Key Points
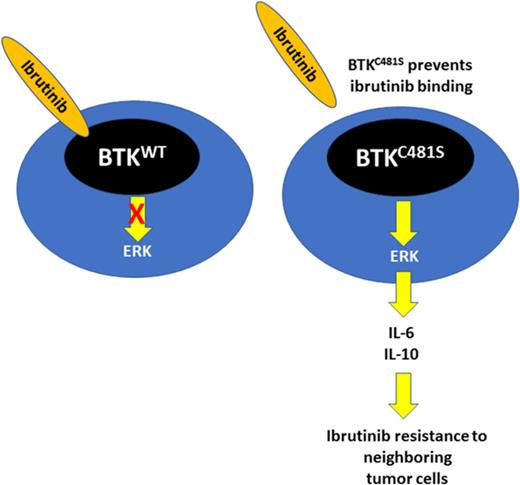
BTKCys481 mutation results in ERK1/2 mediated survival signaling and ibrutinib resistance in MYD88-mutated cells.
BTKCys481 mutation confers a protective effect against ibrutinib on neighboring BTK wild-type cells through a paracrine mechanism.
Abstract
Acquired ibrutinib resistance due to BTKCys481 mutations occurs in B-cell malignancies, including those with MYD88 mutations. BTKCys481 mutations are usually subclonal, and their relevance to clinical progression remains unclear. Moreover, the signaling pathways that promote ibrutinib resistance remain to be clarified. We therefore engineered BTKCys481Ser and BTKWT expressing MYD88-mutated Waldenström macroglobulinemia (WM) and activated B-cell (ABC) diffuse large B-cell lymphoma (DLBCL) cells and observed reactivation of BTK-PLCγ2-ERK1/2 signaling in the presence of ibrutinib in only the former. Use of ERK1/2 inhibitors triggered apoptosis in BTKCys481Ser-expressing cells and showed synergistic cytotoxicity with ibrutinib. ERK1/2 reactivation in ibrutinib-treated BTKCys481Ser cells was accompanied by release of many prosurvival and inflammatory cytokines, including interleukin-6 (IL-6) and IL-10 that were also blocked by ERK1/2 inhibition. To clarify if cytokine release by ibrutinib-treated BTKCys481Ser cells could protect BTKWT MYD88-mutated malignant cells, we used a Transwell coculture system and showed that nontransduced BTKWT MYD88-mutated WM or ABC DLBCL cells were rescued from ibrutinib-induced killing when cocultured with BTKCys481Ser but not their BTKWT-expressing counterparts. Use of IL-6 and/or IL-10 blocking antibodies abolished the protective effect conferred on nontransduced BTKWT by coculture with BTKCys481Ser expressing WM or ABC DLBCL cell counterparts. Rebound of IL-6 and IL-10 serum levels also accompanied disease progression in WM patients with acquired BTKCys481 mutations. Our findings show that the BTKCys481Ser mutation drives ibrutinib resistance in MYD88-mutated WM and ABC DLBCL cells through reactivation of ERK1/2 and can confer a protective effect on BTKWT cells through a paracrine mechanism.
Introduction
Bruton tyrosine kinase (BTK) is a nonreceptor tyrosine kinase that can mediate prosurvival signaling in MYD88-mutated Waldenström macroglobulinemia (WM) and activated B-cell (ABC) diffuse large B-cell lymphoma (DLBCL) cells.1,2 Activating mutations in MYD88 can also transactivate hematopoietic cell kinase (HCK) in WM and ABC DLBCL cells, which can trigger prosurvival signaling cascades that include BTK, as well as PI3K/AKT and MAPK/ERK1/2.3 Ibrutinib targets both BTK and HCK and has demonstrated remarkable clinical activity in MYD88-mutated WM and ABC DLBCL patients.2,4 Mutated MYD88 may also trigger BCR signaling through activation of SYK.5 Acquired resistance to ibrutinib is increasingly being recognized with prolonged therapy in chronic lymphocytic leukemia (CLL),6,7 mantle cell lymphoma (MCL),8 and WM patients9 because of somatic mutations affecting BTKCys481 that abrogates BTK-ibrutinib binding. Among these are c.1635G>C and c.1634T>A mutations resulting in a p.Cys481Ser (BTKCys481Ser) that is frequently found in CLL, MCL, and WM patients.5,7,8 The signaling cascade(s) that contribute to BTKCys481Ser related ibrutinib resistance remain to be clarified in MYD88-mutated cells. Moreover, in most CLL, MCL, and WM cases, BTKCys481 mutations are subclonal, and their relevance to clinical disease progression remains unclear.6,9-11 In this study, we engineered MYD88-mutated WM and ABC DLBCL cells to express BTKCys481Ser and sought to clarify mechanism(s) that contribute to individual as well as bystander tumor cell resistance to ibrutinib.
Materials and methods
Cell lines and reagents
MYD88L265P expressing WM (BCWM.1 and MWCL-1) and ABC DLBCL (TMD8, HBL1) cells were used in the studies. WM cell lines were cultured in RPMI 1640 media with 10% fetal bovine serum, and TMD8 and HBL1 were cultured in Iscove's Modified Dulbecco's Medium (IMDM) with 10% and 20% fetal bovine serum, respectively. Media were supplemented with penicillin, streptomycin, and l-glutamine (ThermoFisher). The BTK inhibitor, ibrutinib, and ERK1/2 inhibitors, ulixertinib (BVD-523) and GDC-0994, were purchased from Selleck Chemicals (Houston, TX). Hyaluronic acid (HA) was purchased from Hyalose, LLC (Austin, TX).
Lentiviral transduction experiments
BTKWT or BTKCys481Ser expressing or control lentiviral vectors were transduced into MYD88-mutated WM and ABC DLBCL cells as previously described.1,3 The coding sequences for BTKWT or BTKCys481Ser were cloned into a lentiviral expression vector, pLVX-EF1α-IRES-Puro vector (Clontech Laboratories, Mountain View, CA), and expression was confirmed by Sanger sequencing as before.9 Following lentiviral transduction, stable cell lines were selected by culture with 0.5 to 1.0 μg/mL puromycin. Protein expression levels for BTKWT or BTKCys481Ser transduced cells were confirmed by immunoblotting for BTK. BTKWT or BTKCys481Ser expressing WM and ABC DLBCL cell lines were transduced to also express GFP for mixed coculture study using pLenti-II-CMV-Luc-IRES-GFP lentiviral vector (Applied Biological Materials Inc, Richmond, BC, Canada).
Signaling studies
Immunoblotting for BTK and its downstream proteins was performed using antibodies for BTK-pY223 (Abcam, Cambridge MA); BTK (Santa Cruz Biotechnology, Dallas, TX); PLCγ2-pY759, PLCγ2, ERK1/2-pT202/pY204, ERK1/2, p90RSK-pT359, p90RSK, AKT-pT473, AKT, IkBα-pS32, IkBα (Cell Signaling Technologies, Danvers, MA). GAPDH antibody (Santa Cruz Biotechnology) was used for loading control. BTKWT or BTKCys481Ser expressing WM and ABC DLBCL cells were treated for 2 hours with either dimethyl sulfoxide (DMSO), or 0.1 to 0.5 µM of ibrutinib, ulixertinib, GDC-0994, or a combination of either ibrutinib with ulixertinib or GDC-0994 prior to immunoblotting.
Cytotoxicity studies
The CellTiter-Glo Luminescent cell viability assay (Promega, Madison WI) was used to assess the dose-response of inhibitors alone or in combination.1,3 Cells were seeded into 384- or 96-well plates with the EL406 Combination Washer Dispenser (BioTek Instruments, Inc), and inhibitors were injected into culture media with the JANUS Automated Workstation (PerkinElmer Inc, Waltham, MA). Cells were incubated with inhibitors for 72 hours at 37°C. Luminescent measurements to assess cell viability were performed using the 2104 Envision Multilabel Reader (PerkinElmer Inc). Drug interactions were assessed by CalcuSyn 2.0 software (Biosoft, Cambridge UK) based on the method of Chou.12 Cell survival was assessed following the treatment with inhibitors using Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining with the Apoptosis Detection Kit I (BD Pharmingen, San Jose, CA). In coculture studies of green fluorescent protein (GFP) positive BTKWT or BTKCys481Ser transduced cells with their nontransduced BTKWT counterpart cells, survival analysis was performed on GFP-negative population with Annexin V-APC staining.
Ibrutinib washout experiment
Washout experiments were also performed to clarify if differences in survival and cytokine release of interleukin-6 (IL-6) and IL-10 were related to non-BTK covalent ibrutinib interactions. For these experiments, vector only, BTKWT or BTKCys481Ser transduced MYD88-mutated WM, and ABC DLBCL cells were treated with or without ibrutinib for 6 hours, then washed twice, and media replaced without drug. Cell viabilities were assessed at 72 hours as described above.
Coculture experiments
For coculture experiments, BTKWT or BTKCys481Ser expressing GFP+ BCWM.1 or TMD8 cells were cultured with ibrutinib (0.5-1.0 μM) for 6 hours. Nontransduced BTKWT GFP-negative BCWM.1 or TMD8 cells were then added to wells and cocultured for 24 to 48 hours in the absence or presence of ibrutinib (0.5-1.0 μM) before performing apoptosis analysis. For Transwell coculture experiments, BTKWT or BTKCys481Ser expressing BCWM.1 or TMD8 cells were pretreated with serially diluted ibrutinib in the upper chambers of 0.4μm filtered 96-well plates for 6 hours. Nontransduced BTKWT BCWM.1 or TMD8 cells were then seeded into the lower chambers. Fresh ibrutinib was added to maintain the same concentration as the upper chamber, and Transwells were incubated for 72 hours. Nontransduced cells in the lower compartment were then assessed for viability using the CellTiter-Glo assay. For anti-IL-6 and/or anti–IL-10 blocking experiments, 10 µg/mL of neutralizing antibodies (R&D Systems, Inc) were added to nontransduced cells in the lower Transwell chambers. Because HA is a major extracellular matrix component and a keystone molecule that supports cytokine effects,13-16 to mimic the impact of microenvironment on the malignant cell survival, we performed the above coculture experiments in the presence of 10 µg/mL of HA. For cytokine rescue experiments, 10 ng/mL CXCL-13, IL-6, IL-10, IL-12, tumor necrosis factor-α (TNF-α), TNF-β, and 100 ng/mL macrophage inflammatory protein-1α (MIP-1α), and RANTES (R&D Systems, Inc, Minneapolis, MN) were added to the cell culture with or without 10 µg/mL HA. HA alone had no impact on growth or survival of BCWM.1 and TMD8 cells alone or in the presence of ibrutinib (data not shown).
Multiplex cytokine and ELISA assays
BTKWT or BTKCys481Ser expressing BCWM.1 or TMD8 cells were treated with vehicle control, ibrutinib (0.02-0.25 μM), ulixertinib (0.5 μM), or in combination for 36 hours at 37°C before the supernatants were harvested, aliquoted, and stored at −80°C. Sample aliquots were thawed on ice before addition to a customized multiplex plate (Invitrogen) containing 21 analytes (APRIL, BAFF, CD27, CXCL13, Eotaxin, IL-10, IL-12p40, IL-17AF, IL-1RA, IL-2R, IL-4, IL-6, MCP-1, MIF, MIP-1a, MIP-1b, MIP-3a, SDF-1a, TNF-α, TNF-β, sRANKL). Enzyme-linked immunosorbent assay (ELISA) was separately performed for RANTES (R&D Systems). For determination of IL-6 and IL-10 cytokine levels in WM patient sera and cell culture supernatants following ibrutinib washout experiments, ELISA assays (R&D Systems) were performed according to manufacturer’s instructions. Sera were stored at −80°C until analysis. The multiplex plate was read on a Bio-Rad Bio-Plex 3D system, and samples were analyzed using xPONENT software. ELISA plates were read on a SpectraMaxM3 Plate Reader (Molecular Devices, Sunnyvale, CA).
Patient samples
Sera were obtained from relapsed/refractory WM patients who received single-agent ibrutinib, and for whom tumor BTK mutation status was established at final serum sampling as previously reported. Three patients in these studies were identified as having BTKCys481 mutations at time of progression, and 6 randomly chosen patients who remained in response were established as having BTKWT disease following last serum sampling. Use of patient samples was approved by the Dana Farber/Harvard Cancer Center Institutional Review Board.
Quantitative polymerase chain reaction
Total RNA was isolated using AllPrep DNA/RNA Mini Kit (QIAGEN), and complementary DNA was synthesized by SuperScript III First-Strand Synthesis SuperMix (Life Technologies). Quantitative detection of messenger RNA (mRNA) levels for IL-6 and IL-10 was performed using TaqMan Gene Expression Assays with TaqMan Gene Expression Master Mix per manufacturer’s instructions using an ABI Prism 7500 Sequence Detection System (Applied Biosystems).
Statistical analysis
The statistical significance of differences was analyzed using 1-way analysis of variance with Tukey's multiple comparisons test by Prism software. Differences were considered significant when P ≤ .05.
Results
BTKCys481Ser mutation promotes ibrutinib resistance and persistent activation of BTK-PLCγ2-ERK1/2 signaling in MYD88-mutated WM and ABC DLBCL cells
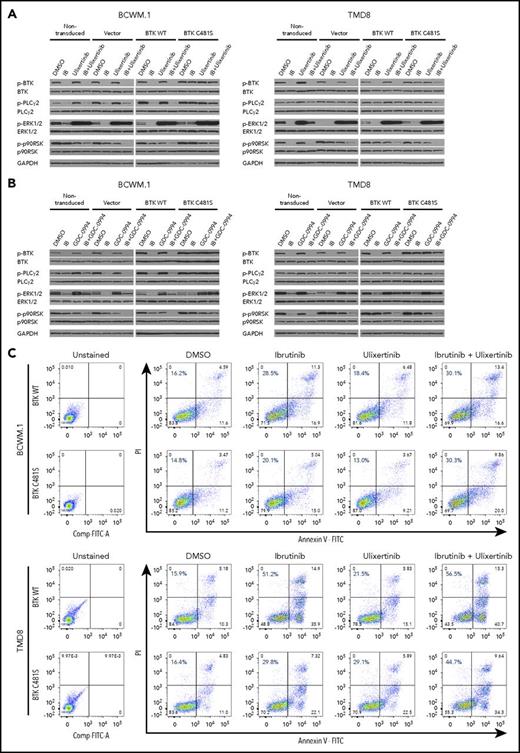
In our previous work, we identified BTKCys481Ser as the most common BTKCys481 mutation in MYD88-mutated WM patients with acquired ibrutinib resistance.9 To explore the functional impact of the BTKCys481Ser mutation on ibrutinib activity, MYD88-mutated WM and ABC DLBCL cells were transduced with either lentiviral vector alone or lentiviral vectors expressing BTKWT or BTKCys481Ser. The transduction of BTKCys481Ser promoted ibrutinib resistance in all MYD88-mutated WM and ABC DLBCL cells with a 1 to 3 log-fold increase in half maximal effective concentration (EC50) vs vector only or BTKWT transduced cells (Figure 1A). Similar protein expression levels for BTKWT or BTKCys481Ser were confirmed by immunoblotting in all MYD88-mutated cells (Figure 1B). Furthermore, to clarify that the protective effects of BTKCys481Ser mutation were likely not a consequence of off-target drug interactions, we performed washout experiments. These studies demonstrated more pronounced protective effects in BTKCys481Ser vs BTKWT or vector only expressing MYD88-mutated WM and ABC DLBCL cells when compared with findings with continuous ibrutinib treatment supporting that loss of on target binding likely accounted for differences in survival (Figure 1C). In contrast to vector only or BTKWT transduced MYD88-mutated cells, all BTKCys481Ser expressing MYD88-mutated cells showed persistent activation of PLCγ2, which is immediately downstream of BTK, as well as ERK1/2 and its immediate downstream kinase, p90RSK, following treatment with ibrutinib (Figure 1D). Whereas BTK, PLCγ2, and ERK1/2 remained activated in BTKCys481Ser-expressing cells, IkBa, AKT, and p38-MAPK were similarly inhibited in vector only, BTKWT and BTKCys481Ser-expressing cells following ibrutinib treatment (data not shown). Total protein levels for these proteins remained unchanged, and GADPH levels confirmed similar protein loading.
BTKCys481Sermutation promotes ibrutinib resistance and persistent activation of BTK and ERK1/2 signaling in MYD88-mutated WM and ABC DLBCL cells. MYD88-mutated WM (BCWM.1, MWCL-1) and ABC DLBCL (TMD8, HBL1) cells were transduced with vector alone, or vectors expressing either BTKWT or BTKCys481Ser (BTKC481S). Dose-dependent survival determined by CellTiter-Glo Luminescent cell viability assay for vector only, BTKWT, or BTKC481S transduced MYD88-mutated WM and ABC DLBCL cells following treatment with ibrutinib for 72 hours. Each data point was calculated relative to DMSO control. The x-axis shows ibrutinib log concentration (A). Similar levels of total BTK protein expression were obtained in MYD88-mutated WM and ABC DLBCL cells following transduction with either BTKWT or BTKC481S vectors (B). Cell viabilities at 72 hours for vector only, BTKWT, or BTKCys481Ser transduced MYD88-mutated WM and ABC DLBCL cells following treatment with ibrutinib for 6 hours and then washout and replacement of media without ibrutinib. Each data point was calculated relative to DMSO control. The x-axis shows ibrutinib log concentration (C). Immunoblotting studies depicting impact of vector only, BTKWT, or BTKC481S expression in MYD88-mutated WM and ABC DLBCL cells on BTK, PLCγ2, ERK1/2, and p90RSK signaling following treatment with vehicle control (DMSO) or ibrutinib (0.5, 0.1 μM) for 2 hours. GAPDH used as protein loading control (D). RLU, relative luminescence unit.
BTKCys481Sermutation promotes ibrutinib resistance and persistent activation of BTK and ERK1/2 signaling in MYD88-mutated WM and ABC DLBCL cells. MYD88-mutated WM (BCWM.1, MWCL-1) and ABC DLBCL (TMD8, HBL1) cells were transduced with vector alone, or vectors expressing either BTKWT or BTKCys481Ser (BTKC481S). Dose-dependent survival determined by CellTiter-Glo Luminescent cell viability assay for vector only, BTKWT, or BTKC481S transduced MYD88-mutated WM and ABC DLBCL cells following treatment with ibrutinib for 72 hours. Each data point was calculated relative to DMSO control. The x-axis shows ibrutinib log concentration (A). Similar levels of total BTK protein expression were obtained in MYD88-mutated WM and ABC DLBCL cells following transduction with either BTKWT or BTKC481S vectors (B). Cell viabilities at 72 hours for vector only, BTKWT, or BTKCys481Ser transduced MYD88-mutated WM and ABC DLBCL cells following treatment with ibrutinib for 6 hours and then washout and replacement of media without ibrutinib. Each data point was calculated relative to DMSO control. The x-axis shows ibrutinib log concentration (C). Immunoblotting studies depicting impact of vector only, BTKWT, or BTKC481S expression in MYD88-mutated WM and ABC DLBCL cells on BTK, PLCγ2, ERK1/2, and p90RSK signaling following treatment with vehicle control (DMSO) or ibrutinib (0.5, 0.1 μM) for 2 hours. GAPDH used as protein loading control (D). RLU, relative luminescence unit.
ERK1/2 activation promotes prosurvival signaling in ibrutinib-resistant MYD88-mutated WM and ABC DLBCL cells
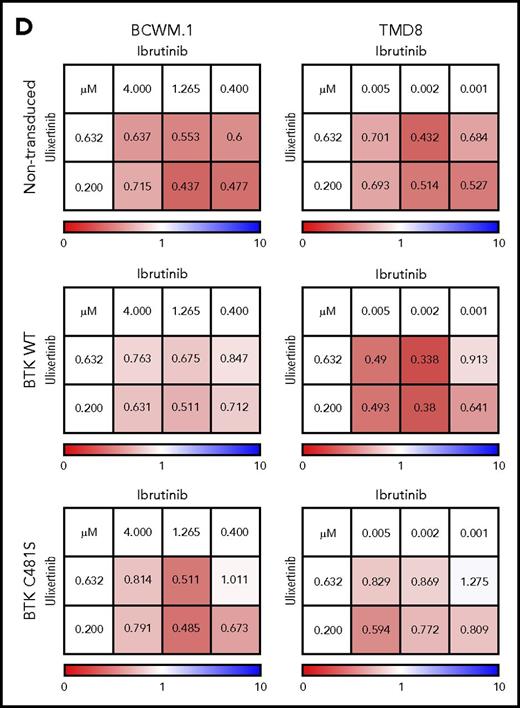
Given the persistent activation of ERK1/2 in BTKCys481Ser expressing MYD88-mutated WM and ABC DLBCL cells following ibrutinib treatment, we next sought to determine the importance of ERK1/2 in mediating ibrutinib resistance. We used 2 highly selective ERK1/2 inhibitors (ulixertinib, GDC-0994), both under active clinical investigation, to clarify the importance of ERK1/2 signaling in mediating ibrutinib resistance in BTKCys481Ser-expressing cells. Ulixertinib is an allosteric inhibitor and prompts hyperphosphorylation of ERK1/2 while blocking its activity.17 As such, phospho-p90RSK, an immediate downstream kinase of ERK1/2, is used to assess ERK1/2 activity (Figure 2A-B). Following ulixertinib or GDC-0994 treatment, phospho-p90RSK was reduced in nontransduced, as well as vector only, BTKWT, and BTKCys481Ser expressing BCWM.1 WM and TMD8 ABC DLBCL cells. More robust reduction in ERK1/2 was also observed in nontransduced, as well as vector only, BTKWT, and BTKCys481Ser expressing BCWM.1 WM and TMD8 ABC DLBCL cells treated with ulixertinib or GDC-0994 and ibrutinib. These findings show that combining an ERK inhibitor with ibrutinib overcomes ERK1/2 reactivation in the presence of ibrutinib, and that such suppression (as confirmed by downstream phospho-p90RSK) is greater with ibrutinib and ERK inhibitors combined, even in cells bearing the BTKCys481 mutation (Figure 2A-B). Importantly, BCWM.1 WM and TMD8 ABC DLBCL cells treated with the combination of ulixertinib with ibrutinib showed higher levels of apoptosis in BTKCys481Ser expressing BCWM.1 WM and TMD8 ABC DLBCL cells vs either drug alone, with similar levels of apoptosis to their BTKWT counterpart cells (Figure 2C). Moreover, the combination of ulixertinib with ibrutinib produced higher levels of tumor cell killing vs either agent alone in both BTKWT and BTKCys481Ser expressing BCWM.1 WM and TMD8 ABC DLBCL cells by CellTiter-Glo assay and showed synergistic interactions with CI values <1.0 at most pharmacologically relevant concentrations (Figure 2D). Similar findings were also observed with GDC-0994 (data not shown). These findings demonstrate that inhibition of ERK1/2 can overcome ibrutinib resistance in MYD88-mutated WM and ABC DLBCL cells.
ERK1/2 activation promotes prosurvival signaling in ibrutinib-resistant MYD88-mutated WM and ABC DLBCL cells. Immunoblotting studies depicting BTK, PLCγ2, ERK1/2, and p90RSK signaling in nontransduced, vector only, BTKWT, or BTKCys481Ser (BTKC481S) MYD88-mutated WM (BCWM.1) and ABC DLBCL (TMD8) cells following treatment with vehicle control, ibrutinib, or ERK1/2 inhibitors (ulixerinib, GDC-0994) alone or with ibrutinib for 2 hours. GAPDH was used as protein loading control. Data for ulixertinib are shown in panel A and for GDC-0994 in panel B. Cellular apoptosis determined by Annexin V-FITC and PI staining for BTKWT or BTKC481S expressing WM and ABC DLBCL cells following treatment with vehicle control, ibrutinib (1.0 µM), or ulixertinib (2.0 µM) alone or in combination. Percent of cells staining for both Annexin V and PI is depicted in each panel. Results are representative from studies performed in triplicate (C). Dose-dependent survival determined by CellTiter-Glo Luminescent cell viability assay for vector only, BTKWT, or BTKC481S transduced MYD88-mutated WM and ABC DLBCL cells following treatment with ibrutinib and ulixertinib at pharmacologically relevant dosimetry for 72 hours. Synergism was assessed by combination index (CI) analysis, with the heat maps depicting the CI values at varying dosimetry for ibrutinib and ulixertinib. CI values <1 denote synergistic interactions (D). IB, ibrutinib.
ERK1/2 activation promotes prosurvival signaling in ibrutinib-resistant MYD88-mutated WM and ABC DLBCL cells. Immunoblotting studies depicting BTK, PLCγ2, ERK1/2, and p90RSK signaling in nontransduced, vector only, BTKWT, or BTKCys481Ser (BTKC481S) MYD88-mutated WM (BCWM.1) and ABC DLBCL (TMD8) cells following treatment with vehicle control, ibrutinib, or ERK1/2 inhibitors (ulixerinib, GDC-0994) alone or with ibrutinib for 2 hours. GAPDH was used as protein loading control. Data for ulixertinib are shown in panel A and for GDC-0994 in panel B. Cellular apoptosis determined by Annexin V-FITC and PI staining for BTKWT or BTKC481S expressing WM and ABC DLBCL cells following treatment with vehicle control, ibrutinib (1.0 µM), or ulixertinib (2.0 µM) alone or in combination. Percent of cells staining for both Annexin V and PI is depicted in each panel. Results are representative from studies performed in triplicate (C). Dose-dependent survival determined by CellTiter-Glo Luminescent cell viability assay for vector only, BTKWT, or BTKC481S transduced MYD88-mutated WM and ABC DLBCL cells following treatment with ibrutinib and ulixertinib at pharmacologically relevant dosimetry for 72 hours. Synergism was assessed by combination index (CI) analysis, with the heat maps depicting the CI values at varying dosimetry for ibrutinib and ulixertinib. CI values <1 denote synergistic interactions (D). IB, ibrutinib.
BTKCys481Ser expressing WM and ABC DLBCL cells provide a protective effect to BTKWT malignant cells through paracrine mediated prosurvival signaling
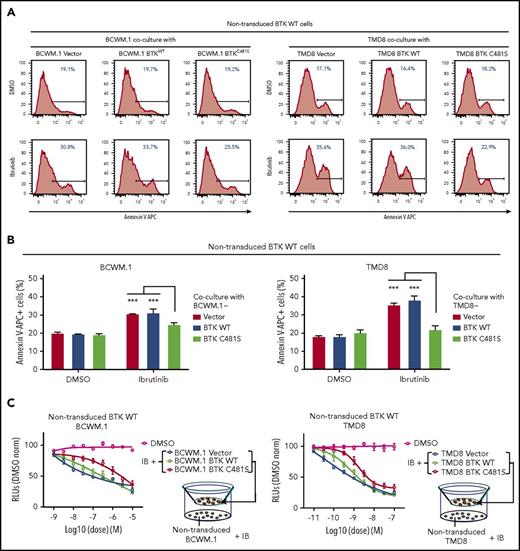
Acquired BTKCys481 mutations following ibrutinib have been reported in CLL, MCL, and WM; however, in most cases the mutation burden remains low despite clinical disease progression.6,9,10 To understand how BTKCys481 mutations might impact neighboring BTKWT malignant cells, we first performed mixed coculture experiments using nontransduced GFP− BCWM.1 WM or TMD8 ABC DLBCL cells with their GFP+ BTKWT or BTKC481S cells models. While under Ibrutinib treatment, nontransduced GFP− BCWM.1 or TMD8 cells that were cocultured with their respective GFP+ BTKCys481Ser expressing showed decreased apoptosis, vs coculture with their BTKWT counterparts (Figure 3A-B). Given the protective effect that BTKCys481Ser-expressing cells provide to nontransduced BTKWT MYD88-mutated malignant cells, we next sought to clarify if such a protective effect was mediated by direct cell-cell contact or through a paracrine mechanism without direct cell-cell contact. To address this question, we used a Transwell coculture system that separated nontransduced BTKWT BCWM.1 or TMD8 cells from direct contact with their corresponding vector only, BTKWT, or BTKCys481Ser transduced counterparts in the presence of ibrutinib. These experiments showed that BTKCys481Ser-expressing cells conferred a protective effect on their counterpart nontransduced BTKWT cells vs vector only, or BTKWT transduced cells with an ∼1 log-fold increase in EC50 (Figure 3C). The findings support that BTKCys481Ser expressing MYD88-mutated WM and ABC DLBCL cells can protect their BTKWT counterparts against ibrutinib through a paracrine mechanism.
BTKCys481Serexpressing WM and ABC DLBCL cells confer a protective effect to BTKWTmalignant cells through paracrine mediated prosurvival signaling. For coculture experiments, BTKWT or BTKCys481Ser expressing GFP+ BCWM.1 or TMD8 cells were cultured with ibrutinib (0.5-1.0 µM) for 6 hours. Nontransduced BTKWT GFP− BCWM.1 or TMD8 cells were then added to wells, and cocultured for 24 to 48 hours in the absence or presence of ibrutinib (0.5-1.0 µM) Annexin V–APC staining was used to assess apoptotic changes on nontransduced GFP− cells. Experiments were done in triplicate, and the representative plots with the percentage of apoptotic cells are shown in panel A. The statistics for all 3 experiments are displayed in panel B. For Transwell coculture experiments, BTKWT or BTKCys481Ser expressing BCWM.1 WM or TMD8 ABC DLBCL cells were pretreated with vehicle control or ibrutinib at indicated concentrations in the upper chambers of 0.4-μm filtered plates for 6 hours. Nontransduced counterparts were then cultured for 72 hours with added ibrutinib at the indicated concentrations in the lower compartments and assessed for viability using the CellTiter-Glo assay. The single (DMSO) line shows viability of nontransduced cells with drug vehicle control alone. The x-axis shows ibrutinib log concentration (C). ***P < .001.
BTKCys481Serexpressing WM and ABC DLBCL cells confer a protective effect to BTKWTmalignant cells through paracrine mediated prosurvival signaling. For coculture experiments, BTKWT or BTKCys481Ser expressing GFP+ BCWM.1 or TMD8 cells were cultured with ibrutinib (0.5-1.0 µM) for 6 hours. Nontransduced BTKWT GFP− BCWM.1 or TMD8 cells were then added to wells, and cocultured for 24 to 48 hours in the absence or presence of ibrutinib (0.5-1.0 µM) Annexin V–APC staining was used to assess apoptotic changes on nontransduced GFP− cells. Experiments were done in triplicate, and the representative plots with the percentage of apoptotic cells are shown in panel A. The statistics for all 3 experiments are displayed in panel B. For Transwell coculture experiments, BTKWT or BTKCys481Ser expressing BCWM.1 WM or TMD8 ABC DLBCL cells were pretreated with vehicle control or ibrutinib at indicated concentrations in the upper chambers of 0.4-μm filtered plates for 6 hours. Nontransduced counterparts were then cultured for 72 hours with added ibrutinib at the indicated concentrations in the lower compartments and assessed for viability using the CellTiter-Glo assay. The single (DMSO) line shows viability of nontransduced cells with drug vehicle control alone. The x-axis shows ibrutinib log concentration (C). ***P < .001.
ERK1/2 activation in BTKCys481Ser expressing MYD88-mutated cells triggers prosurvival and inflammatory cytokine release in ibrutinib treated cells
Because the above studies showed that BTKCys481Ser-expressing cells provided a protective effect on neighboring BTKWT malignant cells through a paracrine mechanism, we next sought to identify cytokine(s) that could possibly afford this protective effect. Moreover, because ERK1/2 remains activated in BTKCys481Ser-expressing cells under ibrutinib treatment, and ERK1/2 is known to trigger prosurvival and inflammatory cytokine release,18-20 we also sought to delineate its role in paracrine-mediated ibrutinib resistance. We performed a multiplex assay to detect cytokines released by either BTKWT or BTKCys481Ser expressing BCWM.1 WM or TMD8 ABC DLBCL cells following treatment with vehicle control, ibrutinib, or ulixertinib alone, or in combination. Cytokine release following drug treatment of 36 hours is shown in Figure 4. Higher levels of cytokines, many with a prosurvival and/or inflammatory role, were observed in ibrutinib-treated BTKCys481Ser vs BTKWT-expressing cells and included CXCL-13, IL-6, IL-10, IL-12, MIP-1α, MIP-3α, RANTES, TNF-α, TNF-β (in BCWM.1 WM cells), and CXCL-13, IL-6, IL-10, IL-12, MIP-1α, MIP-1β, TNF-α, TNF-β (in TMD8 ABC DLBCL) cells (Figure 4). Importantly, the release for most of these cytokines was reduced by ulixertinib, particularly in combination with ibrutinib. The absolute levels of cytokines produced by BTKWT or BTKCys481Ser-expressing cells following vehicle control or drug treatment are shown in supplemental Figure 1, available on the Blood Web site.
ERK1/2 activation in BTKCys481Serexpressing MYD88-mutated cells triggers prosurvival and inflammatory cytokine release in ibrutinib-treated cells. Cytokine production relative to untreated BTKWT or BTKC481S expressing BCWM.1 WM (A) and TMD8 ABC DLBCL (B) cells is shown following treatment with either vehicle control (DMSO), ibrutinib, ulixertinib, or combination of ibrutinib and ulixertinib. Fold changes are indicated by the color scale, and dark red boxes indicate scales above the highest range (≥2.39).
ERK1/2 activation in BTKCys481Serexpressing MYD88-mutated cells triggers prosurvival and inflammatory cytokine release in ibrutinib-treated cells. Cytokine production relative to untreated BTKWT or BTKC481S expressing BCWM.1 WM (A) and TMD8 ABC DLBCL (B) cells is shown following treatment with either vehicle control (DMSO), ibrutinib, ulixertinib, or combination of ibrutinib and ulixertinib. Fold changes are indicated by the color scale, and dark red boxes indicate scales above the highest range (≥2.39).
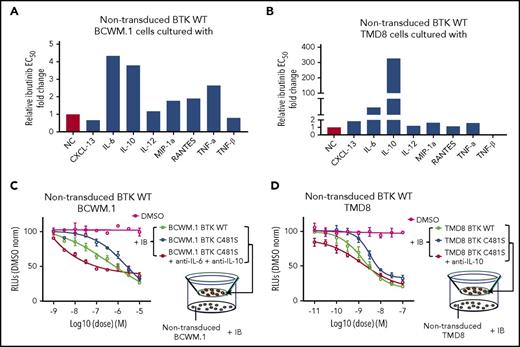
IL-6 and/or IL-10 production by BTKCys481Ser-expressing cells confer a protective effect on BTKWT MYD88-mutated WM and ABC DLBCL cells treated with ibrutinib
To delineate the cytokine(s) released by BTKCys481Ser cells that protected BTKWT against ibrutinib, we evaluated those cytokines that showed a significant reduction following ibrutinib in BTKWT but not BTKCys481Ser-expressing cells. CXCL-13, IL-6, IL-10, IL-12, MIP-1α, RANTES, TNF-α, and TNF-β were therefore evaluated on ibrutinib-treated nontransduced BTKWT BCWM.1 and TMD8 cells. To mimic the impact of microenvironment on the malignant cell survival, we performed the experiments with or without 10 µg/mL HA, a major extracellular matrix component and a keystone molecule that supports cytokine effects.13-16 HA itself had no impact on growth or survival of BCWM.1 and TMD8 cells alone or in the presence of ibrutinib, but enhanced IL-6 protective effects in BCWM.1 cells (data not shown). In BCWM.1 cells, the addition of IL-6 or IL-10 protected against ibrutinib-related cytotoxicity with about a fourfold increase in EC50; MIP-1α, RANTES, TNF-α also showed a protective effect against ibrutinib, with more moderate shifts in EC50 following ibrutinib treatment (Figure 5A). In TMD8 cells, IL-10 provided a highly robust rescue effect against ibrutinib-related cytotoxicity, with EC50 increased by 300-fold. Relative to IL-10, IL-6 provided a more modest (ie, fivefold) increase in EC50 against ibrutinib in TMD8 cells (Figure 5B). Last, both IL-6 and IL-10 were decreased in washout studies with ibrutinib in BTKWT but not BTKCys481Ser expressing BCWM.1 and TMD8 cells informing that such differences were likely not a consequence of off-target drug interactions (supplemental Figure 2).
IL-6 and/or IL-10 production by BTKCys481Ser-expressing cells confer a protective effect on BTKWTMYD88-mutated WM and ABC DLBCL cells treated with ibrutinib. Nontransduced BTKWT BCWM.1 (A) and TMD8 (B) cells were treated with a serial diluted ibrutinib in the presence or absence of cytokines (10 ng/mL CXCL-13, IL-6, IL-10, IL-12, TNF-α, TNF-β and 100 ng/mL MIP-1α, RANTES), and the relative fold change to no cytokine control in EC50 for ibrutinib is shown. For Transwell coculture experiments, BTKWT or BTKCys481Ser expressing BCWM.1 (C) or TMD8 (D) cells were pretreated with vehicle control or a serial diluted ibrutinib at indicated concentrations in the upper chambers of 0.4-μm filtered plates for 6 hours. Nontransduced counterparts were then added to lower chambers with or without blocking antibodies to IL-6 and IL-10 (10 μg/mL) for BCWM.1 WM cells, and to IL-10 alone (10 μg/mL) for TMD8 ABC DLBCL cells. Ibrutinib was then added to the lower chambers to keep the same concentrations and incubated for 72 hours. Viability was assessed by the CellTiter-Glo assay. The single (DMSO) line shows viability of nontransduced cells with drug vehicle control alone. The x-axis shows ibrutinib log concentration.
IL-6 and/or IL-10 production by BTKCys481Ser-expressing cells confer a protective effect on BTKWTMYD88-mutated WM and ABC DLBCL cells treated with ibrutinib. Nontransduced BTKWT BCWM.1 (A) and TMD8 (B) cells were treated with a serial diluted ibrutinib in the presence or absence of cytokines (10 ng/mL CXCL-13, IL-6, IL-10, IL-12, TNF-α, TNF-β and 100 ng/mL MIP-1α, RANTES), and the relative fold change to no cytokine control in EC50 for ibrutinib is shown. For Transwell coculture experiments, BTKWT or BTKCys481Ser expressing BCWM.1 (C) or TMD8 (D) cells were pretreated with vehicle control or a serial diluted ibrutinib at indicated concentrations in the upper chambers of 0.4-μm filtered plates for 6 hours. Nontransduced counterparts were then added to lower chambers with or without blocking antibodies to IL-6 and IL-10 (10 μg/mL) for BCWM.1 WM cells, and to IL-10 alone (10 μg/mL) for TMD8 ABC DLBCL cells. Ibrutinib was then added to the lower chambers to keep the same concentrations and incubated for 72 hours. Viability was assessed by the CellTiter-Glo assay. The single (DMSO) line shows viability of nontransduced cells with drug vehicle control alone. The x-axis shows ibrutinib log concentration.
To further clarify whether the protective effect on nontransduced BTKWT malignant cells by BTKCys481Ser-expressing cells was due to their release of IL-6 and/or IL-10, we next performed cytokine blocking experiments. Because a rescue effect was similarly prompted by IL-6 and IL-10 in BCWM.1 WM cells, and robustly by IL-10 alone in TMD8 cells, anti–IL-6 and anti–IL-10 blocking antibodies were added to Transwell coculture with nontransduced BTKWT BCWM.1/BTKCys481Ser BCWM.1 cells pair, and only anti–IL-10 blocking antibodies to Transwell coculture with nontransduced BTKWT TMD8/BTKCys481Ser TMD8 cells pair. Importantly, the addition of IL-6 and IL-10 blocking antibodies to Transwell cocultures with nontransduced BTKWT BCWM.1/BTKCys481Ser BCWM.1 cells pair, and anti–IL-10 blocking antibodies with nontransduced BTKWT TMD8/BTKCys481Ser TMD8 cells pair abrogated the rescue effects conferred by BTKCys481Ser cells (Figure 5C-D).
Increased IL-6 and IL-10 serum levels accompanies disease progression on ibrutinib in WM patients with acquired BTKCys481Ser mutations
Serial serum samples obtained from relapsed/refractory WM patients who received single-agent ibrutinib, and for whom tumor BTK mutation status was established at final serum sampling, were evaluated for IL-6 and IL-10. Their clinical course and methods for determining their BTK mutation status were previously reported. Three patients in these studies were identified as having BTKCys481 mutations at time of progression, and 6 randomly chosen patients who remained in response were established as having BTKWT disease following last serum sampling. All patients achieved a major response as their best response. The findings from these studies showed that IL-6 and IL-10 levels declined in all patients at time of response and rebounded at time of progression in those patients in whom BTKCys481 mutations were detected. Among patients who continued to respond, IL-6 and IL-10 serum levels remained decreased (Figure 6A-B).
IL-6 and IL-10 cytokine levels in MYD88-mutated WM patient sera following ibrutinib treatment. The (A) IL-6 and (B) IL-10 cytokine levels were detected by ELISA in MYD88-mutated WM patients with acquired BTKCys481Ser mutation and progressed on ibrutinib as well as patients with BTKWT and continued response to ibrutinib. Blue arrows indicate ibrutinib start date. Red arrows indicate date at BTKCys481 mutations were first noted in patients with disease progression, whereas green arrows show determination point for BTK wild-type status for those patients with ongoing response. All patients achieved a major response, and their clinical course and determination of BTK mutation status were previously reported.8
IL-6 and IL-10 cytokine levels in MYD88-mutated WM patient sera following ibrutinib treatment. The (A) IL-6 and (B) IL-10 cytokine levels were detected by ELISA in MYD88-mutated WM patients with acquired BTKCys481Ser mutation and progressed on ibrutinib as well as patients with BTKWT and continued response to ibrutinib. Blue arrows indicate ibrutinib start date. Red arrows indicate date at BTKCys481 mutations were first noted in patients with disease progression, whereas green arrows show determination point for BTK wild-type status for those patients with ongoing response. All patients achieved a major response, and their clinical course and determination of BTK mutation status were previously reported.8
IL-6 and IL-10 are triggered by sustained ERK1/2 activation in ibrutinib-treated BTKCys481Ser-expressing cells and can be abrogated by ERK1/2 inhibitors
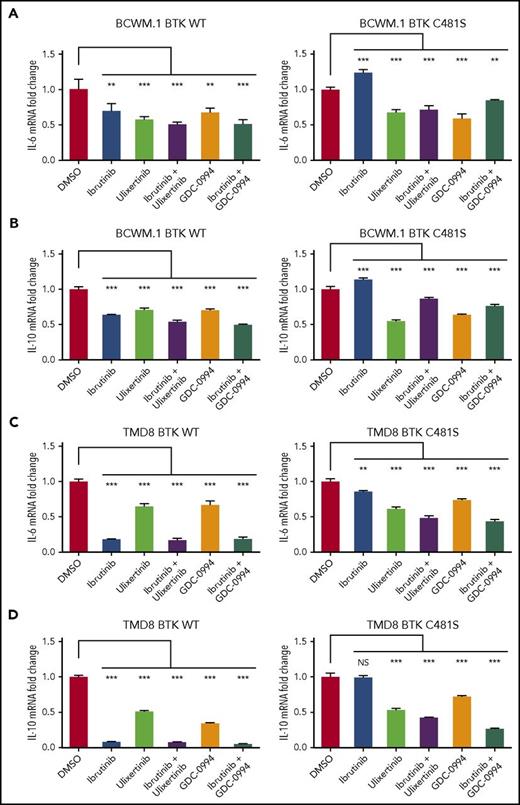
To delineate if the persistent production of IL-6 and IL-10 by BTKCys481Ser-expressing cells under ibrutinib treatment is due to sustained ERK1/2 activation, we treated BTKWT and BTKCys481Ser expressing BCWM.1 WM or TMD8 ABC DLBCL cells with ibrutinib, ulixertinib, or GDC-0994 alone or in combination, and assessed IL-6 and IL-10 mRNA expression. Compared with their BTKWT transduced counterparts, BTKCys481Ser-expressing cells showed significantly higher levels of IL-6 and IL-10 transcription under treatment with ibrutinib (Figure 7). Moreover, IL-6 and IL-10 transcription were both markedly reduced in BCWM.1 WM and TMD8 ABC DLBCL cells following treatment with the ERK1/2 inhibitors ulixertinib or GDC-0994 (Figure 7).
IL-6 and IL-10 are triggered by sustained ERK1/2 activation in ibrutinib-treated BTKCys481Serexpressing cells and can be abrogated by ERK1/2 inhibitors. BTKWT and BTKCys481Ser expressing BCWM.1 WM or TMD8 ABC DLBCL cells were treated with ibrutinib, ulixertinib, or GDC-0994 alone or in combination and assessed for IL-6 and IL-10 mRNA expression by TaqMan Gene Expression Assays. BTKCys481Ser expressing BCWM.1 WM and TMD8 ABC DLBCL cells showed sustained IL-6 (A,C) and IL-10 (B,D) transcription following ibrutinib treatment that was abrogated by the ERK1/2 inhibitors ulixertinib and GDC-0994. ***P < .001; **P < .005; NS, not significant (P ≥ .05).
IL-6 and IL-10 are triggered by sustained ERK1/2 activation in ibrutinib-treated BTKCys481Serexpressing cells and can be abrogated by ERK1/2 inhibitors. BTKWT and BTKCys481Ser expressing BCWM.1 WM or TMD8 ABC DLBCL cells were treated with ibrutinib, ulixertinib, or GDC-0994 alone or in combination and assessed for IL-6 and IL-10 mRNA expression by TaqMan Gene Expression Assays. BTKCys481Ser expressing BCWM.1 WM and TMD8 ABC DLBCL cells showed sustained IL-6 (A,C) and IL-10 (B,D) transcription following ibrutinib treatment that was abrogated by the ERK1/2 inhibitors ulixertinib and GDC-0994. ***P < .001; **P < .005; NS, not significant (P ≥ .05).
Discussion
In this study, we sought to delineate the pathway(s) responsible for acquired ibrutinib resistance related to BTKCys481 mutations in MYD88-mutated B-cell malignancies. BTKCys481 mutations that abolish BTK-ibrutinib binding are the most commonly acquired mutations observed in CLL, MCL, and WM patients progressing on ibrutinib, particularly the BTKCys481Ser somatic variant.7-9 BTKCys481 mutations may be detected up to a year before clinical progression,9,11,21 and multiple BTKCys481 mutations can occur within individual patients.9,21 However, in most progressing patients, BTKCys481 variants are found in a minority of tumor clones,9,10,22 raising the possibility that non-BTKCys481 mutations also coexist that contribute to disease progression, or that BTKCys481 mutated cells are able to confer resistance to non-BTK mutated malignant cells. Although the former continues to be sought, few coincidental mutations to BTKCys481 such as PLCγ2 and CARD11 have been identified by targeted and whole exome sequencing. For this reason, we sought to clarify if BTK mutated cells could promote resistance of neighboring non-BTK mutated malignant cells.
Our findings showed that expression of the BTKCys481Ser mutation resulted in uniform loss of BTK pathway suppression in the presence of ibrutinib, with activation of downstream PLCγ2 signaling observed in all MYD88-mutated WM and ABC DLBCL cells. The introduction of BTKCys481 showed higher levels of resistance to ibrutinib in ABC DLBCL vs WM cells and may reflect differences in noncovalent interactions with other prosurvival targets such as HCK.3 Importantly, sustained activation of ERK1/2 along with its immediate downstream kinase, p90RSK, uniformly occurred in all BTKCys481 mutated WM and ABC DLBCL despite ibrutinib treatment. In contrast, an ibrutinib suppressive effect on ERK1/2 signaling was present in ibrutinib-treated WM and ABC DLBCL cells transduced with vector only or BTKWT. Although both ERK1/2 and AKT are known to be transactivated by BTK,23,24 only sustained activation of ERK1/2 following ibrutinib treatment was observed in BTKCys481Ser MYD88-mutated WM and ABC DLBCL cells. The importance of sustained ERK1/2 signaling to ibrutinib resistance in MYD88-mutated WM and ABC DLBCL cells was demonstrated by cotreatment of BTKCys481Ser-expressing cells with ibrutinib and selective ERK1/2 inhibitors. The combined use of an ERK1/2 inhibitor with ibrutinib resulted in synergistic tumor cell killing in BTKCys481Ser-expressing cells vs either agent alone. The findings could reflect a continued role for ibrutinib against BTKCys481 mutated cells, because ibrutinib is known to suppress the activity of other kinases such as HCK that support growth and survival signaling in MYD88-mutated cells.3 Although these studies, to our knowledge, provide the first experimental evidence that sustained ERK1/2 activation accompanies mutated BTKCys481 signaling in ibrutinib-resistant cells, the reactivation of ERK1/2 was also observed in serially obtained CLL cells from a patient that initially responded to, and subsequently progressed on, ibrutinib after acquisition of a BTKCys481 mutation.25 Therefore, the relevance of ERK1/2 activation following acquisition of a BTKCys481 mutation may apply to non–MYD88-mutated B-cell diseases and warrant further studies. Other treatment options, including targeting BCL2 with venetoclax, and use of noncovalent inhibitors of BTK that rely on binding to non-BTKCys481 targets such as vecabrutinib (SNS-062), represent other strategies for overcoming BTKCys481-dependent ibrutinib resistance and are under investigation. A clinical trial examining venetoclax (NCT02677324) in previously treated WM patients, including those that progressed on ibrutinib, is underway in WM patients. A clinical trial with vecabrutinib in B-lymphoid malignancies is also underway (NCT03037645).
A novel finding from these studies was the recognition that BTKCys481 mutated cells could bestow a protective effect on BTKWT cells. BTKCys481 are typically subclonal, and these findings provide an explanation for how subclonal BTK mutated cells could promote widespread resistance against ibrutinib. A paracrine-mediated mechanism was surmised from results showing that a protective effect was conferred by both direct and indirect (via Transwell) coculture of BTKCys481Ser and BTKWT MYD88-mutated WM and ABC DLBCL cells. Given these findings, we sought to clarify if cytokine(s) prompted the protective effects afforded by BTKCys481 mutated cells. ERK1/2 signaling is triggered by BTK and is responsible for a wide range of inflammatory and prosurvival cytokine signaling. Using a customized multiplex cytokine assay system, we evaluated cytokines with a putative prosurvival role in MYD88-mutated B-cell diseases, and those blocked by ibrutinib in patients. The findings herein showed that loss of ibrutinib mediated suppression of many inflammatory and prosurvival cytokines accompanied expression of BTKCys481Ser and were directly related to sustained ERK1/2 activation. Use of ERK1/2 inhibitors abolished the release of most aberrantly expressed cytokines that we evaluated as well as the transcription and release of IL-6 and IL-10 that mediated the most impressive prosurvival effects against ibrutinib in MYD88-mutated cells. Both IL-6 and IL-10 are known triggers of strong prosurvival signaling, including JAK/STAT in MYD88-mutated WM and ABC DLBCL cells.26-30 Many of the other ERK1/2 triggered cytokines in BTKCys481Ser, such as RANTES, which themselves showed modest direct prosurvival effects on ibrutinib-treated MYD88-mutated cells, could act as triggers of microenvironmental IL-6 or IL-10 release.31 In previous work, we observed that both IL-6 and IL-10 serum levels decreased in WM patients responding to ibrutinib, and further to this work, we show in these studies their subsequent rebound in progressing patients with acquired BTKCys481 mutations consistent with the in vitro findings presented herein.32 Other cytokines may also contribute to disease resistance related to BTKCys481 mutations. Ahn et al21 observed rebound in CCL3 and CCL4 levels after initial decrease in CLL patients with acquired BTK Cys481 mutations that progressed on ibrutinib. Decreased CCL4 but not CCL3 was also observed by us in ibrutinib-responding WM patients, although changes in the former were less pronounced vs those observed in IL-6 and IL-10.32
Several ERK1/2 inhibitors are currently under clinical investigation17,33,34 and could be used with ibrutinib in patients with BTKCys481 mutations. Such a strategy would have the benefit of continuing ibrutinib to suppress BTKWT clones and non-BTK kinases, such as HCK within BTKCys481 mutated clones that promote MYD88 directed growth and survival, whereas the addition of an ERK1/2 inhibitor would suppress prosurvival signaling triggered by the BTKCys481 mutation, and its paracrine-mediated protective effects on BTKWT clones. Conceivably, intervention with an ERK1/2 inhibitor could also occur before clinical progression occurs on ibrutinib, because BTKCys481 mutations can often be detected up to a year beforehand. Studies to address the use of ERK1/2 inhibitors and optimal timing for drug intervention are needed to address these points. Last, the use of IL-6 and/or IL-10 blocking agents is also suggested by these studies as an alternative strategy to overcome ibrutinib resistance in patients with BTKCys481 mutations.
Because PLCγ2 activating mutations are also found in patients with acquired ibrutinib resistance, including those with and without MYD88-mutated disease,6,9,21,22,35,36 these findings may also be relevant because ERK1/2 activation is downstream of both BTK and PLCγ2. Further work to elucidate the importance of ERK1/2 signaling, and the potential use of ERK1/2 inhibitors to overcome PLCγ2 mutations, appears warranted.
In summary, our findings provide experimental evidence for the first time that an acquired BTKCys481 mutation drives ibrutinib resistance in MYD88-mutated WM and ABC DLBCL cells through sustained ERK1/2 activation that can confer a protective effect on BTKWT cells through a paracrine mechanism. Use of ERK1/2 inhibitors along with ibrutinib abolished the protective effects triggered by ERK1/2 activation. The findings provide a framework for investigation of ERK1/2 inhibitors to overcome ibrutinib-mediated resistance in MYD88-mutated diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the WM patients who provided samples for these studies.
The authors also gratefully acknowledge the generous support of the Peter Bing, the International Waldenstrom’s Macroglobulinemia Foundation, the Leukemia and Lymphoma Society, the Edward and Linda Nelson Fund for WM Research, the Kerry Robertson Fund for WM Research, and the Bauman Family Trust.
Authorship
Contribution: G.Y. and S.P.T. conceived and designed the experiments, and wrote the manuscript; G.Y., G.G.C., and Z.R.H. performed the data analysis; J.G.C. and X.L. performed transduction experiments, PCR-based studies, and immunoblotting; J.G.C. and G.Y. performed drug treatment and cell viability assessments; J.G.C. and M.M. performed the multiplex cytokine studies; L.X., N.T., M.G.D., M.L.G., and A.K. prepared samples and performed genotyping studies; J.W., S.J.B., and N.S.G. provided medicinal chemistry expertise; J.J.C., T.D., P.S., K.M., J.G., C.J.P., and S.P.T. provided patient care, obtained consent, and were responsible for sample collection.
Conflict-of-interest disclosure: S.P.T. and J.J.C. have received research funding, consulting fees, and/or honoraria from Pharmacyclics Inc and Janssen Oncology Inc. The remaining authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström's Macroglobulinemia, Dana Farber Cancer Institute, M548, 450 Brookline Ave, Boston, MA 02115; e-mail: steven_treon@dfci.harvard.edu.