In this issue of Blood, Chen et al1 report about novel mechanisms of ibrutinib resistance related to BTK Cys481 point mutations in Waldenström macroglobulinemia (WM). They transfected WM and diffuse large B-cell lymphoma (DLBCL) cells with vectors containing wild-type (BTKWT) or Cys481Ser mutated (BTKCys481Ser) BTK, and examined effects of the transfected genes on ibrutinib sensitivity and signaling pathways, especially on ERK activation. The authors report that BTKCys481Ser promotes ibrutinib resistance via reactivation of ERK1/2 signaling (see figure). Next, they examined how WM and DLBCL cells carrying BTKCys481Ser can confer survival benefit to BTKWT cells, an important question because BTK resistance mutations often only affect a subpopulation of the malignant B cells. A prosurvival effect on WT cells was seen when mixing mutated and WT cells, which apparently did not depend on cell-cell contact, as demonstrated in micropore filter experiments to separate BTKWT from BTKCys481Ser cells. In this setting, BTKCys481Ser cells still conferred protection of BTKWT cells in a paracrine fashion, via secretion of cytokines, especially interleukin-6 (IL-6) and IL-10, which were found to be elevated in supernatants from BTKCys481Se but not from BTKWT cells.
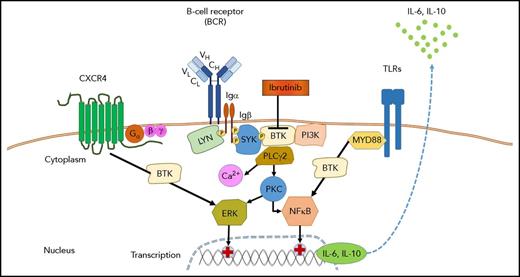
Role of BTK in signaling of the BCR, CXCR4, and TLRs in WM. Antigen binding by the BCR and/or ligand-independent tonic BCR signaling induces the formation of a signaling complex that is initiated by phosphorylation of immunoreceptor tyrosine-based activation motif residues on the cytoplasmic tails of CD79A (Igα) and CD79B (Igβ). In turn, this event recruits spleen tyrosine kinase (SYK), which is followed by the activation of BTK, PI3K, and phospholipase Cγ2 (PLCγ2). Further downstream responses include calcium (Ca2+) mobilization, activation of protein kinase C (PKC), and the ERK (MAPK) and nuclear factor-κB (NFκB) pathways. Most WM cells carry MYD88 and/or CXCR4 warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM)–like mutations, which result in enhanced signaling of CXCR4 and toll-like receptors (TLRs). Ibrutinib targets BTK and thereby can inhibit multiple pathways, including BCR signaling, CXCR4, and TLR signaling. BTKCys481Ser mutated WM cells emerge when WM patients develop ibrutinib resistance; such resistant cells have restored ERK signaling and secrete higher levels of IL-6 and IL-10, which can promote survival of BTKWT cells in a paracrine fashion.
Role of BTK in signaling of the BCR, CXCR4, and TLRs in WM. Antigen binding by the BCR and/or ligand-independent tonic BCR signaling induces the formation of a signaling complex that is initiated by phosphorylation of immunoreceptor tyrosine-based activation motif residues on the cytoplasmic tails of CD79A (Igα) and CD79B (Igβ). In turn, this event recruits spleen tyrosine kinase (SYK), which is followed by the activation of BTK, PI3K, and phospholipase Cγ2 (PLCγ2). Further downstream responses include calcium (Ca2+) mobilization, activation of protein kinase C (PKC), and the ERK (MAPK) and nuclear factor-κB (NFκB) pathways. Most WM cells carry MYD88 and/or CXCR4 warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM)–like mutations, which result in enhanced signaling of CXCR4 and toll-like receptors (TLRs). Ibrutinib targets BTK and thereby can inhibit multiple pathways, including BCR signaling, CXCR4, and TLR signaling. BTKCys481Ser mutated WM cells emerge when WM patients develop ibrutinib resistance; such resistant cells have restored ERK signaling and secrete higher levels of IL-6 and IL-10, which can promote survival of BTKWT cells in a paracrine fashion.
