembro. (N = 4) . | 50 × 106 AUTO3 D14 Pembro. (N = 3) . | 150 × 106-450 × 106 AUTO3 D14 Pembro. (N = 8) . | 150 × 106-450 × 106 AUTO3 D1 Pembro. inpatient (N = 17) . | 150 × 106-450 × 106 AUTO3 D1 Pembro. OPD (N = 20) . | Total (N = 52) . |
|---|---|---|---|---|---|---|
| Maximum grade CRS (ASTCT criteria) | ||||||
| G1 | 1 (25%) | 0 | 2 (25%) | 4 (23.5%) | 4 (20%) | 11 (21.2%) |
| G2 | 0 | 0 | 2 (25%) | 1 (5.9%) | 4 (20%) | 7 (13.5%) |
| G3 | 0 | 0 | 0 | 0 | 1 (5%)∗ | 1 (1.9%) |
| G4/5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Maximum G neurotoxicity† | ||||||
| All grades | 1 (25%) | 0 | 0 | 1 (5.9%) | 2 (10%) | 4 (7.7%) |
| ≥G 3 | 1 (25%) | 0 | 0 | 0 | 1 (5%) | 2 (3.8%) |
| HLH | ||||||
| All grades | 0 | 0 | 1 (12.5%) | 1 (5.9%) | 0 | 2 |
| ≥G3 | 0 | 0 | 1 (12.5%) | 1 (5.9%) | 0 | 2 |
| Cytopenias at D30 | ||||||
| ≥G3 Neutropenia | 2 (50%) | 1 (33.3%) | 2 (25%) | 5 (29.4%) | 7 (35%) | 17 (32.7%) |
| ≥G3 thrombocytopenia | 3 (75%) | 3 (100%) | 3 (37.5%) | 5 (29.4%) | 7 (35%) | 21 (40.4%) |
| Maximum grade infections | ||||||
| All grades | 0 | 1 (33.3%) | 7 (87.5%) | 6 (35.3%) | 7 (35%) | 21 (40.4%) |
| ≥G3 | 0 | 0 | 6 (75%) | 3 (17.6%) | 3 (15%) | 12 (23.1%) |
| Hypogammaglobuliemia (≤4 g/L IgG) | ||||||
| All grades | 0 | 0 | 1(12.5%) | 1 (5.9%) | 0 | 2 (3.8%) |
| ≥G3 | 0 | 0 | 0 | 0 | 0 | 0 |
ASTCT, American Society for Transplantation and Cellular Therapy; D, day; IgG, immunoglobulin G; Pembro, pembrolizumab.
One patient who had no CRS with primary infusion developed G3 CRS (severe hypoxia) with retreatment 1 year later, which happened in a setting of no CAR-T and significant disease burden in lung that had been treated with radiation.
NT grading based on general NT by NCI-CTCAE and CARTOX-10.
Treatment emergent adverse events occurring anytime after AUTO3 infusion in at least 10% patients, regardless of relationship to AUTO3, based on the preferred term (safety set)
| Preferred term . | Total (N = 52) . | |
|---|---|---|
| All grades n (%) . | Grade ≥ n (%) . | |
| Any TEAE | 50 (96.2) | 41 (78.8) |
| Anemia | 27 (51.9) | 21 (40.4) |
| Neutropenia | 20 (38.5) | 19 (36.5) |
| CRS | 19 (36.5) | 1 (1.9) |
| Pyrexia | 17 (32.7) | 1 (1.9) |
| Fatigue | 16 (30.8) | 0 |
| Platelet count decreased | 16 (30.8) | 11 (21.2) |
| Neutrophil count decreased | 15 (28.8) | 15 (28.8) |
| Thrombocytopenia | 12 (23.1) | 11 (21.2) |
| Constipation | 10 (19.2) | 0 |
| Diarrhea | 9 (17.3) | 1 (1.9) |
| Headache | 9 (17.3) | 1 (1.9) |
| Febrile neutropenia | 8 (15.4) | 7 (13.5) |
| Hypotension | 8 (15.4) | 2 (3.8) |
| Edema peripheral | 7 (13.5) | 0 |
| Chills | 6 (11.5) | 0 |
| Dizziness | 6 (11.5) | 0 |
| Hypophosphatemia | 6 (11.5) | 3 (5.8) |
| White blood cell count decreased | 6 (11.5) | 6 (11.5) |
| Preferred term . | Total (N = 52) . | |
|---|---|---|
| All grades n (%) . | Grade ≥ n (%) . | |
| Any TEAE | 50 (96.2) | 41 (78.8) |
| Anemia | 27 (51.9) | 21 (40.4) |
| Neutropenia | 20 (38.5) | 19 (36.5) |
| CRS | 19 (36.5) | 1 (1.9) |
| Pyrexia | 17 (32.7) | 1 (1.9) |
| Fatigue | 16 (30.8) | 0 |
| Platelet count decreased | 16 (30.8) | 11 (21.2) |
| Neutrophil count decreased | 15 (28.8) | 15 (28.8) |
| Thrombocytopenia | 12 (23.1) | 11 (21.2) |
| Constipation | 10 (19.2) | 0 |
| Diarrhea | 9 (17.3) | 1 (1.9) |
| Headache | 9 (17.3) | 1 (1.9) |
| Febrile neutropenia | 8 (15.4) | 7 (13.5) |
| Hypotension | 8 (15.4) | 2 (3.8) |
| Edema peripheral | 7 (13.5) | 0 |
| Chills | 6 (11.5) | 0 |
| Dizziness | 6 (11.5) | 0 |
| Hypophosphatemia | 6 (11.5) | 3 (5.8) |
| White blood cell count decreased | 6 (11.5) | 6 (11.5) |
TEAE, treatment emergent adverse event.
CRS and HLH
Any grade (G) CRS occurred in 19 of 52 (36.5%) patients at a median onset duration of 2 days (range, 1-36 days)16 for a median duration of 3 days (range, 1-19 days). Eleven G1 events (21.2%), 7 G2 events (13.5%), and 1 G3 event (1.9%) were observed, and tocilizumab was administered to 9 patients (17.3%). Corticosteroids or admission to the intensive care unit for CRS was not required. Serum cytokine levels were low across the study cohort, consistent with the low CRS burden observed (supplemental Figure 3).
There was no significant correlation between CRS and AUTO3 dose received, timing of pembrolizumab administration, or pre-LD disease burden owing to LDH and SPD, albeit the sample size was small. HLH was observed in 2 patients, both G3 and having late onset (at day 182 and at day 192 after AUTO3 infusion). In one case, the patient recovered with administration of corticosteroids and anakinra. In the other case, the patient developed HLH with cytopenias and was treated with immunosuppressive therapy. This patient developed multiple infections leading to G5 septicemia.
Neurotoxicity
Neurotoxicity affected 4 of 52 (7.7%) patients, of whom 2 had events higher or equal to G3. In all cases, neuroimaging, electroencephalogram, and lumbar puncture were uninformative, and all 4 patients received corticosteroids. Of the events higher or equal to G3, 1 patient presented with facial and arm weakness at day 53 after AUTO3 administration, which fully resolved within 9 days and mirrored a similar presentation >10 years earlier of unknown etiology, and 1 patient presented with encephalopathy at day 10 in the context of sepsis and multiorgan failure, from which the patient later died.
Cytopenia, hypogammaglobulinemia, and infection
Events higher than or equal to G3 cytopenias were common (neutropenia, 61.5%; thrombocytopenia, 40.4%; and anemia, 40.4%), with ongoing events higher than or equal to G3 neutropenia in 17 patients (32.7%) and ongoing events higher than or equal to G3 thrombocytopenia in 21 patients (40.4%) at 30 days after AUTO3 infusion. Among patients who had events higher than or equal to G3 neutropenia within 30 days of AUTO3 infusion, the median time to recovery to events lower than or equal to G2 neutropenia was 15 days (confidence interval [CI], 12-15). Hypogammaglobulinemia (serum immunoglobulin G ≤4 g/L) was observed in 2 patients (the baseline immunoglobulin G levels for these 2 patients were 3.4 and 2.5 g/L), but neither of them received immunoglobulin replacement. Infection events were observed in 21 patients (40.4%), with events higher than or equal to G3 recorded in 12 patients (23.1%) and death from sepsis in 1. Additional information regarding timing of infections are described in supplemental Table 6.
Immune-related adverse events related to pembrolizumab
Immune-related adverse events were not reported on study after single or repeated dosing pembrolizumab schedules.
AUTO3 PK: expansion and persistence
In all 52 patients who received infusion, peak AUTO3 expansion (maximum concentration [Cmax]) determined using peripheral blood quantitative polymerase chain reaction (qPCR) was 4400 copies per μg genomic DNA (Geo-mean coefficient of variation [CV%] 294) at a median of 12 days after infusion (range, 7-58 days) and AUTO3 expansion over the first 28 days after infusion (area under the curve from time zero to day 28) was 38 110 days × copies per μg guide DNA (Geo-mean CV% 370). Analysis for correlations of Cmax did not show an overt association between the treatment cohort receiving AUTO3 and the timing of pembrolizumab administration (day 14+ vs day 1; Figure 2A; supplemental Table 5). Cmax was significantly associated with neither high baseline disease burden, as measured using SPD ≥ 20 cm2 (Figure 2B), nor elevated LDH ≥400 IU per mL, nor baseline CD19 or CD22 expression measured using immunohistochemistry, as assessed using H-score (Figure 2C). Cmax was affected neither by the proportion of T-naive (Tn) and T-central memory (Tcm) cells in the AUTO3 product nor with shorter product doubling time in vitro (data not shown). Cmax was higher in patients achieving CR/partial remission (PR vs stable disease (SD)/PD at month 1 (Figure 2D; Table 4).
AUTO3 engraftment and persistence. (A) Peak AUTO3 using qPCR and AUTO3 dose or timing of pembrolizumab administration. (B) Peak AUTO3 using qPCR, and pre-LD disease burden using SPD <20 vs ≥20. Data missing for 3 patients. (C) Peak AUTO3 using qPCR and baseline CD19/22 status, in which CD19+ or CD22+ is reflected through H-score ≥150. CD19lo/– and CD22lo/– represent H-scores <150. (D) Peak AUTO3 using qPCR and disease response at month 1. (E) AUTO3 persistence using qPCR and timing of pembrolizumab. (F) AUTO3 persistence using qPCR and Tn and Tcm populations in the drug product for patients with short engraftment ≤28 days. (G) AUTO3 persistence for all patients using qPCR based on the dose. Pem, pembrolizumab. (H) AUTO3 persistence for all patients using qPCR based on the response.
AUTO3 engraftment and persistence. (A) Peak AUTO3 using qPCR and AUTO3 dose or timing of pembrolizumab administration. (B) Peak AUTO3 using qPCR, and pre-LD disease burden using SPD <20 vs ≥20. Data missing for 3 patients. (C) Peak AUTO3 using qPCR and baseline CD19/22 status, in which CD19+ or CD22+ is reflected through H-score ≥150. CD19lo/– and CD22lo/– represent H-scores <150. (D) Peak AUTO3 using qPCR and disease response at month 1. (E) AUTO3 persistence using qPCR and timing of pembrolizumab. (F) AUTO3 persistence using qPCR and Tn and Tcm populations in the drug product for patients with short engraftment ≤28 days. (G) AUTO3 persistence for all patients using qPCR based on the dose. Pem, pembrolizumab. (H) AUTO3 persistence for all patients using qPCR based on the response.
Summary of CAR-T kinetic parameters based on best overall response, as measured in the peripheral blood using qPCR
| Parameter . | Statistics . | CR (N = 23) . | PR (N = 8) . | SD (N = 2) . | PD (N = 14) . | Total (N = 47) . |
|---|---|---|---|---|---|---|
| Cmax (copies per μg DNA) | Geo-mean | 6 222.7 | 5 645.9 | 2 067.7 | 1 956.3 | 4 205.4 |
| Geo-CV% | 197.1 | 104.9 | 305.2 | 668.4 | 290.9 | |
| Tmax (d) | Median | 12.2 | 9.9 | 13.4 | 12.0 | 11.9 |
| Min-max | 7-58 | 7-14 | 10-17 | 7-28 | 7-58 | |
| AUC 0-28 d (day × copies per μg DNA) | Geo-mean | 55 968.7 | 53 883.6 | 24 922.9 | 13 284.7 | 35 750.8 |
| Geo-CV% | 218.2 | 140.2 | 130.8 | 1 043.8 | 377.0 | |
| AUC 0-84 d (day × copies per μg DNA) | Geo-mean | 87 617.6 | 87 081.8 | 4 163.9 | 43 630.5 | |
| Geo-CV% | 248.0 | 227.6 | 260.8 | 577.2 | ||
| Tlast (d) | Median | 108.2 | 41.9 | 39.1 | 19.8 | 50.0 |
| Min-max | 16-734 | 14-168 | 28-50 | 7-55 | 7-734 |
| Parameter . | Statistics . | CR (N = 23) . | PR (N = 8) . | SD (N = 2) . | PD (N = 14) . | Total (N = 47) . |
|---|---|---|---|---|---|---|
| Cmax (copies per μg DNA) | Geo-mean | 6 222.7 | 5 645.9 | 2 067.7 | 1 956.3 | 4 205.4 |
| Geo-CV% | 197.1 | 104.9 | 305.2 | 668.4 | 290.9 | |
| Tmax (d) | Median | 12.2 | 9.9 | 13.4 | 12.0 | 11.9 |
| Min-max | 7-58 | 7-14 | 10-17 | 7-28 | 7-58 | |
| AUC 0-28 d (day × copies per μg DNA) | Geo-mean | 55 968.7 | 53 883.6 | 24 922.9 | 13 284.7 | 35 750.8 |
| Geo-CV% | 218.2 | 140.2 | 130.8 | 1 043.8 | 377.0 | |
| AUC 0-84 d (day × copies per μg DNA) | Geo-mean | 87 617.6 | 87 081.8 | 4 163.9 | 43 630.5 | |
| Geo-CV% | 248.0 | 227.6 | 260.8 | 577.2 | ||
| Tlast (d) | Median | 108.2 | 41.9 | 39.1 | 19.8 | 50.0 |
| Min-max | 16-734 | 14-168 | 28-50 | 7-55 | 7-734 |
Time to Cmax is the time to reach peak CAR-T concentration. (Only summarized for patients with PET-positive disease before preconditioning.)
AUC, area under the curve; Tlast, time to last measurable in blood (days); Tmax, time to Cmax (days).
AUTO3 persistence (Tlast) was demonstrated via qPCR of the peripheral blood at last follow-up among 28 of 52 (53.8%) patients, at a median of 4.2 months (CI: 1.9-not evaluable [NE]). Tlast was not significantly affected by AUTO3 dose, the timing of pembrolizumab infusion (Figure 2E; supplemental Table 5), or baseline disease burden; however, there was a trend toward a higher proportion of Tn and Tcm cells within the AUTO3 product among patients with persistence beyond 28 days after infusion (Figure 2F). PD-1/TIM3 expression and product T-cell doubling time in vitro was not significantly lower in persisting products (data not shown). Engraftment and persistence measured using qPCR for all patients who underwent treatment based on the specific treatment cohort and response are illustrated in Figure 2G-H, and supplemental Table 5 summarizes PK results for all cohorts.
Loss of functional persistence (ie, relapse despite ongoing AUTO3 persistence measured using qPCR at the point of relapse) affected 15 of 33 (45.5%) patients who relapsed during the study period.
Response rates and survival
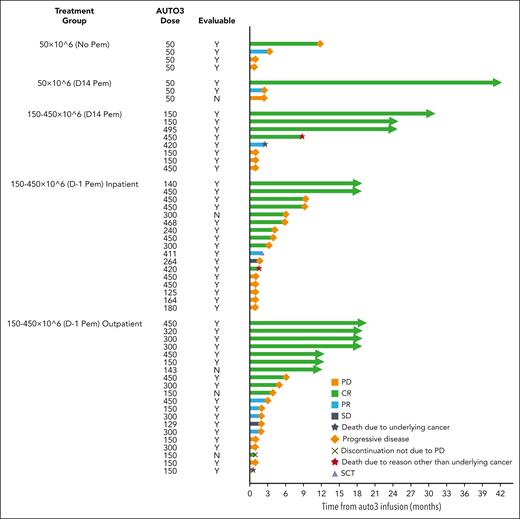
Median follow-up was 21.6 months (range, 15.1-51.3 months) at the data cutoff date (28 February 2022). Forty-seven of 52 patients were eligible for evaluation of response, and individual patient outcomes are illustrated in Figure 3. Five of 52 patients were not eligible for evaluation of response to AUTO3 because they achieved complete metabolic response (CMR) on PET-CT following bridging therapy and prior to LD. Best ORR was 66.0% (31/47 patients), with CMR observed in 48.9% of patients eligible for evaluation (Table 5). The ORR in the intention to treat analysis was 58.1% (36/62 patients). The median DOR was 8.3 months (CI: 3.0 to NE) among all responders (Figure 4A). For patients with CR, median DOR was not reached, with 54.4% (CI: 32.8-71.7) projected to remain progression-free beyond 12 months after onset of remission (Figure 4B). Among all patients who received infusion, the median PFS was 3.32 months (CI, 1.94-6.05), and the median OS was 13.8 months (CI: 7.2 to NE). PFS at 6 and 12 months was 38.6% (CI, 25.3%-51.7%) and 25.8% (CI, 14.5%-38.7%), respectively (Figure 4C). OS at 6 and 12 months was 72.3% (CI: 57.7%-82.6%) and 53.7% (38.4%-66.8%), respectively (Figure 4D).
AUTO3 disease response. Swimmer plot, for total infused cohort. SCT, stem cell transplant; N, no; Y, yes.
AUTO3 disease response. Swimmer plot, for total infused cohort. SCT, stem cell transplant; N, no; Y, yes.
Summary of responses based on the AUTO3 dose received
| . | 50 × 106 AUTO3 No Pembro. (N = 4) . | 50 × 106 AUTO3 D14 Pembro. (N = 3) . | 150 × 106-450 × 106 AUTO3 D14 Pembro. (N = 8) . | 150 × 106-450 × 106 AUTO3 D-1 Pembro. inpatient (N = 17) . | 150 × 106-450 × 106 AUTO3 D-1 Pembro. OPD (N = 20) . | Total (N = 52) . |
|---|---|---|---|---|---|---|
| N evaluable∗ | 4 | 2 | 8 | 16 | 17 | 47 |
| ORR (CR + PR) | 2 (50%) | 2 (100%) | 5 (62.5%) | 10 (62.5%) | 12 (70.6%) | 31 (66.0%) |
| CR | 1 (25%) | 1 (50%) | 4 (50%) | 9 (56.3%) | 8 (47.1%) | 23 (48.9%) |
| PR | 1 (25%) | 1 (50%) | 1 (12.5%) | 1 (6.3%) | 4 (23.5%) | 8 (17%) |
| . | 50 × 106 AUTO3 No Pembro. (N = 4) . | 50 × 106 AUTO3 D14 Pembro. (N = 3) . | 150 × 106-450 × 106 AUTO3 D14 Pembro. (N = 8) . | 150 × 106-450 × 106 AUTO3 D-1 Pembro. inpatient (N = 17) . | 150 × 106-450 × 106 AUTO3 D-1 Pembro. OPD (N = 20) . | Total (N = 52) . |
|---|---|---|---|---|---|---|
| N evaluable∗ | 4 | 2 | 8 | 16 | 17 | 47 |
| ORR (CR + PR) | 2 (50%) | 2 (100%) | 5 (62.5%) | 10 (62.5%) | 12 (70.6%) | 31 (66.0%) |
| CR | 1 (25%) | 1 (50%) | 4 (50%) | 9 (56.3%) | 8 (47.1%) | 23 (48.9%) |
| PR | 1 (25%) | 1 (50%) | 1 (12.5%) | 1 (6.3%) | 4 (23.5%) | 8 (17%) |
D, day; PR, partial response rate; Pembro, pembrolizumab.
PET-positive disease before LD.
Duration of response. (A) Duration of response among all responders. (B) DOR based on best overall response. (C) PFS of all patients who received infusion. (D) OS of all patients who received infusion.
Duration of response. (A) Duration of response among all responders. (B) DOR based on best overall response. (C) PFS of all patients who received infusion. (D) OS of all patients who received infusion.
DOR for all responding patients was 8.3 months (95% CI, 3.0 to NE) with 42.6% (CI, 25.2-59.0) projected to remain progression-free beyond 12 months after onset of remission (Figure 4A). Table 5 summarizes responses based on the dosing cohort.
Analysis for correlations of response (ORR and CR) showed that the following baseline demographic features were associated with outcome: lymphoma subtype, international prognostic index, SPD, and LDH pre-LD (supplemental Figure 4A-B). There was no clear correlation between response and AUTO3 dose, response and pembrolizumab single vs repeat dosing, or response and the timing of administration.
There appears to be a trend toward higher Tn and Tcm populations in patients achieving CR/PR compared to those achieving SD/PD 1 month after AUTO3 (supplemental Figure 5A). Early loss of AUTO3 persistence at ≤28 days after infusion appears to be associated with a lower CR rate at 1 month (supplemental Figure 5B). The presence of CD19lo/–CD22lo/– vs CD19+/CD22+ disease at baseline appears to trend toward increased evolution to PD/SD from CR/PR for 6 months (supplemental Figure 5C), albeit this may also relate to higher baseline tumor burden estimation using SPD (supplemental Figure 5D). In line with the literature,2 there was a clear association between CAR-T expansion and associated outcomes, with higher Cmax, area under the curve from time zero to day 84, and longer Tlast observed in patients achieving CR than in patients with PD (Table 4). Patients who maintained response for ≥6 months showed a trend toward higher peak expansion than those who never achieved CR (supplemental Figure 5E). This suggests that initial expansion may be important for durability of response. High baseline ferritin appeared to be associated with absence of CR (supplemental Figure 5F).
Paired tissue biopsy analysis at baseline and relapse
Baseline lymph node biopsies were available from 33 of 52 (63%) patients who received infusion. Among these, 17 of 33 (51%) patients had sequential biopsies, 4 of these 17 (23%) were performed when the patients were in remission and 13 of 17 (76%) were performed at relapse. CD19 and CD22 levels were assessed using immunohistochemistry H-score, in which a score >150 was considered “strongly positive.”19 PD-L1 staining using SP142 was considered significant when it was beyond 10%.20 Supplemental Figure 6A-C depict heat maps of CD19, CD22, and PD-L1 expression at baseline and at relapse in matched patient samples. CD19 expression at baseline was strong in 9 of 13 patients, and CD19lo/– escape was not the predominant mode of relapse, with only 2 of 13 patients showing complete loss of CD19 at relapse (<10/high power field [hpf]). Notably, both patients had PD <3 months after AUTO3 infusion. Of 5 late relapses (beyond month 3), 4 had preserved CD19 expression >150.
CD22 expression, assessed using immunohistochemistry, was low at baseline, with 0 of 13 samples at >150/hpf and 5 of 13 samples with <10/hpf. At relapse, 1 of 13 samples received an H-score >150 and 7 of 13 samples received a score <10. PD-L1 levels were generally low at baseline, with only 1 of 13 samples showing >10%. At relapse, 3 of 13 samples had PD-L1 levels >10%, and 2 of these had early PD at 1 month.
Feasibility of OPD treatment with AUTO3
Twenty of 52 (38%) patients who underwent treatment were enrolled into the OPD cohort. Demographics and disease characteristics for this population were not significantly different from those of the other cohorts. Median patient age was 59.5 years (range, 27-76 years). The majority had stage IV disease (65%), diffuse large B-cell lymphoma—not otherwise specified (65%), ECOG performance status score of 1 (60%), extranodal disease before LD (65%), SPD before LD <20 cm2 (70%), and LDH before LD ≥200 U/L (65%).
Sixteen of 20 (80%) patients completed LD and AUTO3 infusion exclusively in the OPD setting. Four of 20 (20%) required hospital admission before LD and AUTO3 infusion was completed; in 1 case, admission was to manage an adverse event other than CRS or immune effector cell–associated neurotoxicity syndrome (ICANS), and in 3 cases, admission was for technical, societal, or practical reasons. Among the 16 patients who completed LD and AUTO3 infusion exclusively in the OPD setting, 10 were admitted to hospital at a median of 2.5 days (range, 2-90 days) from AUTO3 infusion. For the whole OPD cohort (n = 20), the median total duration of hospitalization after AUTO3 infusion was 5 days (range, 0-19 days), as compared with a median of 19 days (range, 0-199 days) in the inpatient cohort.
CRS was described in 9 of 20 (45%) patients, including 4 × G1, 4 × G2, and 1× G3, at a median onset of 2 days after AUTO3 infusion, lasting a median of 3 days (range, 1-4 days). Five of 20 patients received tocilizumab, 0 of 20 received steroids, and 0 of 20 required the intensive care unit. ICANS affected 2 of 20 (10%) patients with 1 × G4 event. Both patients required corticosteroids, but both cases were resolved.
Discussion
Cumulative clinical data has established that sustained responses can be achieved in 30% or 40% of adult patients with r/r LBCL receiving CD19-directed CAR–T therapy.1,2,21 Of the 60% or 70% patients whose disease did not respond or relapse, antigen loss may be an explanation in one-third of the patients4,5,19,22 and CAR-T exhaustion in the remaining.6,7 Dual antigen targeting has the potential to prevent relapse from antigen downregulation or loss, and several cotargeting strategies are possible, some of which have been tested in early studies.11-13 Wang et al described sequential infusion of 5 × 106 CD19 and 5 × 106 CD22 single targeting CAR-T products in 38 patients with B-cell non–Hodgkin lymphoma. The incidence of events higher than or equal to G3 ICANS and CRS was 13.5% and 22%, respectively, and 50% of patients achieved CMR. Of the patients who relapsed, repeat biopsy (if provided) did not demonstrate CD19 or CD22 loss as the cause for relapse.23
Tan-CARs are single CARs that recognize multiple targets. Tan-CARs targeting CD19 or CD20 were tested in a dose-escalation basket study that included patients with LBCL.24 Of the 22 patients who received infusion, 13 either did not respond or relapsed, and relapse biopsies uniformly showed preserved CD19 expression. Furthermore, there was no difference in the mean expression of CD19 or CD20, assessed using H-score, on tumor cells at baseline vs relapse to determine individual relapse risk.
Spiegel et al described a CD19/CD22 tan-CAR approach in LBCL19 that was safe and well-tolerated, with an ORR of 62% and a CR rate of 29%. CD19lo/– relapse occurred in 4 of 14 patients (29%), as assessed using flow cytometric analysis of lymph node fine needle aspirate material, but CD22lo/– relapse was not observed.19 They observed impaired CD22CAR-T function within their tan-CAR design compared with single CAR format, suggesting that steric hindrance or mechanical factors could be contributory. Future tan-CAR designs with alternate spacers and high-affinity binders may improve these approaches in the clinical setting.
Bicistronic vectors, which allow coexpression of 2 independent receptors, may have advantages over tan-CARs.3 We previously described the bicistronic CD19/CD22 CAR product AUTO3 in a phase 1 study of r/r pediatric B-ALL (the AMELIA study). AUTO3 was infused in 15 patients and 86% achieved CR at month 1, but relapse was observed in 9 of 13 responders, 3 of these 9 relapses were associated with CD19dim/–, 2 of 9 with CD22dim/–, and the remaining with CD19+/CD22+. Antigen-positive relapse was associated with the loss of CAR persistence, and CAR-T product analysis on AMELIA showed enrichment for differentiated CAR-T phenotypes. We hypothesized that AUTO3 might be better suited to r/r LBCL, in which long-term persistence is potentially not needed for durable outcome. We also postulated that combination of AUTO3 with pembrolizumab may overcome CAR-T exhaustion.
In the phase 1 ALEXANDER study among adults with LBCL, AUTO3 manufacture was successful in all participants who underwent leukapheresis, which further supports the use of closed-system, semiautomated CAR-T manufacture systems. Minimal CAR-T toxicity was observed despite dual antigen targeting and concurrent PD-1 blockade, with only a single case of G3 CRS and 2 cases of events higher than or equal to G3 neurotoxicity observed across all dosing cohorts. Consistent with this, we found no substantial elevation of serum cytokines after AUTO3 administration, even among patients with high disease burden. HLH G3 was observed in 2 patients.
The ORR was 66% across all cohorts, but response was not clearly correlated with AUTO3 dose or pembrolizumab dosing or timing. Rather, predictors of response to AUTO3 included low disease burden before LD, as estimated using SPD and LDH, which has also been shown to be predictive of response in single antigen targeting CARs for LBCL.18 This potentially supports the concept of effective bridging therapy to debulk patients ahead of dual-targeting CAR-T. Younger age (<65 years) and fewer prior lines of therapy was also predictive of response, which may reflect better fitting T-cell harvests for AUTO3 manufacture. Aligned with this, AUTO3 products showed a trend toward central and stem cell memory populations25-27 in patients achieving CR/PR vs SD/PD, and there was a significant association between AUTO3 expansion and CR,2 particularly among patiests in whom response was maintained for ≥6 months. AUTO3 expansion did not strongly correlate with CAR-T or pembrolizumab dose.
With an overall 12-month PFS of 25.8%, a significant proportion of patients relapsed after AUTO3. As a potential driver for relapse, we assessed antigen escape through paired biopsies (baseline vs relapse) from 13 patients. CD19lo/– escape was observed in 2 of 13 (15%) samples, both of which developed PD <3 months after AUTO3 infusion. Of 5 late relapses (beyond month 3), 4 of 5 had preserved CD19 expression >150/hpf. CD19 loss as a mode of relapse in B-ALL is determined easily via analysis of blood and bone marrow samples. CD19 loss often occurs early (<6 months post-CAR-T) and is associated with high CAR-T expansion in patients with bulky disease.28,29 The availability of similar data sets for patients with LBCL is limited because of the inaccessibility of tissue biopsies at relapse and the technical limitations of the H-score in accurately defining antigen expression.19,22 Alternative flow cytometry–based assessments of antigen density in LBCL may supersede H-scores in the future, establishing thresholds for effective antigen targeting, to potentially inform patient selection.
In the ALEXANDER study, CD22lo/– expression was observed in 7 of 13 patients who relapsed. Assessment of the impact of CD22 CAR in reducing or delaying antigen escape after AUTO3 administration is difficult, but several relapses with CD22lo/– disease imply some activity of the CD22CAR component in the AUTO3 construct.
It is likely that the LBCL tumor microenvironment directly and indirectly affects response to CAR-T. Data show that CD8 T–cell exhaustion is associated with poor response to axi-cel in LBCL,25 and high PD-L1 expression is reported in 62% of PD biopsies.5 After the ALEXANDER study was designed, other studies have explored single-antigen targeting CAR-T plus PD1/PD-L1 checkpoint blockade in LBCL. To date, improved ORR and CAR-T expansion has not been reported using these combinations.30
An important focus for the CAR-T field from a health economics perspective is the development of a product that can be safely administered in the OPD setting. In the ALEXANDER study, OPD administration was safe and reduced hospital stay by a median of 14 hospital days per patient. There were no clear demographic differences between patients treated in the inpatient and those treated in the OPD setting to confound findings, specifically, patients were not skewed toward low burden disease or younger age. There were similarities in the response rates of those observed in the inpatient cohort and in the immunotoxicity. Overall, OPD administration in the ALEXANDER study was performed safely and represented a potentially significant cost-saving for the hospital and potential quality of life benefits for the patient and carer.
The ALEXANDER study adds to the growing efforts towards improved outcomes for r/r LBCL beyond the 30% to 40% currently afforded by standard CD19CARs. Dual targeting of CD19 and CD22 in r/r LBCL using bicistronic CAR-Ts with pembrolizumab was well-tolerated and associated with encouraging early responses. However, neither dual antigen targeting nor pembrolizumab improved outcome.
The variable expression of CD22 observed with ALEXANDER and other studies should prompt the evaluation of additional or alternative CAR-T target antigens for LBCL.3,31 Furthermore, a deeper understanding of the potential technical advantages and disadvantages of dual-targeting CAR–T should be explored. Loss of persistence for dual-targeting CARs remains an issue. For bicistronic designs, it is possible that expression of 2 CARs plus native T-cell receptor on a single T cell leads to the depletion of downstream signaling proteins and impaired CAR function.32,33 This requires further study.
Future optimization may include improved manufacture and design toward enhanced CAR potency at low antigen density to result in better AUTO3 expansion and persistence in vivo. Efforts to mitigate the LBCL microenvironment beyond the PD-1/PD-L1 axis, eg, modulation of inhibitory signals, such as transforming growth factor β, may also improve CAR-T activity in LBCL.
Acknowledgments
The authors thank Muzaffar Qazilbash, Jason Westin, and Kevin Carroll for providing study oversight as the Independent Data Monitoring Committee. The authors thank all patients and their families, participating sites and their staff for their support of the study.
This study was funded by Autolus. M.A.P., K.M.A., C.R., K.S.P., and D.C.L. were supported by the University College London NIHR Biomedical Research Centre.
Authorship
Contribution: C.R. and M.A.P. conceived the study and supervised the project; C.R., C.L.B., L.J.L., W.O., M.A.V.M., E.T., D.I., C.B., E.L.B., M.A.O., L.W., A.R., K.M.A., K.S.P., and D.C.L. treated patients and/or acquired clinical samples and data; C.B. contributed to the clinical trial design and patient enrollment; C.R., M.A.P., W.B., Y.Z., M.R., S.B., F.A.V., K.D., Y.H., V.G.R.P., and N.K. interpreted the results and wrote the first draft of the paper; Y.H. and Y.Z. performed statistical analysis; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: M.A.P. owns stock in and is employed by Autolus Therapeutics and is an inventor on patents licensed to Autolus Therapeutics, for which he receives a share of revenues. C.L.B. consults and serves on the advisory board for BMS, Seattle Genetics, Kite, Karyopharm, TG Therapeutics, ADC Therapeutics, AbbVie, Genentech, and Treeline Bioscience; receives research fundings from Epizyme, Autolus Therapeutics, Roche, and Vincerx; and received honoraria from Dava Oncology, TouchIME, and Medscape. W.O. reports fees from Roche, Takeda, Pfizer, Servier, Kite Gilead, MSD, Novartis, Beigene, AstraZeneca, Syneos, Autolus, Kyowa Kirin, AbbVie, Incyte, BMS/Celgene, and Janssen. K.S.P. is a shareholder and consultant of Autolus Therapeutics. E.T. receives speaker fees from and serves in the advisory boards for Novartis, Kite/Gilead, Janssen, and BMS/Celgene. D.I. receives speaker fees from Kite/Gilead. The remaining authors declare no competing financial interests.
Correspondence: Martin A. Pule, UCL Cancer Institute, 72 Huntley St, London WC1E 6DD, United Kingdom; e-mail: m.pule@ucl.ac.uk.
References
Author notes
Data are available on request from the corresponding author, Martin A. Pule (m.pule@ucl.ac.uk).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal