Key Points
In human CalDAG-GEFI deficiency, αIIbβ3 activation was impaired, but not agonist-induced neutrophil β2 integrin activation.
Delayed αIIbβ3 activation kinetics was associated with severe bleeding tendency in CalDAG-GEFI deficiency.
Abstract
Affinity regulation of integrin αIIbβ3 for fibrinogen by inside-out signaling plays a critical role in hemostasis. Calcium and diacylglycerol (DAG)-regulated guanine nucleotide exchange factor I (CalDAG-GEFI) was identified as a Rap1-activating molecule, and its role in inside-out αIIbβ3 activation was established in CalDAG-GEFI–deficient mice. However, little information regarding CalDAG-GEFI in human platelets is available. Here, we report a 16-year-old girl with CalDAG-GEFI deficiency who has been suffering from severe bleeding tendency. Although talin and kindlin-3 were normally detected, CalDAG-GEFI was undetectable in her platelets by western blotting. Genetic analysis revealed compound heterozygous CalDAG-GEFI mutations, Lys309X and Leu360del, which were responsible for CalDAG-GEFI deficiency. The functional analysis demonstrated impaired αIIbβ3 activation by various agonists except for phorbol myristate acetate, normal calcium mobilization, and impaired Rap1 activation, which were consistent with the phenotype of CalDAG-GEFI–deficient mice. Despite substantial αIIbβ3 activation at high agonist concentrations, she had severe bleeding tendency. Further functional analysis demonstrated markedly delayed αIIbβ3 activation velocity and decreased shear-induced thrombus formation. Contrary to CalDAG-GEFI–deficient mice, which showed integrin-dependent neutrophil functional abnormality, neutrophil β2 integrin activation was not impaired in the patient. Our results demonstrate the critical role of CalDAG-GEFI in rapid αIIbβ3 activation of human platelets.
Introduction
The affinity regulation of fibrinogen receptor integrin αIIbβ3 (GPIIb-IIIa) by inside-out signaling is critical for normal platelet hemostatic functions.1 Upon agonist stimulation, activated Rap1 recruits talin to β3 cytoplasmic tail.2 At the final common step of inside-out signaling, the interaction of talin3-5 and kindlin-36,7 with β3 integrin is necessary for αIIbβ3 activation.8,9 Calcium and diacylglycerol (DAG)-regulated guanine nucleotide exchange factor I (CalDAG-GEFI) was identified as a Rap1-activating molecule,10 and its role in calcium-dependent platelet activation has been established in CalDAG-GEFI–deficient mice.11-14 Recently, its role in humans has also become available by the first report of the patient with CalDAG-GEFI mutation.15 Here, we report our patient with CalDAG-GEFI deficiency who presents severe bleeding symptoms. In agreement with phenotypes in CalDAG-GEFI–deficient mice, the patient’s platelets showed impaired inside-out αIIbβ3 activation, granule release, and platelet aggregation. In addition, the analysis of αIIbβ3 activation kinetics by initial velocity assay16,17 and shear-induced thrombus formation suggests that the delayed αIIbβ3 activation is associated with severe bleeding symptoms in our patient. In sharp contrast, N-formylmethionyl-leucyl-phenylalanine (fMLP) or phorbol myristate acetate (PMA)-induced neutrophil β2 integrin activation was not impaired. Our results, in conjunction with the information from genetically modified mice, clearly demonstrate the importance of CalDAG-GEFI in inside-out αIIbβ3 activation, especially rapid αIIbβ3 activation, for human platelet hemostatic functions.
Study design
The functional and genetic analysis of our patient and her parents was performed after they provided informed consent in accordance with the Declaration of Helsinki under protocols reviewed by the Osaka University ethics committees. Platelets were analyzed by aggregometer, western blotting, and flow cytometry. αIIbβ3 activation kinetics was assessed by initial velocity assay.16,17 Sequence of CalDAG-GEFI was analyzed using Sanger sequencing. Detailed methods are included in the supplemental Methods, available on the Blood Web site.