Given the increasing use of ibrutinib and other kinase inhibitors targeting B-cell receptor (BCR) signaling in patients with B-cell malignancies, the topic of resistance development is important, and this study adds new insight into possible mechanisms of resistance development. Primary sensitivity or resistance to ibrutinib in patients with B-cell malignancies generally mirrors the importance of BCR signaling and other signaling pathways involving BTK (such as signaling of chemokine receptors and adhesion molecules) for growth and survival of the respective neoplastic B cells.2 For example, chronic lymphocytic leukemia (CLL) cells are exquisitely sensitive to ibrutinib, mirroring the importance of the BCR in CLL biology, and consequently, primary resistance to ibrutinib occurs only in patients with disease transformation (Richter transformation). On the other hand, patients with germinal center B-cell DLBCL, a lymphoma subtype not dependent on active BCR signaling, almost uniformly lack responsiveness to ibrutinib.3 Secondary resistance to BTK inhibitors is best characterized in CLL, where it can manifest as Richter transformation during the first year of therapy, or as progressive CLL at later stages in a relatively small fraction of high-risk patients, characteristically those with del(17p) and/or complex cytogenetics. CLL progression often coincides with an expansion of clones carrying BTK mutations at the ibrutinib binding site (C481S) or mutations in the BCR signaling-related molecule PLCG2 (R665W, L845F, S707Y).4,5 Although BTK mutations generally reduce binding and thereby the efficacy of the kinase inhibitor, activating PLCG2 mutations result in pathway activation that is independent from BTK. In addition, ibrutinib therapy can also promote the expansion of CLL subclones carrying del(8p), with additional driver mutations.6 Based on a highly sensitive droplet method for detection of single cells with somatic gene mutations,6 it is apparent that miniscule populations of resistant cells already can be present before therapy initiation, which then become selected and expand, as an example of clonal evolution under therapeutic pressure. Patients with WM generally have durable remissions while on ibrutinib therapy, and, as in CLL, BTK C481S mutations emerge in those patients developing resistance. In contrast, development of resistance during therapy is more common in patients with mantle cell lymphoma (MCL), where C481S BTK mutations can be associated with resistance, along with additional PI3K-AKT and CDK4 resistance pathway activity.7 Besides infrequent C481S BTK mutations, resistance to ibrutinib in MCL has been shown to arise from adaptive changes in the kinome usage in tumor cells, in particular, enhanced PI3K-AKT signaling.7,8 In part, adaptive changes appear to be facilitated by integrin β1 signaling and tumor microenvironment interactions.8
Given that the study by Chen et al is largely based on in vitro models with vector-based transfection of WT vs mutated BTK into WM and DLBCL cell lines, a key question becomes how much these findings related to the situation in actual patients. To this end, the authors tested serial plasma samples from WM patients during ibrutinib therapy for changes in IL-6 and IL-10 concentrations, the cytokines that were found to be upregulated in and secreted by BTKCys481Ser cells. Although IL-6 and IL-10 levels remained stable and low in responders, those patients who developed resistance developed increasing levels of both cytokines at time of resistance (n = 3; Figure 6 in Chen et al), translating this aspect of their work into the clinical context. This appears to be different from findings in CLL patients, where high plasma concentrations of CCL3 (MIP-1α), a key BCR signaling response gene and chemokine to attract T cells and macrophages, rapidly normalize when patients start ibrutinib therapy and reappear once CLL patients develop resistance,4 which apparently was not the case in WM, suggesting that BTKCys481Ser may have entirely different biologic consequences in different diseases. In favor of this concept is the fact that the primary targets of ibrutinib in CLL vs WM may be different. Although BCR signaling generally is considered the primary target of ibrutinib in CLL, the majority of WM cells carry MYD88 and/or CXCR4 WHIM syndrome-like mutations, which result in enhanced signaling activation in a BTK-sensitive fashion, predicting for clinical responsiveness to ibrutinib and pointing toward these activating mutations as primary targets of ibrutinib.9 Fortunately, most patients with CLL and WM have durable responses to ibrutinib; CLL patients developing resistance generally respond to the BCL2 antagonist venetoclax.10 Whether this agent also can salvage WM patients developing ibrutinib resistance, or whether ERK signaling-inhibitors would be more suitable as suggested by the data by Chen et al, will ultimately be answered in clinical trials.
Conflict-of-interest disclosure: J.A.B. received research support from Pharmacyclics and Gilead.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal