Key Points
VWF gene variants that modify FVIII binding influence FVIII PK.
Variants in clearance receptors for VWF-FVIII influence FVIII PK.
Abstract
Factor VIII (FVIII) pharmacokinetic (PK) properties show high interpatient variability in hemophilia A patients. Although previous studies have determined that age, body mass index, von Willebrand factor antigen (VWF:Ag) levels, and ABO blood group status can influence FVIII PK, they do not account for all observed variability. In this study, we aim to describe the genetic determinants that modify the FVIII PK profile in a population of 43 pediatric hemophilia A patients. We observed that VWF:Ag and VWF propeptide (VWFpp)/VWF:Ag, but not VWFpp, were associated with FVIII half-life. VWFpp/VWF:Ag negatively correlated with FVIII half-life in patients with non-O blood type, but no correlation was observed for type O patients, suggesting that von Willebrand factor (VWF) half-life, as modified by the ABO blood group, is a strong regulator of FVIII PK. The FVIII-binding activity of VWF positively correlated with FVIII half-life, and the rare or low-frequency nonsynonymous VWF variants p.(Arg826Lys) and p.(Arg852Glu) were identified in patients with reduced VWF:FVIIIB but not VWF:Ag. Common variants at the VWF, CLEC4M, and STAB2 loci, which have been previously associated with plasma levels of VWF and FVIII, were associated with the FVIII PK profile. Together, these studies characterize the mechanistic basis by which VWF clearance and ABO glycosylation modify FVIII PK in a pediatric population. Moreover, this study is the first to identify non-VWF and non-ABO variants that modify FVIII PK in pediatric hemophilia A patients.
Introduction
Historically, prophylactic dosing regimens of factor VIII (FVIII) concentrates for hemophilia A patients have been based on body mass and in vivo recovery. However, the pharmacokinetic (PK) profile of FVIII is highly variable within the hemophilia A population, with the half-life of FVIII varying more than fourfold (6-25 hours) in adult populations.1-3 Interindividual variability is greater than intraindividual variability, with terminal half-life and weight-adjusted clearance highly reproducible over a patient’s lifetime, despite age-related changes, suggesting a genetic basis for this observation.4 In more recent years, there has been an increased emphasis on personalized prophylactic FVIII dosing to minimize patient time below critical trough levels (0.01-0.03 IU/mL) to prevent spontaneous joint bleeding.5 The dose and frequency of personalized FVIII administration can vary greatly; however, the factors that modulate FVIII PK are incompletely understood.
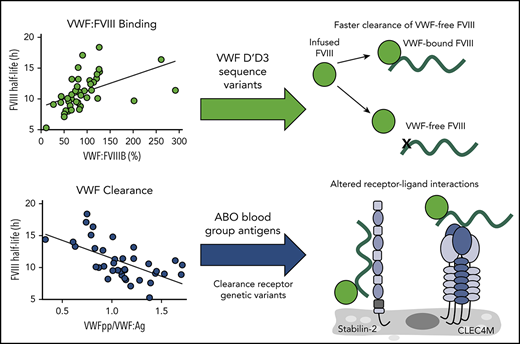
Factors that influence plasma levels of endogenous FVIII coagulant activity (FVIII:C) in normal individuals may also modify FVIII PK in patients with hemophilia A. FVIII circulates in the plasma in a noncovalent complex with von Willebrand factor (VWF), with VWF acting as a carrier to protect FVIII from accelerated proteolysis by activated protein C and clearance by liver- and spleen-expressed lectin and scavenger receptors.6-10 In normal individuals, elevated VWF plasma levels are associated with increased FVIII activity,11,12 and endogenous von Willebrand factor antigen (VWF:Ag) levels have previously been demonstrated to associate with FVIII PK parameters in hemophilia A patients.1,13,14 In normal individuals, common and rare variants within the VWF gene can modify plasma VWF:Ag levels through biosynthetic or clearance-related mechanisms, thus modifying FVIII activity.15,16 Additionally, variants within the D′D3 FVIII-binding region of VWF can modify VWF:Ag and FVIII activity levels in normal individuals (heterozygous inheritance), as well as in type 2N von Willebrand disease (VWD) (homozygous or compound heterozygous inheritance).15,17-19 However, the influence of VWF gene variants on FVIII PK has not been studied.
In addition to variability in the VWF gene, variants within the ABO blood group locus account for ∼50% of the heritability, or genetic influence, on plasma FVIII levels in normal individuals.20 ABO blood group status has been shown to modify endogenous VWF and FVIII levels through a mechanism involving accelerated clearance of type-O VWF and, presumably, of type-O VWF-bound FVIII.21 ABO blood group has previously been shown to associate with FVIII PK, although the detailed mechanistic basis by which VWF and ABO blood group factors modify FVIII PK have not been confirmed.1,13 Moreover, these factors cannot fully explain the high interindividual variability of FVIII PK.
Genetic variability outside VWF and the ABO blood group locus that associates with plasma VWF or FVIII levels has been identified through whole-genome linkage analysis and genome-wide association studies.16 The CHARGE genome-wide association studies meta-analysis identified 400 single nucleotide variants (SNVs) that associate with plasma VWF and/or FVIII levels in normal subjects of European ancestry at 8 loci: VWF, ABO, STAB2, SCARA5, TC2N, STXBP5, STX2, and CLEC4M.16 These associations were recently confirmed in a multiethnic population of >46 000 normal individuals.22 STXBP5 and STX2 encode proteins involved in endothelial cell biosynthetic pathways and, although the function of TC2N is unknown, SCARA5, STAB2 (stabilin-2), and CLEC4M encode endocytic receptors expressed in the liver and spleen that have been shown to bind, internalize, and/or regulate the clearance of VWF-FVIII and therefore, may modify FVIII PK.23-25
In this study, we performed FVIII PK analysis in 43 pediatric patients with severe hemophilia A. We assessed the association between measurements of FVIII PK and plasma VWF properties, including levels of VWF:Ag, FVIII-binding activity, and surrogate markers of VWF synthesis/secretion (VWF propeptide [VWFpp]) and clearance (VWFpp/VWF:Ag).26,27 We sequenced the FVIII-binding region of the VWF gene to identify missense variants and characterized the influence of the identified rare and low-frequency variants on VWF:Ag and FVIII-binding activity in these patients. Finally, study subjects were genotyped for VWF- or FVIII-modifying SNVs identified in the CHARGE study, as well as the CLEC4M variable number tandem repeat (VNTR) polymorphism, and their association with measurements of FVIII PK was assessed. This study represents the most comprehensive analysis of the genetic regulation of FVIII PK in a pediatric population to date.
Patients, materials, and methods
Study subjects
A total of 43 children and adolescent study subjects (age, 6 to 17.7 years) with severe hemophilia A was recruited from 3 large academic pediatric hemophilia centers (the Hospital for Sick Children in Toronto, the Children’s Hospital at the Medical University of Vienna, and the Division of Pediatric Hematology/Oncology at the University of Leuven) between October 2012 and August 2015. Informed consent was obtained for patient participation following approval from institutional ethics review boards. Subjects had a baseline plasma FVIII:C < 1% and no evidence of current anti-FVIII inhibitors or nonneutralizing anti-FVIII immunoglobulin G. Subject demographics can be found in Table 1 and supplemental Table 1 (available on the Blood Web site).
Summary of study subject characteristics, FVIII PK profile, and plasma VWF properties
| . | Median . | Range . |
|---|---|---|
| Patient traits | ||
| Age, y | 10.6 | 6.0-17.7 |
| Weight, kg | 40.6 | 17.6-132.5 |
| Height, cm | 143.0 | 109.6-177.2 |
| BMI, kg/m2 | 19.3 | 14.4-42.8 |
| BSA, m2 | 1.3 | 0.7-2.4 |
| FVIII PK | ||
| Cl, mL/h | 149 | 47-400 |
| Vd, L | 2.12 | 1.20-4.29 |
| k, h−1 | 0.067 | 0.038-0.130 |
| Half-life, h | 10.38 | 5.32-18.43 |
| AUC, h·mIU/dL | 137.36 | 62.39-291.99 |
| Recovery, (IU/dL)/(IU/kg) | 2.18 | 1.3-5.14 |
| VWF | ||
| VWF:Ag, % | 86.74 | 39.94-141.64 |
| VWFpp, % | 85.84 | 43.46-156.63 |
| VWFpp/VWF:Ag | 1.09 | 0.33-1.71 |
| VWF:FVIIIB, % | 80.70 | 11.30-292.15 |
| . | Median . | Range . |
|---|---|---|
| Patient traits | ||
| Age, y | 10.6 | 6.0-17.7 |
| Weight, kg | 40.6 | 17.6-132.5 |
| Height, cm | 143.0 | 109.6-177.2 |
| BMI, kg/m2 | 19.3 | 14.4-42.8 |
| BSA, m2 | 1.3 | 0.7-2.4 |
| FVIII PK | ||
| Cl, mL/h | 149 | 47-400 |
| Vd, L | 2.12 | 1.20-4.29 |
| k, h−1 | 0.067 | 0.038-0.130 |
| Half-life, h | 10.38 | 5.32-18.43 |
| AUC, h·mIU/dL | 137.36 | 62.39-291.99 |
| Recovery, (IU/dL)/(IU/kg) | 2.18 | 1.3-5.14 |
| VWF | ||
| VWF:Ag, % | 86.74 | 39.94-141.64 |
| VWFpp, % | 85.84 | 43.46-156.63 |
| VWFpp/VWF:Ag | 1.09 | 0.33-1.71 |
| VWF:FVIIIB, % | 80.70 | 11.30-292.15 |
AUC, area under the curve; BMI, body mass index; BSA, body surface area; Cl, clearance; k, elimination rate constant; Vd, volume of distribution.
PK study
Participants were dosed with recombinant standard half-life FVIII concentrates at 50 IU/kg, rounded as appropriate to vial size. Recombinant FVIII products used can be found in supplemental Table 1. Blood was collected under resting conditions at the following intervals: preinfusion (within 30 minutes prior to infusion) and postinfusion 1 hour (±5 minutes), 9 hours (±1 hour), 24 hours (±2 hours), and 40 to 48 hours, in accordance with a previous International Society on Thrombosis and Haemostasis–recommended schedule of sampling.28,29 The dosing history for each subject (up to 72 hours prior to the PK study) was collected; therefore, a wash-out period was not required.
PK analysis
FVIII activity was measured by a 1-stage clotting assay using a BCS XP Hemostasis System (Siemens, Munich, Germany) or an STA compact hemostasis system (Diagnostica Stago, Parsippany, NJ) at a single site at Queen’s University. FVIII PK parameters were evaluated using TCIWorks 10.0-RC1 program, as previously described.13,30 TCIWorks is a 1-compartment model that has been previously validated for use in assessing FVIII PK parameters for plasma-derived and recombinant products.30 FVIII PK characteristics, including clearance, elimination rate constant, terminal half-life, and AUC, were reported.
Plasma assays
Plasma levels of VWFpp and VWF:Ag were measured by enzyme-linked immunosorbent assay (Immucor GTI Diagnostics, Waukesha, WI). VWF:FVIIIB was measured by solid-phase binding assay, as described, using 1.25 U/mL recombinant FVIII (ADVATE; Baxter, Deerfield, IL) and a horseradish peroxidase–conjugated polyclonal sheep anti-human FVIII antibody (Affinity Biologicals, Ancaster, ON, Canada) for detection.31
Genotyping
Genotyping for SNVs rs868875, rs2726953, rs9644133, rs4981022, rs12229292, and rs10133762 was performed using TaqMan Real-Time PCR analysis, in accordance with the manufacturer’s protocols (Thermo Fisher Scientific). Genotyping of VWF variants (rs1063856, rs1063857) and analysis of D′D3 and N-linked glycan-containing exonic sequence of VWF were performed as previously described.32 Analysis of the CLEC4M VNTR genotype was performed as previously described.24,33
Statistical analysis
Associations between continuous variables and FVIII PK parameters were determined by a 2-tailed Pearson correlation coefficient using GraphPad Prism 7.03 (GraphPad Software, La Jolla, CA). Linear regression analysis was performed to quantify influences for each SNV; they were adjusted for age and ABO blood group status using IBM-SPSS Version 25.0 for Windows 2018 (Armonk, NY). Differences between genotypes were assessed by the Mann-Whitney U test or by Fisher’s exact test using GraphPad Prism 7.03. P ≤ .05 was considered statistically significant; because this is an exploratory study, no adjustments were made for multiple comparisons.
Results
Patient characteristics and VWF:Ag, VWFpp, and VWF:FVIIIB measurements
Patient physical characteristics and FVIII PK profiles can be found in Table 1 and supplemental Table 1. We first confirmed the association between patient age and body mass index, which have previously been associated with FVIII PK using Pearson’s correlation coefficient (Table 2). Both parameters were significantly associated with FVIII PK.
Association between FVIII PK profile and study subject physical characteristics and plasma VWF properties
| . | Cl, mL/h . | k, h−1 . | Half-life, h . | AUC, h·mIU/mL . |
|---|---|---|---|---|
| Age, y | r = 0.534 | r = −0.039 | r = 0.005 | r = 0.202 |
| P = .002 | P = .8027 | P = .9738 | P = .1939 | |
| BMI, kg/m2 | r = 0.512 | r = −0.008 | r = 0.0213 | r = 0.118 |
| P = .0005 | P = .9580 | P = .8941 | P = .0258 | |
| VWF:Ag, % | r = −0.615 | r = −0.741 | r = 0.777 | r = 0.656 |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |
| VWFpp, % | r = −.0657 | r = −0.277 | r = 0.2112 | r = 0.036 |
| P = .6949 | P = .0922 | P = .202 | P = .8288 | |
| VWFpp/VWF:Ag | r = 0.634 | r = 0.538 | r = −0.598 | r = −0.644 |
| P < .0001 | P = .0005 | P < .0001 | P < .0001 | |
| VWF:FVIIIB, % | r = −0.510 | r = −0.499 | r = 0.484 | r = 0.317 |
| P = .006 | P = .0008 | P = .0012 | P = .0408 |
| . | Cl, mL/h . | k, h−1 . | Half-life, h . | AUC, h·mIU/mL . |
|---|---|---|---|---|
| Age, y | r = 0.534 | r = −0.039 | r = 0.005 | r = 0.202 |
| P = .002 | P = .8027 | P = .9738 | P = .1939 | |
| BMI, kg/m2 | r = 0.512 | r = −0.008 | r = 0.0213 | r = 0.118 |
| P = .0005 | P = .9580 | P = .8941 | P = .0258 | |
| VWF:Ag, % | r = −0.615 | r = −0.741 | r = 0.777 | r = 0.656 |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |
| VWFpp, % | r = −.0657 | r = −0.277 | r = 0.2112 | r = 0.036 |
| P = .6949 | P = .0922 | P = .202 | P = .8288 | |
| VWFpp/VWF:Ag | r = 0.634 | r = 0.538 | r = −0.598 | r = −0.644 |
| P < .0001 | P = .0005 | P < .0001 | P < .0001 | |
| VWF:FVIIIB, % | r = −0.510 | r = −0.499 | r = 0.484 | r = 0.317 |
| P = .006 | P = .0008 | P = .0012 | P = .0408 |
The P values were determined using a 2-tailed test of significance. Bold results are statistically significant.
r, Pearson’s correlation.
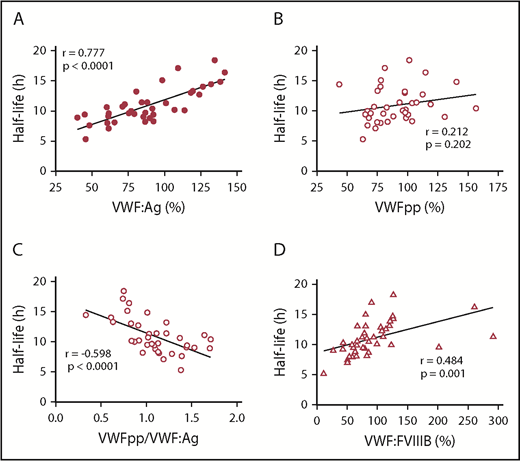
We next assessed the influence of baseline plasma VWF properties on FVIII PK. We confirmed that plasma VWF:Ag levels were strongly associated with FVIII PK parameters, including FVIII half-life (P < .0001) and clearance (P < .0001), in our pediatric population (Figure 1A; Table 2), which is consistent with previous observations.1,4,13,34 Plasma VWF:Ag levels are influenced by pathways that modify its synthesis and secretion, as well as by mechanisms that regulate its clearance from the plasma. We measured VWFpp as a surrogate marker of endothelial cell or platelet VWF secretion, as well as the VWFpp/VWF:Ag ratio, which serves as an indirect measurement of the rate of VWF clearance. Interestingly, we observed no significant association between plasma VWFpp levels and FVIII PK (half-life: P = .202) (Figure 1B; Table 2), although there was a strong association between PK and VWFpp/VWF:Ag (half-life: P < .0001) (Figure 1C; Table 2), indicating that the rate at which VWF is cleared from the plasma, but not the rate of VWF secretion, strongly modifies FVIII PK in this cohort of patients. We also observed a significant association between the FVIII binding ability of VWF (VWF:FVIIIB) and FVIII PK (half-life: P = .0012) (Figure 1D; Table 2).
Association between VWF, VWFpp, and VWF:FVIIIB and FVIII half-life. The Pearson correlation coefficient of continuous variables was assessed by a 2-tailed test of significance. (A) Strong positive correlation between VWF:Ag and FVIII half-life (P < .0001). (B) Weak positive correlation between VWFpp (a surrogate marker for VWF secretion) and FVIII half-life (P = .202). (C) Moderate negative correlation between VWFpp/VWF:Ag ratio (a surrogate marker for VWF clearance) and FVIII half-life (P < .0001). (D) Moderate positive correlation between VWF:FVIIIB and FVIII half-life (P = .0012).
Association between VWF, VWFpp, and VWF:FVIIIB and FVIII half-life. The Pearson correlation coefficient of continuous variables was assessed by a 2-tailed test of significance. (A) Strong positive correlation between VWF:Ag and FVIII half-life (P < .0001). (B) Weak positive correlation between VWFpp (a surrogate marker for VWF secretion) and FVIII half-life (P = .202). (C) Moderate negative correlation between VWFpp/VWF:Ag ratio (a surrogate marker for VWF clearance) and FVIII half-life (P < .0001). (D) Moderate positive correlation between VWF:FVIIIB and FVIII half-life (P = .0012).
VWF gene variants
Common and rare variants within the VWF gene can modify VWF plasma levels, the rate of VWF clearance, and the FVIII-binding ability of VWF.15-17 We performed sequencing of the FVIII-binding and N-linked glycan sites in the VWF gene.35 No variant at the Asn–X–Ser/Thr consensus N-linked glycan sequences previously confirmed to be occupied was observed. We next assessed the influence of nonsynonymous variants found within the FVIII-binding region of VWF (exons 17-21, 24-27). Five patients heterozygous for 2 rare or low-frequency nonsynonymous variants were identified: c.2477G>A, p.(Arg826Lys) (n = 1) and c.2555G>A, p.(Arg852Glu) (n = 4). Of note, the patient heterozygous for the p.(Arg826Lys) variant had the shortest FVIII half-life (5.3 hours) and fastest rate of FVIII clearance (400 mL/h) in this cohort (Table 3). In silico pathogenicity assessment predicted that the p.(Arg826Lys) substitution may influence VWF function, whereas the p.(Arg852Glu) variant may be tolerated (supplemental Table 2). Interestingly, when we compared the patients with a rare or low-frequency VWF D′D3 variant with the rest of the patient cohort, we did not observe significant differences in VWF:Ag (P = .159) (Figure 2A), although VWF:FVIIIB (P = .05) was reduced (Figure 2B). Expression of the p.(Arg826Lys) and p.(Arg852Glu) variants cloned into human VWF complementary DNA did not modify VWF secretion in a heterologous HEK 293T cell system (supplemental Figure 1A). A VWF:FVIII binding assay using recombinant human VWF demonstrated that p.(Arg826Lys) and p.(Arg852Glu) had reduced binding activity compared with wild-type VWF (supplemental Figure 1B).
Summary of rare and low VWF D′D3 variants and associated FVIII PK
| Genetic variant . | Reference allele . | c.[2477G>A]+[=] . | c.[2555G>A]+[=] . |
|---|---|---|---|
| Protein variant | N/A | p.(R826K) | p.(R852Q) |
| Sample size | n = 38 | n = 1 | n = 4 |
| FVIII PK | |||
| Cl, mL/h | 149 (47-322) | 400 | 132 (63-260) |
| k, h−1 | 0.0668 (0.0377-0.098) | 0.13 | 0.069 (0.04-0.084) |
| Half-life, h | 10.38 (7.09-18.43) | 5.3 | 10.06 (8.23-17.05) |
| AUC (h·mIU/dL) | 137.36 (73.34-267.79) | 62.39 | 137.33 (93.65-202.65) |
| VWF | |||
| VWF:Ag, % | 87.49 (39.93-141.64) | 45.7 | 78.82 (44.99-109.37) |
| VWFpp/VWF:Ag | 1.1 (0.329-1.71) | 1.38 | 0.99 (74.13-145.32) |
| VWF:FVIIIB, % | 83.0 (27.0-291.5) | 11.3 | 66.62 (43.0-90.3) |
| Genetic variant . | Reference allele . | c.[2477G>A]+[=] . | c.[2555G>A]+[=] . |
|---|---|---|---|
| Protein variant | N/A | p.(R826K) | p.(R852Q) |
| Sample size | n = 38 | n = 1 | n = 4 |
| FVIII PK | |||
| Cl, mL/h | 149 (47-322) | 400 | 132 (63-260) |
| k, h−1 | 0.0668 (0.0377-0.098) | 0.13 | 0.069 (0.04-0.084) |
| Half-life, h | 10.38 (7.09-18.43) | 5.3 | 10.06 (8.23-17.05) |
| AUC (h·mIU/dL) | 137.36 (73.34-267.79) | 62.39 | 137.33 (93.65-202.65) |
| VWF | |||
| VWF:Ag, % | 87.49 (39.93-141.64) | 45.7 | 78.82 (44.99-109.37) |
| VWFpp/VWF:Ag | 1.1 (0.329-1.71) | 1.38 | 0.99 (74.13-145.32) |
| VWF:FVIIIB, % | 83.0 (27.0-291.5) | 11.3 | 66.62 (43.0-90.3) |
Data are median (range).
N/A, not applicable.
Influence of rare and low-frequency VWF D′D3 variants on VWF:Ag and VWF:FVIIIB. (A) Comparison of VWF:Ag between study subjects heterozygous for a rare or low-frequency VWF D′D3 variant and subjects homozygous for the reference allele [excluding the p.(Thr789Ala)/p.(Tyr795=) variants]. (B) Comparison of VWF:FVIIIB between study subjects heterozygous for a rare or low-frequency VWF D′D3 variant and subjects homozygous for the reference allele [excluding the p.(Thr789Ala)/p.(Tyr795=) variants]. Data are mean ± standard error. ○, wild-type; ▪, p.(Arg852Glu); □, p.(Arg826Lys). *P ≤ .05.
Influence of rare and low-frequency VWF D′D3 variants on VWF:Ag and VWF:FVIIIB. (A) Comparison of VWF:Ag between study subjects heterozygous for a rare or low-frequency VWF D′D3 variant and subjects homozygous for the reference allele [excluding the p.(Thr789Ala)/p.(Tyr795=) variants]. (B) Comparison of VWF:FVIIIB between study subjects heterozygous for a rare or low-frequency VWF D′D3 variant and subjects homozygous for the reference allele [excluding the p.(Thr789Ala)/p.(Tyr795=) variants]. Data are mean ± standard error. ○, wild-type; ▪, p.(Arg852Glu); □, p.(Arg826Lys). *P ≤ .05.
ABO blood group
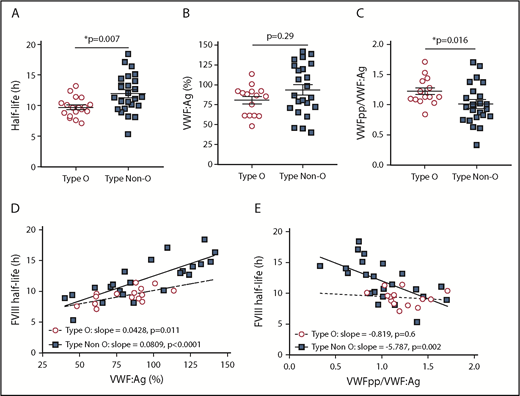
Although the ABO blood group has been previously shown to modify FVIII PK, the mechanistic basis for this association has not been demonstrated.1,13 Consistent with previous studies, we observed that non-O blood group was associated with a longer FVIII half-life (P = .007) compared with type O subjects (Figure 3A; Table 4). Although there was a modest increase in VWF:Ag in the non-O subjects (Figure 3B; Table 4), this influence was not statistically significant (P = .299). Importantly, the non-O blood group was associated with a significant (P = .016) decrease in VWFpp/VWF:Ag, indicating a longer VWF half-life (Figure 3C; Table 4). We next performed association analyses to assess the influence of ABO blood group on FVIII half-life. We observed a positive association between VWF:Ag and FVIII half-life for type O patients (P = .011) and non-O patients (P < .0001), although the association was stronger for non-O individuals (Figure 3D). In contrast, we observed a significant association between VWFpp/VWF:Ag for non-O individuals (P = .002) but not type O subjects (P = .6) (Figure 3A), suggesting that, for non-O subjects, the increase in FVIII half-life is related to the longer half-life of VWF.
Association between ABO blood group with FVIII half-life, VWF:Ag, and VWFpp/VWF:Ag. Influence of ABO blood group on FVIII half-life (A), VWF:Ag (B), and VWFpp/VWF:Ag (C). Statistical significance was assessed by the Mann-Whitney U test. Horizontal lines indicate mean ± standard error. (D) Influence of ABO blood group on the association between VWF:Ag and FVIII half-life was assessed by linear regression analysis. The slope for type O (m = 0.0428, P = .0112) and type non-O (m = 0.0809, P < .0001) are significantly different from 0 (null hypothesis) but not from each other (P = .1611). (E) Influence of ABO blood group on the association between VWFpp/VWF:Ag and FVIII half-life was assessed by linear regression analysis. The slope for type 0 (P = .6) is not significantly different from 0 (null hypothesis). The slope for type non-O (P = .002) is significantly different from 0 (null hypothesis) although the 2 slopes are not significantly different from each other (P = .1095). *P ≤ .05
Association between ABO blood group with FVIII half-life, VWF:Ag, and VWFpp/VWF:Ag. Influence of ABO blood group on FVIII half-life (A), VWF:Ag (B), and VWFpp/VWF:Ag (C). Statistical significance was assessed by the Mann-Whitney U test. Horizontal lines indicate mean ± standard error. (D) Influence of ABO blood group on the association between VWF:Ag and FVIII half-life was assessed by linear regression analysis. The slope for type O (m = 0.0428, P = .0112) and type non-O (m = 0.0809, P < .0001) are significantly different from 0 (null hypothesis) but not from each other (P = .1611). (E) Influence of ABO blood group on the association between VWFpp/VWF:Ag and FVIII half-life was assessed by linear regression analysis. The slope for type 0 (P = .6) is not significantly different from 0 (null hypothesis). The slope for type non-O (P = .002) is significantly different from 0 (null hypothesis) although the 2 slopes are not significantly different from each other (P = .1095). *P ≤ .05
Association between ABO blood group and FVIII PK parameters or plasma VWF properties
| . | Type O (n = 18), median (range) . | Non-O (n = 25), median (range) . | P . | Regression analysis β (CI); P . |
|---|---|---|---|---|
| FVIII PK | ||||
| Cl, mL/h | 173 (74-322) | 141 (47-400) | .136 | −34.84 (−74.25 to 4.57); .082 |
| k, h−1 | 0.072 (0.052-0.098) | 0.061 (0.038-0.130) | .007 | −0.011 (−0.021 to 0); .048 |
| Half-life, h | 9.62 (7.09-13.20) | 11.36 (5.32-18.43) | .007 | 2.231 (0.604 to 3.858); .008 |
| AUC, h·mIU/dL | 125.19 (83.42-176.39) | 150.86 (62.39-291.99) | .056 | 32.93 (2.30 to 63.56); .036 |
| VWF | ||||
| VWF:Ag, % | 86.84 (48.22-113.78) | 86.63 (39.94-141.64) | .3 | 13.2 (−5.348 to 31.75); .157 |
| VWFpp/VWF:Ag | 1.13 (0.84-1.70) | 0.963 (0.329-1.701) | .016 | −0.209 (−0.407 to −0.011); .039 |
| VWF:FVIIIB, % | 80.0 (27.0-291.5) | 82.9 (11.30-260.6) | .36 | 0.009 (−0.332 to 0.352); .955 |
| . | Type O (n = 18), median (range) . | Non-O (n = 25), median (range) . | P . | Regression analysis β (CI); P . |
|---|---|---|---|---|
| FVIII PK | ||||
| Cl, mL/h | 173 (74-322) | 141 (47-400) | .136 | −34.84 (−74.25 to 4.57); .082 |
| k, h−1 | 0.072 (0.052-0.098) | 0.061 (0.038-0.130) | .007 | −0.011 (−0.021 to 0); .048 |
| Half-life, h | 9.62 (7.09-13.20) | 11.36 (5.32-18.43) | .007 | 2.231 (0.604 to 3.858); .008 |
| AUC, h·mIU/dL | 125.19 (83.42-176.39) | 150.86 (62.39-291.99) | .056 | 32.93 (2.30 to 63.56); .036 |
| VWF | ||||
| VWF:Ag, % | 86.84 (48.22-113.78) | 86.63 (39.94-141.64) | .3 | 13.2 (−5.348 to 31.75); .157 |
| VWFpp/VWF:Ag | 1.13 (0.84-1.70) | 0.963 (0.329-1.701) | .016 | −0.209 (−0.407 to −0.011); .039 |
| VWF:FVIIIB, % | 80.0 (27.0-291.5) | 82.9 (11.30-260.6) | .36 | 0.009 (−0.332 to 0.352); .955 |
Bold results are statistically significant. Statistical significance was assessed using the Mann-Whitney U test and confirmed by regression analysis.
β, β coefficient; CI, confidence interval.
CHARGE variants
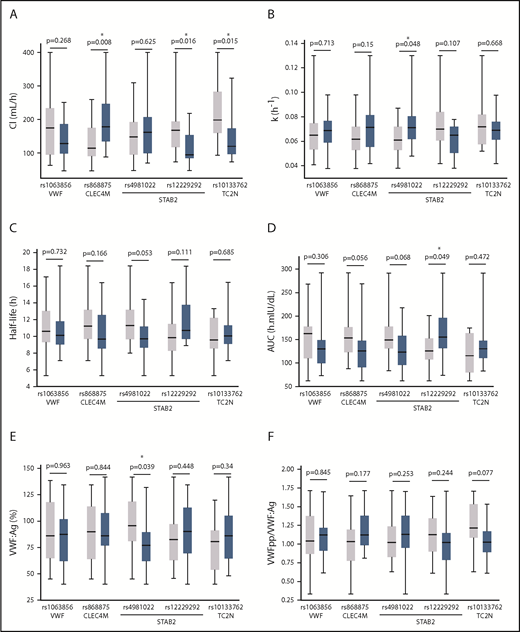
We next assessed the influence of 9 SNVs, which have previously been demonstrated to be associated with plasma VWF or FVIII levels in normal individuals, on FVIII PK parameters in our pediatric population.16,22 The association between the CHARGE SNVs and FVIII PK was assessed using linear regression, which assumes an additive genetic model (Table 5). The Mann-Whitney U test was used to determine the difference between individuals homozygous for the reference allele (gray bar) and heterozygous individuals (blue bar) to investigate nonadditive effects (Figure 4), because, for most variants, the sample size of individuals homozygous for the nonreference allele was too small for this analysis. VWF (P = .05), CLEC4M (P = .039), and TC2N (P = .045) gene variants were associated with FVIII clearance by linear regression (Table 5) and/or the Mann-Whitney U test (Figure 4A). The STAB2 variant rs4981022 was associated with the FVIII elimination rate constant (P = .005), FVIII half-life (P = .007), and plasma VWF:Ag (P = .015) by regression analysis, whereas the rs12229292 variant was associated with an increased rate of clearance (P = .016) in patients heterozygous for the STAB2 variant compared with patients homozygous for the reference allele (Mann-Whitney U test) (Figure 4A). Neither SCARA5 gene variant was associated with FVIII PK or plasma VWF measurements by regression analysis (Table 5) or the Mann-Whitney U test (data not shown).
Association between common CHARGE variants and FVIII PK parameters or plasma VWF properties
| . | VWF . | CLEC4M . | SCARA5 . | STAB2 . | TC2N . | |||
|---|---|---|---|---|---|---|---|---|
| rs1063856/rs1063857 . | rs868875 . | rs2726953 . | rs9644133 . | rs4981022 . | rs12229292 . | rs10133762 . | ||
| MAF = 0.297 . | MAF = 0.209 . | MAF = 0.263 . | MAF = 0.224 . | MAF = 0.221 . | MAF = 0.337 . | MAF = 0.5 . | ||
| FVIII PK | ||||||||
| Cl, mL/h | −32.21 (−64.43 to 0.02) | 39.50 (2.19 to 76.83) | −1.26 (−38.55 to 36.04) | −7.33 (−48.35 to 33.69) | 22.08 (−13.66 to 57.81) | −10.11 (−35.73 to 15.51) | −32.61 (−64.41 to −0.82) | |
| P = .05 | P = .039 | P = .946 | P = .719 | P = .219 | P = .43 | P = .045 | ||
| k, h−1 | −0.004 (−0.013 to 0.005) | 0.008 (-0.002 to 0.018) | 0.004 (−0.006 to 0.013) | −0.005 (−0.015 to 0.006) | 0.013 (0.004 to 0.022) | −0.006 (−0.013 to 0.001) | −0.006 (−0.014 to 0.003) | |
| P =.391 | P = .109 | P = .444 | P = .387 | P = .005 | P = .079 | P = .186 | ||
| Half-life, h | 0.591 (−0.794 to 1.977) | −1.052 (−2.645 to 0.54) | −0.647 (−2.133 to 0.84) | 0.826 (−0.806 to 2.458) | −1.941 (−3.308 to 0.573) | 0.854 (−0.176 to 1.883) | 0.917 (−0.406 to 2.24) | |
| P =.393 | P = .189 | P = .393 | P = .311 | P = .007 | P = .102 | P = .168 | ||
| AUC, h·mIU/dL | −3.61 (−2.99 to 2.27) | −3.45 (−6.3 to 5.92) | 0.68 (−28.18 to 29.54) | 0.12 (−3.17 to 31.93) | −27.04 (-53.97 to −0.011) | 14.36 (−0.005 to 33.88) | 22.45 (−0.25 to 47.39) | |
| P = .783 | P = .019 | P = .962 | P = .994 | P = .049 | P = .145 | P = .076 | ||
| Plasma levels | ||||||||
| VWF:Ag, % | 4.509 (−11.14 to 20.15) | −0.899 (−19.41 to 17.61) | 2.119 (−13.42 to 17.66) | 7.251 (−9.71 to 24.2) | −19.399 (−3484 to −3.96) | 4.966 (−6.73 to 16.67) | 9.866 (−3.79 to 23.53) | |
| P = .562 | P = .922 | P = .783 | P = .391 | P = .015 | P = .394 | P = .151 | ||
| VWFpp/VWF:Ag | 0.022 (−0.146 to 0.189) | 0.127 (−0.066 to 0.319) | 0.011 (−0.155 to 0.177) | −0.027 (−0.21 to 0.156) | 0.076 (−0.102 to 0.254) | 0.025 (−0.101 to 0.151) | −0.09 (−0.237 to 0.057) | |
| P = .795 | P = .191 | P = .896 | P = .767 | P = .395 | P = .685 | P = .222 | ||
| . | VWF . | CLEC4M . | SCARA5 . | STAB2 . | TC2N . | |||
|---|---|---|---|---|---|---|---|---|
| rs1063856/rs1063857 . | rs868875 . | rs2726953 . | rs9644133 . | rs4981022 . | rs12229292 . | rs10133762 . | ||
| MAF = 0.297 . | MAF = 0.209 . | MAF = 0.263 . | MAF = 0.224 . | MAF = 0.221 . | MAF = 0.337 . | MAF = 0.5 . | ||
| FVIII PK | ||||||||
| Cl, mL/h | −32.21 (−64.43 to 0.02) | 39.50 (2.19 to 76.83) | −1.26 (−38.55 to 36.04) | −7.33 (−48.35 to 33.69) | 22.08 (−13.66 to 57.81) | −10.11 (−35.73 to 15.51) | −32.61 (−64.41 to −0.82) | |
| P = .05 | P = .039 | P = .946 | P = .719 | P = .219 | P = .43 | P = .045 | ||
| k, h−1 | −0.004 (−0.013 to 0.005) | 0.008 (-0.002 to 0.018) | 0.004 (−0.006 to 0.013) | −0.005 (−0.015 to 0.006) | 0.013 (0.004 to 0.022) | −0.006 (−0.013 to 0.001) | −0.006 (−0.014 to 0.003) | |
| P =.391 | P = .109 | P = .444 | P = .387 | P = .005 | P = .079 | P = .186 | ||
| Half-life, h | 0.591 (−0.794 to 1.977) | −1.052 (−2.645 to 0.54) | −0.647 (−2.133 to 0.84) | 0.826 (−0.806 to 2.458) | −1.941 (−3.308 to 0.573) | 0.854 (−0.176 to 1.883) | 0.917 (−0.406 to 2.24) | |
| P =.393 | P = .189 | P = .393 | P = .311 | P = .007 | P = .102 | P = .168 | ||
| AUC, h·mIU/dL | −3.61 (−2.99 to 2.27) | −3.45 (−6.3 to 5.92) | 0.68 (−28.18 to 29.54) | 0.12 (−3.17 to 31.93) | −27.04 (-53.97 to −0.011) | 14.36 (−0.005 to 33.88) | 22.45 (−0.25 to 47.39) | |
| P = .783 | P = .019 | P = .962 | P = .994 | P = .049 | P = .145 | P = .076 | ||
| Plasma levels | ||||||||
| VWF:Ag, % | 4.509 (−11.14 to 20.15) | −0.899 (−19.41 to 17.61) | 2.119 (−13.42 to 17.66) | 7.251 (−9.71 to 24.2) | −19.399 (−3484 to −3.96) | 4.966 (−6.73 to 16.67) | 9.866 (−3.79 to 23.53) | |
| P = .562 | P = .922 | P = .783 | P = .391 | P = .015 | P = .394 | P = .151 | ||
| VWFpp/VWF:Ag | 0.022 (−0.146 to 0.189) | 0.127 (−0.066 to 0.319) | 0.011 (−0.155 to 0.177) | −0.027 (−0.21 to 0.156) | 0.076 (−0.102 to 0.254) | 0.025 (−0.101 to 0.151) | −0.09 (−0.237 to 0.057) | |
| P = .795 | P = .191 | P = .896 | P = .767 | P = .395 | P = .685 | P = .222 | ||
Bold results are statistically significant. Statistical significance was determined using regression analysis. Data are presented as β (CI) and P value.
MAF, minor allele frequency.
Association between common CHARGE variants and FVIII PK parameters. Analysis of the association between CHARGE variants in VWF, CLEC4M, STAB2, and TC2N and FVIII PK or VWF levels. Gray boxes correspond to homozygous inheritance of the major (reference) allele, and blue boxes correspond to heterozygous inheritance. Homozygous inheritance of the minor (nonreference) allele was not assessed because of small sample sizes. The box plots correspond to median and interquartile range, and the whiskers denote minimum and maximum values. Statistical significance was assessed by Mann-Whitney U. (A) Association between CHARGE variants and FVIII clearance (Cl). (B) Association between CHARGE variants and FVIII elimination rate constant (k). (C) Association between CHARGE variants and FVIII half-life. (D) Association between CHARGE variants and FVIII AUC. (E) Association between CHARGE variants and VWF:Ag. (F) Association between CHARGE variants and VWFpp/VWF:Ag.
Association between common CHARGE variants and FVIII PK parameters. Analysis of the association between CHARGE variants in VWF, CLEC4M, STAB2, and TC2N and FVIII PK or VWF levels. Gray boxes correspond to homozygous inheritance of the major (reference) allele, and blue boxes correspond to heterozygous inheritance. Homozygous inheritance of the minor (nonreference) allele was not assessed because of small sample sizes. The box plots correspond to median and interquartile range, and the whiskers denote minimum and maximum values. Statistical significance was assessed by Mann-Whitney U. (A) Association between CHARGE variants and FVIII clearance (Cl). (B) Association between CHARGE variants and FVIII elimination rate constant (k). (C) Association between CHARGE variants and FVIII half-life. (D) Association between CHARGE variants and FVIII AUC. (E) Association between CHARGE variants and VWF:Ag. (F) Association between CHARGE variants and VWFpp/VWF:Ag.
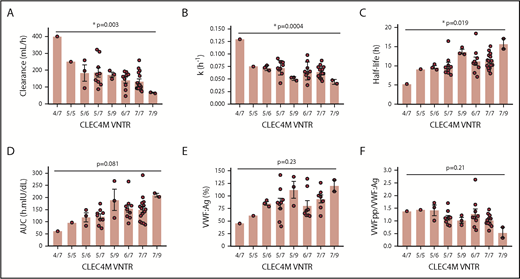
The CLEC4M SNV rs868875 is in linkage disequilibrium with the VNTR polymorphism that has been shown to modulate the ligand-binding affinity of CLEC4M.24 We genotyped our study subjects for the CLEC4M VNTR and observed, using 1-way ANOVA, that there was a significant association between VNTR genotype and FVIII clearance (P = .003) (Figure 5A), elimination rate constant (P = .0004) (Figure 5B), and half-life (P = .019) (Figure 5C). Associations between CLEC4M VNTR genotype and AUC (P = .081), VWF:Ag (P = .23), and VWFpp/VWF:Ag (P = .21) were not statistically significant (Figure 5D-F). Tukey’s post hoc analysis multiple-comparisons tests for differences between VNTR genotypes were not statistically significant, likely as a result of the small number of study subjects.
Association between CLEC4M VNTR variant and FVIII PK parameters. Analysis of the association between the CLEC4M VNTR variant and FVIII PK or VWF levels by 1-way ANOVA. (A) Association between the CLEC4M VNTR variant and FVIII clearance. (B) Association between the CLEC4M VNTR variant and FVIII elimination rate constant (k). (C) Association between the CLEC4M VNTR variant and FVIII half-life. (D) Association between the CLEC4M VNTR variant and FVIII AUC. (E) Association between the CLEC4M VNTR variant and VWF:Ag. (F) Association between the CLEC4M VNTR variant and VWFpp/VWF:Ag. *P ≤ .05.
Association between CLEC4M VNTR variant and FVIII PK parameters. Analysis of the association between the CLEC4M VNTR variant and FVIII PK or VWF levels by 1-way ANOVA. (A) Association between the CLEC4M VNTR variant and FVIII clearance. (B) Association between the CLEC4M VNTR variant and FVIII elimination rate constant (k). (C) Association between the CLEC4M VNTR variant and FVIII half-life. (D) Association between the CLEC4M VNTR variant and FVIII AUC. (E) Association between the CLEC4M VNTR variant and VWF:Ag. (F) Association between the CLEC4M VNTR variant and VWFpp/VWF:Ag. *P ≤ .05.
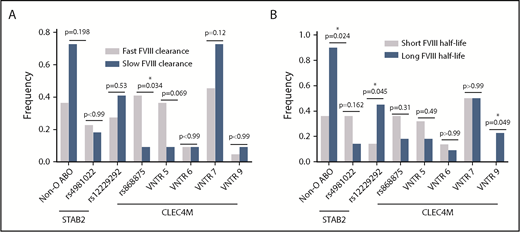
Finally, we assessed the genetic characteristics in the quartile (Q1) of patients (n = 11) with the fastest FVIII clearance and the quartile (Q4) with the slowest FVIII clearance (n = 11) and the quartile (Q1) of patients (n = 11) with the shortest FVIII half-life compared with the quartile (Q4) of patients (n = 11) with the longest FVIII half-life. We observed a significant association of the CLEC4M rs868875 variant with fast FVIII clearance (P = .034), whereas the CLEC4M VNTR 5 allele trended toward significance (P = .069) (Figure 6A) using Fisher’s exact test. We also observed a significant association between a longer FVIII half-life and non-O blood group (P = .024), STAB2 rs122292929 (P = .045), and CLEC4M VNTR 9 allele (P = .049) using Fisher’s exact test.
Influence of CHARGE variants on FVIII PK phenotypic quartiles. The frequency of genotypes were compared between patients in the quartile with the shortest FVIII half-life (n = 11) and patients in the quartile with the longest FVIII half-life (n = 11) (A) and between patients in the quartile with the slowest rate of FVIII clearance (n = 11) and patients with the fastest rate of FVIII clearance (n = 11) (B). *P ≤ .05.
Influence of CHARGE variants on FVIII PK phenotypic quartiles. The frequency of genotypes were compared between patients in the quartile with the shortest FVIII half-life (n = 11) and patients in the quartile with the longest FVIII half-life (n = 11) (A) and between patients in the quartile with the slowest rate of FVIII clearance (n = 11) and patients with the fastest rate of FVIII clearance (n = 11) (B). *P ≤ .05.
Discussion
FVIII PK properties are likely influenced by genetic and environmental factors; although the heritability of FVIII PK has not been determined, it is likely similar to endogenous FVIII:C in normal individuals, in whom the genetic contribution has been estimated to be between 57% and 82%.36,37 There is very little information available concerning the non-ABO pharmacogenomic regulation of FVIII PK; patient F8 mutation type, and LRP1 genotypes have not been shown to be associated with these parameters, although a recent report has described the c.1773C>T, p.(Asn591=) variant in LDLR as being associating with FVIII clearance and volume of distribution.13,38
In this study of FVIII PK determinants in 43 boys with severe hemophilia A, we observed that the rate at which endogenous VWF is cleared from the plasma and the relative FVIII-binding activity of VWF are important determinants of FVIII PK. Within the FVIII-binding D′D3 region of the VWF gene, we identified 1 rare [p.(Arg826Lys)], 1 low-frequency [p.(Arg852Glu)], and 2 common [p.(Thr789Ala) and p.(Tyr795=)] variants in our patient population. An analysis generated from the 1000 Genomes database has previously observed 2.5 VWF gene SNVs per individual, with rare or novel nonsynonymous coding region variants identified in >6% of normal individuals.39 When the patients with the rare and low-frequency variants p.(Arg826Lys) and p.(Arg852Glu) were pooled, we observed a significant decrease in VWF:FVIIIB, but not VWF:Ag levels in these patients (Figure 2), which is consistent with studies involving recombinant VWF variants (supplemental Figure 1). The p.(Arg826Lys) variant was predicted to have pathogenic function by two thirds of in silico software analyses (supplemental Table 2). p.(Arg826Lys) is not listed in the VWF variant database (http://www.vwf.group.shef.ac.uk/index.html), although 1 abstract has reported an association with type 2N VWD.40 In contrast, although the p.(Arg852Glu) variant is not associated with type 2N VWD, it has been shown to be associated with a modest reduction in VWF:FVIIIB activity in hemophilia A patients.41 Collectively, these data suggest that heterozygous inheritance of VWF D′D3 variants that modify VWF-FVIIIB may influence FVIII PK.
We also observed that the common VWF variants p.(Thr789Ala) and p.(Tyr795=) (rs1063856 and rs1063857), which are in strong linkage disequilibrium, were associated with decreased rates of FVIII clearance (Figure 4; Table 5). This observation is consistent with previous studies that have determined that these variants are associated with increased VWF:Ag and FVIII:C in normal individuals and, consequently, with an increased risk for venous thromboembolism.16,19,42-47 Previously published in vitro and in vivo studies suggest that these variants can increase the rate of synthesis/secretion and decreased clearance without altering FVIII-binding activity.19,41,42 Therefore, it follows that these variants may influence FVIII clearance in this population by altering the rate at which endogenous VWF is cleared.
We next assessed the influence of the ABO blood group on FVIII PK, which accounts for the greatest proportion of heritable variability in endogenous plasma VWF and FVIII levels.12,20 Importantly, we have previously demonstrated that the influence of the ABO blood group locus on the VWF life cycle in pediatric populations may be significantly different than in adults. Although VWF:Ag and FVIII:C levels are consistently elevated in non-O middle- and old-age adults, this effect is less profound in pediatric cohorts.26 Here, we observed an increase in FVIII half-life in non-O patients, although VWF:Ag levels were not statistically different between the 2 groups (Figure 3; Table 4).13 This effect is likely related to ABO-mediated influences on VWF clearance, because the VWFpp/VWF:Ag ratio was significantly decreased in the non-O group. Interestingly, the variability in FVIII PK parameters is larger for the non-O group than for the O group, likely because of the diversity of non-O genotypes (AA, AB, AO, BB, BO) within this population.
In addition to VWF and ABO, a number of genetic variants have been shown to be associated with plasma levels of VWF and FVIII in normal adult populations.16 Given the observed association between FVIII PK parameters and VWF clearance in our pediatric population, we looked at variants in the STAB2, CLEC4M, and SCARA5 genes that encode cell surface receptors thought to modify VWF-FVIII clearance, as well as TC2N, which has an unknown function. In our pediatric patients, the STAB2, CLEC4M, and TC2N variants exhibited the same directionality and similar effect size on VWF levels that was previously reported in the CHARGE study (supplemental Table 6).16
STAB2 encodes stabilin-2, a class H scavenger receptor expressed on the sinusoidal endothelial cells of the liver and spleen. Stabilin-2–expressing cells can bind and internalize VWF in vitro, and stabilin-2–knockout mice demonstrate an increased half-life for human VWF and VWF-bound FVIII, but not for VWF-free FVIII.23 Additionally, stabilin-2 regulates the clearance of the murine propeptide,48 suggesting that the VWFpp/VWF:Ag ratio may fail to capture the effect of all non-VWF gene variants that modify the rate of VWF clearance in vivo. Rare pathogenic variants in the STAB2 gene have previously been associated with elevated levels of VWF:Ag in the normal population and an increased risk for venous thromboembolism15,49,50 or VWF:Ag in patients with “low-VWF” or type 1 VWD.23 Here, we observed that both CHARGE STAB2 variants were associated with FVIII PK parameters by regression analysis or the Mann-Whitney U test (Figure 4; Table 5). Furthermore, the rs4981022 variant was associated with FVIII half-life, elimination rate constant, and AUC, as well as a relatively large ∼19% decrease in VWF:Ag. Although this effect size is greater than expected given the magnitude reported in the CHARGE study, in which the median age of study subjects ranged from 44.9 to 72.3 years,16 there may be STAB2-dependent differences in VWF clearance in pediatric vs adult populations.
CLEC4M is a mannose-specific lectin receptor expressed by endocytic endothelial cells in the liver, spleen, and lymphatics. The neck region of CLEC4M consists of a VNTR region (3-9 repeats), which supports tetramerization of the molecule and modulates its ligand-binding affinity.51 We have previously demonstrated that CLEC4M-expressing cells are capable of binding and internalizing the VWF-FVIII complex and VWF-free FVIII.24,52 The CLEC4M VNTR variant is in linkage disequilibrium with the rs868875 variant, and these variants are associated with VWF:Ag or activity levels in patients with “low-VWF” or type 1 VWD.24,53 In this study, we observed that rs868875 is associated with FVIII clearance and AUC (Figure 4; Table 5) and that the VNTR variant is associated with FVIII clearance, elimination rate constant, half-life, and AUC but not VWF:Ag or VWFpp/VWF:Ag (Figure 5), although the sample sizes for each genotype precluded a multiple-comparison test. The absence of an association between the CLEC4M variants and VWF:Ag or VWFpp/VWF:Ag may reflect the small sample size, as well as the observation that CLEC4M can regulate the clearance of VWF-free FVIII.52 We also observed an increased prevalence of rs868875 and VNTR 5 in individuals with rapid FVIII clearance, as well as an increased prevalence of VNTR 9 in individuals with a long FVIII half-life (Figure 6). This observation is consistent with our previous in vitro studies that demonstrated that the CLEC4M VNTR 9 variant was associated with impaired VWF internalization compared with VNTR 6 and VNTR 7 in an in vitro assay.24
The association among VWF:Ag, age, and FVIII PK has been well characterized, with older age associated with longer FVIII half-life and elevated VWF:Ag.4,13,34 The basis of this observation is thought to be related to the accumulation of fewer pathobiological or environmental influences on plasma VWF levels in younger cohorts. Studies on VWF heritability have consistently produced higher estimates of the proportion of VWF variability influenced by genetic factors in younger populations.36,37,54-56 Thus, it follows that the heritability of FVIII PK may also be higher in a pediatric population, and the replication or confirmation of these observations in an adult cohort may be warranted.
Plasma levels of VWF are highly variable and continuously distributed and, in the normal population, can be modified by environmental factors, as well as variants in the VWF gene and at external genetic loci, making this a complex quantitative trait.57 Thus, the PK profile of FVIII is also likely to be regulated by environmental and multigenic influences. In this study, we assessed the influence of each variant in isolation, because the relatively large number of variants tested and the small sample size of this study preclude the possibility of a multivariable analysis. However, future studies with larger subject populations may allow for testing of the overall model or for the influence of individual variants within the model.
Collectively, these studies demonstrate for the first time that FVIII PK is predominantly regulated by the relative rate at which VWF is cleared from the plasma. Common genetic modifiers of VWF clearance, including ABO blood group, VWF gene variants, and variants in the VWF clearance receptors CLEC4M and stabilin-2, can also influence patient FVIII PK profiles. Future studies of FVIII PK determinants may focus on searching for rare gain- and loss- of-function variants within these genes that make larger contributions to FVIII PK. Moreover, the findings in this report demonstrate that differences in VWF-FVIII binding can also modify FVIII PK, suggesting that the accelerated clearance of VWF-free FVIII can influence FVIII PK and that the clinical use of VWF D′D3 extended half-life products may prove beneficial in this patient population.58-60 This study has begun to elucidate the pharmacogenomic basis underlying the variability of FVIII PK within the hemophilia A population. Although these studies are not intended to supplant PK analyses for individual patients, they may provide insights into novel strategies for improving the half-life of FVIII replacement products.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Rand, C. Wakefield, E. Wissmann, and J. Vandesande for patient sample and data collection; A. Tuttle, S. Albánez, G. Jones, and S. Tinlin for laboratory technical assistance; and C. Zhang, K. Usuba, P. Collins, and D. Ignas for assistance with set up and validation of the TCIWorks program.
This work was supported by the Baxalta (now Takeda) Canadian Hemophilia Epidemiology Research Program and by a Foundation Grant from the Canadian Institutes of Health Research (FDN154285). D.L. is a recipient of a Canada Research Chair in Molecular Hemostasis, and L.L.S. was supported by a Canadian Institutes of Health Research Fellowship.
Authorship
Contribution: L.L.S. and D.L. conceived and designed the study; L.L.S., K.O., O.R., C.B., and I.G. performed experiments, laboratory assays, and/or genetic analyses; L.L.S., K.O., O.R., and D.L. analyzed and interpreted data; K.O. performed PK analysis; L.L.S. and W.H. performed statistical analyses; K.T., V.L., C.M., V.S.B., and M.D.C. recruited study subjects and collected PK samples; L.L.S. wrote the manuscript; and D.L. edited the manuscript.
Conflict-of-interest disclosure: O.R. has received research funding and honoraria for participating on advisory boards for CSL Behring. C.M. has received research grants from CSL Behring and Shire (now Takeda). V.L. has received research support from Baxalta. V.S.B. has received speakers fees/honoraria from Bayer, Bioverativ/Sanofi, Novo Nordisk, Pfizer, Roche, Shire, and Spark Therapeutics; is a member of Data Safety Monitoring Boards for Octapharma and Shire; has received research support from Bayer Health Care, Bioverativ, and Shire; and is a chairman of the International Prophylaxis Study Group, which is supported by grants from Bayer Health Care, Bioverativ, Novo Nordisk, Pfizer, Shire, and Spark Therapeutics. M.D.C. has received research support from Bayer, Bioverativ/Sanofi, CSL-Behring, Novo Nordisk, Octapharma, Pfizer, and Shire and has received honoraria for speaking/participating on advisory boards for Bayer, Bioverativ/Sanofi, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, and Shire. D.L. has received research grants from Shire, Bayer, Bioverativ, CSL Behring, and Octapharma. The remaining authors declare no competing financial interests.
Correspondence: David Lillicrap, Richardson Laboratory, Queen’s University, 88 Stuart St, Kingston, ON K7L 3N6, Canada; e-mail: david.lillicrap@queensu.ca.

![Figure 2. Influence of rare and low-frequency VWF D′D3 variants on VWF:Ag and VWF:FVIIIB. (A) Comparison of VWF:Ag between study subjects heterozygous for a rare or low-frequency VWF D′D3 variant and subjects homozygous for the reference allele [excluding the p.(Thr789Ala)/p.(Tyr795=) variants]. (B) Comparison of VWF:FVIIIB between study subjects heterozygous for a rare or low-frequency VWF D′D3 variant and subjects homozygous for the reference allele [excluding the p.(Thr789Ala)/p.(Tyr795=) variants]. Data are mean ± standard error. ○, wild-type; ▪, p.(Arg852Glu); □, p.(Arg826Lys). *P ≤ .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/134/11/10.1182_blood.2019000190/7/m_bloodbld2019000190f2.png?Expires=1769081382&Signature=ATSITWhdL7pxh3gHzjCXSjsKxDLjoCmMeQQF0Pd9KtB9s3c5FZe8UqexU3XHN7A3JLX3Snobcqhn4EITc1hhkYdYZ6IvhgQYurnBpnoMEM57G-2pkqxQWrt3dZtbpVHXfI~ITmOJbNHbHQL6FXtIzxkO-qqGIFoZYJkb1zBBWntBPgrWvBdBwl4P8fT4DLldTdK-tL7p5d~6OYkOpyBhtj1wpZikYzTOI4ab~B71WWsNPoJzueTcVnl8R-LdBBOVe15x20brMT1rvXxf5FJ07haNHlAGPH1-9Gr29KngOWrwX8j-0HKl3KMGka~a2n4ap17IwnluRbr4gqvIV7HaMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)