Results and discussion
The proband, a 16-year-old Japanese girl born from nonconsanguineous parents, has been suffering from severe bleeding tendency. Although her parents had no bleeding problems, she had severe epistaxis 3 times that required red blood cell and platelet transfusion before she was 3 years of age. Thereafter, desmopressin was administered to prevent severe bleeding, which showed some improvement in her bleeding time. However, at 14 years of age, she had massive menorrhagia with hemorrhagic shock that required red blood cell transfusion. To investigate the cause of her severe bleeding tendency, she was referred to Osaka University Hospital.
The patient constantly showed normal leukocyte count and differential. Although her platelet count and coagulation functions were normal (supplemental Table 1), her bleeding time was markedly prolonged to >30 minutes. In platelet aggregometer, her platelets aggregated poorly even with high-concentration adenosine 5′-diphosphate (ADP) stimulation (Figure 1A). Slow and impaired platelet aggregation at 1 μg/mL collagen, but relatively normal aggregation at 2 μg/mL collagen, was observed (Figure 1B). Ristocetin-induced aggregation (supplemental Figure 1) and platelet glycoprotein expression were comparable to those of healthy control platelets (supplemental Figure 2).
Impaired inside-out αIIbβ3 activation in patient platelets. (A-B) Aggregation response of control (black) and patient (gray) platelets activated with ADP (A) and collagen (B). (C-E) Flow cytometry analysis of integrin αIIbβ3 activation by activated αIIbβ3-specific antibody PAC-1 (n = 3: mean ± standard deviation [SD]; Student t test, *P < .05, **P < .01). Mean fluorescence intensity (MFI) is indicated after subtracting the MFI of basal PAC-1 binding. (C) Washed platelets were stimulated with ADP, CRP, PAR1-AP, and U46619. (D) Washed platelets were treated with αIIbβ3-activating antibody PT-25-2, or 100 ng/mL PMA. (E) Washed platelets were simultaneously stimulated with the cocktail of 50 μM ADP, 200 μM PAR1-AP, and 20 μM U46619. (F) Electron microscopy image of platelets from patient. The arrow and arrowhead indicate α-granules and dense granule. Image of control platelet is shown in supplemental Figure 4. (G) Western blot analysis of talin, FAK, Kindlin-3, and CalDAG-GEFI in platelet lysate of control and patient. β-Actin was probed as a loading control. (H) Pull-down assay for activated Rap1 in platelets. Washed control and patient platelets were stimulated with 100 μM PAR1-AP for indicated time. Platelet lysates were incubated GST-RalGDS, and the binding of active GTP-Rap1 was analyzed by western blotting (representative image from 3 independent experiments). The density of each band was measured, and the relative changes of Rap1 activation to unstimulated platelets were indicated as a fold change (n = 3: mean ± SD; Student t test, *P < .05). (I) Time course of intraplatelet calcium mobilization. Washed control and patient fluo-4–loaded platelets were stimulated with 100 μM PAR1-AP, 5.0 μg/mL CRP, or A23187, and fluorescence was analyzed by flow cytometry (MFI).
Impaired inside-out αIIbβ3 activation in patient platelets. (A-B) Aggregation response of control (black) and patient (gray) platelets activated with ADP (A) and collagen (B). (C-E) Flow cytometry analysis of integrin αIIbβ3 activation by activated αIIbβ3-specific antibody PAC-1 (n = 3: mean ± standard deviation [SD]; Student t test, *P < .05, **P < .01). Mean fluorescence intensity (MFI) is indicated after subtracting the MFI of basal PAC-1 binding. (C) Washed platelets were stimulated with ADP, CRP, PAR1-AP, and U46619. (D) Washed platelets were treated with αIIbβ3-activating antibody PT-25-2, or 100 ng/mL PMA. (E) Washed platelets were simultaneously stimulated with the cocktail of 50 μM ADP, 200 μM PAR1-AP, and 20 μM U46619. (F) Electron microscopy image of platelets from patient. The arrow and arrowhead indicate α-granules and dense granule. Image of control platelet is shown in supplemental Figure 4. (G) Western blot analysis of talin, FAK, Kindlin-3, and CalDAG-GEFI in platelet lysate of control and patient. β-Actin was probed as a loading control. (H) Pull-down assay for activated Rap1 in platelets. Washed control and patient platelets were stimulated with 100 μM PAR1-AP for indicated time. Platelet lysates were incubated GST-RalGDS, and the binding of active GTP-Rap1 was analyzed by western blotting (representative image from 3 independent experiments). The density of each band was measured, and the relative changes of Rap1 activation to unstimulated platelets were indicated as a fold change (n = 3: mean ± SD; Student t test, *P < .05). (I) Time course of intraplatelet calcium mobilization. Washed control and patient fluo-4–loaded platelets were stimulated with 100 μM PAR1-AP, 5.0 μg/mL CRP, or A23187, and fluorescence was analyzed by flow cytometry (MFI).
We then carefully performed functional analysis of αIIbβ3 and found that agonist-induced binding of PAC-1, which specifically recognizes activated αIIbβ3,18 was significantly reduced in the patient. In addition to ADP and collagen-related peptide (CRP), thrombin receptor activating peptide (PAR1-AP)- or thromboxane analog U46619-induced PAC-1 binding was also impaired (Figure 1C). In contrast, PMA- or anti-αIIbβ3–activating antibody PT25-2-induced PAC-1 binding was comparable to those of control (Figure 1D). Because released platelet granular contents act in an autocrine fashion for αIIbβ3 activation,19 the impaired granule release (supplemental Figure 3) may cause reduced αIIbβ3 activation. However, impaired PAC-1 binding by simultaneous stimulation with PAR1-AP, ADP, and U46619 suggested that inside-out αIIbβ3 activation itself was impaired (Figure 1E). Transmission electron microscopy showed no apparent abnormality in granule contents (Figure 1F), which further denied the possibility of storage pool deficiency. Collectively, these findings indicate that αIIbβ3 activation, inside-out signaling, is impaired in her platelets. Impaired αIIbβ3 activation by any agonist except for PMA stimulation suggested that CalDAG-GEFI and/or its related signaling molecules may be impaired in her platelets.11 We then analyzed these critical molecules regarding αIIbβ3 activation by western blotting. In sharp contrast to the normal amounts of talin, FAK, and kindlin-3, CalDAG-GEFI was undetectable in her platelets (Figure 1G). In contrast to the first patient with dysfunctional CalDAG-GEFI due to p.Gly248Trp,15 our patient is unique because of its complete deficiency. As shown in its knockout mice, CalDAG-GEFI is essential for calcium-induced inside-out αIIbβ3 activation and hemostasis.11 As expected, weaker and delayed Rap1 activation after PAR1-AP stimulation (Figure 1H) despite normal intraplatelet calcium mobilization (Figure 1I) was observed in her platelets.
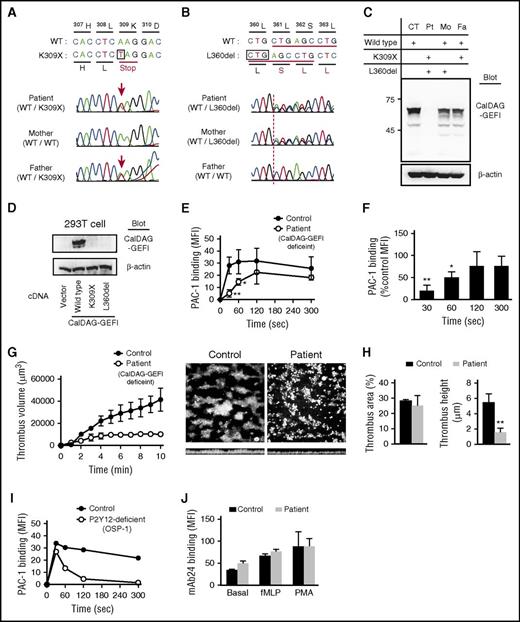
Sequencing analysis revealed novel compound heterozygous mutations in CalDAG-GEFI, an A>T transition c.1178 resulting in a premature stop codon (p.Lys309X) (Figure 2A) and a 3 nucleic acids deletion (c.1331_1333delCTG), resulting in the loss of a single amino acid (p.Leu360del) (Figure 2B). The parents are heterozygous carriers of these mutations with decreased platelet CalDAG-GEFI expression (Figure 2C). No detectable CalDAG-GEFI expression by transfection of mutant CalDAG-GEFI expression vector into 293T cells confirmed that the identified novel mutations are responsible for CalDAG-GEFI deficiency in our patient (Figure 2D).
Compound heterozygous CalDAG-GEFI mutation causes CalDAG-GEFI deficiency in patient platelets and delayed integrin αIIbβ3 activation kinetics. (A-B) Sequencing results of CalDAG-GEFI showing the heterozygous 1178A>T mutation (K309X) in patient (top) and father (bottom) (A) and heterozygous 1331_1333 deletion (L360del) in patient (top) and mother (middle) (B). Genomic DNA isolated from peripheral blood mononuclear cells was used as a template for polymerase chain reaction and amplified DNA fragments were sequenced. Reverse transcription polymerase chain reaction analysis of platelet mRNA also confirmed these mutations (data not shown). (C) Western blot analysis of CalDAG-GEFI in platelets from control, patient, her mother, and her father. (D) Western blot for CalDAG-GEFI in 293T cells transfected empty vector, wild-type CalDAG-GEFI, or mutant CalDAG-GEFI (K309X and L360del) vector. (E) Platelet integrin αIIbβ3 activation kinetics determined by initial velocity assay. Washed control and patient platelets were mixed with 100 μM PAR1-AP at time “zero.” At each indicated time point, 20 μL FITC-PAC-1 was added to the mixture. After 30 seconds of incubation with FITC-PAC-1, bound FITC-PAC-1 was immediately assessed by flow cytometry (n = 3: mean ± SD; Student t test, *P < .05, **P < .01). (F) The percentage of FITC-PAC-1 binding to patient platelets relative to control platelets. PAC-1 binding to patient platelets relative to control platelets at each time point was indicated as percent control MFI (n = 3: mean ± SD; Student t test, *P < .05, **P < .01). (G) Shear-induced thrombus formation. Whole blood from control or patient was perfused over collagen-coated surface at a wall shear rate of 1250 seconds−1. Thrombus volumes were measured by MetaMorph and VoxBlast software every minute (n = 3: mean ± SD). Representative thrombus images after 10 minutes of perfusion were shown (top and side view). (H) Statistical analysis of thrombus area and height. Surface coverage by adhered platelets (left) and thrombus height (right) after 10 minutes of perfusion were analyzed (n = 3). (I) Platelet integrin αIIbβ3 activation kinetics in patient with OSP-1 determined by initial velocity assay. In platelets of OSP-1, rapid initial αIIbβ3 activation at 30 seconds was induced the same as control platelets. However, αIIbβ3 activation was transient and almost no αIIbβ3 activation velocity at 300 seconds. (J) β2 integrin activation in neutrophils. Whole blood was stimulated with 200 nM fMLP or 100 ng/mL PMA, and induced β2 integrin activation was determined by binding of monoclonal antibody 24, which recognizes the activated form of β2 integrin (n = 3: mean ± SD; Student t test). WT, wild type.
Compound heterozygous CalDAG-GEFI mutation causes CalDAG-GEFI deficiency in patient platelets and delayed integrin αIIbβ3 activation kinetics. (A-B) Sequencing results of CalDAG-GEFI showing the heterozygous 1178A>T mutation (K309X) in patient (top) and father (bottom) (A) and heterozygous 1331_1333 deletion (L360del) in patient (top) and mother (middle) (B). Genomic DNA isolated from peripheral blood mononuclear cells was used as a template for polymerase chain reaction and amplified DNA fragments were sequenced. Reverse transcription polymerase chain reaction analysis of platelet mRNA also confirmed these mutations (data not shown). (C) Western blot analysis of CalDAG-GEFI in platelets from control, patient, her mother, and her father. (D) Western blot for CalDAG-GEFI in 293T cells transfected empty vector, wild-type CalDAG-GEFI, or mutant CalDAG-GEFI (K309X and L360del) vector. (E) Platelet integrin αIIbβ3 activation kinetics determined by initial velocity assay. Washed control and patient platelets were mixed with 100 μM PAR1-AP at time “zero.” At each indicated time point, 20 μL FITC-PAC-1 was added to the mixture. After 30 seconds of incubation with FITC-PAC-1, bound FITC-PAC-1 was immediately assessed by flow cytometry (n = 3: mean ± SD; Student t test, *P < .05, **P < .01). (F) The percentage of FITC-PAC-1 binding to patient platelets relative to control platelets. PAC-1 binding to patient platelets relative to control platelets at each time point was indicated as percent control MFI (n = 3: mean ± SD; Student t test, *P < .05, **P < .01). (G) Shear-induced thrombus formation. Whole blood from control or patient was perfused over collagen-coated surface at a wall shear rate of 1250 seconds−1. Thrombus volumes were measured by MetaMorph and VoxBlast software every minute (n = 3: mean ± SD). Representative thrombus images after 10 minutes of perfusion were shown (top and side view). (H) Statistical analysis of thrombus area and height. Surface coverage by adhered platelets (left) and thrombus height (right) after 10 minutes of perfusion were analyzed (n = 3). (I) Platelet integrin αIIbβ3 activation kinetics in patient with OSP-1 determined by initial velocity assay. In platelets of OSP-1, rapid initial αIIbβ3 activation at 30 seconds was induced the same as control platelets. However, αIIbβ3 activation was transient and almost no αIIbβ3 activation velocity at 300 seconds. (J) β2 integrin activation in neutrophils. Whole blood was stimulated with 200 nM fMLP or 100 ng/mL PMA, and induced β2 integrin activation was determined by binding of monoclonal antibody 24, which recognizes the activated form of β2 integrin (n = 3: mean ± SD; Student t test). WT, wild type.
Although the inside-out αIIbβ3 activation was significantly impaired in her platelets, CalDAG-GEFI independent PAC-1 binding and platelet aggregation were still observed (Figure 1A-C). To elucidate why she has severe bleeding problems regardless of some residual αIIbβ3 activity, we determined the kinetics of αIIbβ3 activation by initial velocity assay.16,17 In contrast to the rapid and sustained αIIbβ3 activation in control platelets, αIIbβ3 activation was delayed in patient platelets (Figure 2E), and PAC-1 binding velocity at 30 and 60 seconds after PAR1-AP stimulation was only 19% and 49% of those of control, respectively (Figure 2F). In addition, shear-induced thrombus growth on collagen was markedly impaired in our patient (Figure 2G). Although the surface coverage by adhered platelets was comparable to control, the thrombus height was significantly lower after 10 minutes of perfusion (Figure 2H). In our patient with ADP receptor P2Y12 deficiency (OSP-1),20 rapid and normal αIIbβ3 activation observed at 30 seconds after PAR1-AP stimulation was quickly diminished at 5 minutes (Figure 2I). As shown in our previous report, shear-induced thrombus height in OSP-1 was roughly half of control. However, that is much better than our patient with CalDAG-GEFI deficiency, which is consistent with milder bleeding tendency in OSP-1. These results obtained in a human patient with CalDAG-GEFI deficiency are consistent with the data reported in the mice model13,21 and suggest the importance of rapid αIIbβ3 activation.
Previous studies demonstrated the reduced β2 integrin activation and impaired neutrophil functions in CalDAG-GEFI–deficient mice.22 However, our patient has no history of frequent infections, and fMLP- or PMA-induced β2 integrin activation detected by activated β2 integrin-specific mAb2423 was comparable between control and patient’s CalDAG-GEFI–deficient neutrophils (Figure 2J; supplemental Figure 5). Because no apparent abnormality has been reported in the first CalDAG-GEFI–mutant patient either,15 the difference by CalDAG-GEFI deficiency in neutrophil function may be a species-specific phenomenon. Further studies are needed to define the role of CalDAG-GEFI in neutrophils.
Our results confirmed the critical role of CalDAG-GEFI in human platelet inside-out αIIbβ3 activation. Although the impaired granule release and outside-in signaling also as reported previously11,15 may contribute to her bleeding tendency, delayed αIIbβ3 activation is associated with severe bleeding problems in the patient with CalDAG-GEFI deficiency. Our findings indicate the importance of rapid αIIbβ3 activation for normal hemostatic function
Note added in proof
During our manuscript submission, 2 additional (p.Arg113X and p.Ser381Phe) mutations responsible for CalDAG-GEFI deficiency and/or dysfunction were reported by Lozano et al.24
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Koichi Kokame and Toshiyuki Miyata (National Cerebral and Cardiovascular Center, Suita, Japan) for help with the shear-induced thrombus formation experiment.
This work was supported by a Grant-in Aid for Scientific Research from the Japan Society for the Promotion of Science (grant JP26461403).
Authorship
Contribution: H. Kato designed and performed research, analyzed data, and wrote the paper; Y.N., D.M., and Y. Kurokawa contributed patient samples, performed experiments, and analyzed results; F.B., S.H., Y.M., and H. Kashiwagi performed experiments; Y. Kanakura and Y.T. designed and supervised the research. All coauthors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hisashi Kato, Department of Hematology and Oncology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan; e-mail: hisashi@hp-blood.med.osaka-u.ac.jp.

![Figure 1. Impaired inside-out αIIbβ3 activation in patient platelets. (A-B) Aggregation response of control (black) and patient (gray) platelets activated with ADP (A) and collagen (B). (C-E) Flow cytometry analysis of integrin αIIbβ3 activation by activated αIIbβ3-specific antibody PAC-1 (n = 3: mean ± standard deviation [SD]; Student t test, *P < .05, **P < .01). Mean fluorescence intensity (MFI) is indicated after subtracting the MFI of basal PAC-1 binding. (C) Washed platelets were stimulated with ADP, CRP, PAR1-AP, and U46619. (D) Washed platelets were treated with αIIbβ3-activating antibody PT-25-2, or 100 ng/mL PMA. (E) Washed platelets were simultaneously stimulated with the cocktail of 50 μM ADP, 200 μM PAR1-AP, and 20 μM U46619. (F) Electron microscopy image of platelets from patient. The arrow and arrowhead indicate α-granules and dense granule. Image of control platelet is shown in supplemental Figure 4. (G) Western blot analysis of talin, FAK, Kindlin-3, and CalDAG-GEFI in platelet lysate of control and patient. β-Actin was probed as a loading control. (H) Pull-down assay for activated Rap1 in platelets. Washed control and patient platelets were stimulated with 100 μM PAR1-AP for indicated time. Platelet lysates were incubated GST-RalGDS, and the binding of active GTP-Rap1 was analyzed by western blotting (representative image from 3 independent experiments). The density of each band was measured, and the relative changes of Rap1 activation to unstimulated platelets were indicated as a fold change (n = 3: mean ± SD; Student t test, *P < .05). (I) Time course of intraplatelet calcium mobilization. Washed control and patient fluo-4–loaded platelets were stimulated with 100 μM PAR1-AP, 5.0 μg/mL CRP, or A23187, and fluorescence was analyzed by flow cytometry (MFI).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2016-03-704825/4/m_blood704825f1.jpeg?Expires=1767838200&Signature=5HWIxwgdGDXEN36Rew7TAq2QNAnvfCN~C-IicdH8tUOpzqkLW9j1GQAzeS8BZJOvVg5~9XWvKv6E4vNMi~XrSEdBg6q60WUInnkN5zE5gX0udSWXVz71NP8YId5ettjtSViU4e9Iq1lUIMW6HofSt8cseq1TbAAtGu0cjU3Z-xus2oonUyRIC0a-nfYhvX4ix1Jiji7ftb95~949bc6NMaUyXEXu0wuKOTKRnv4IFjgQvLXGWN2FUnYNQlO4uT7tDMzS1ehn~OMy4iVq5J~ii2WEaEhdf3WbEL05HSmSMvOhPhEQHYemhuK0e3wx3yoaDIuIG0~xbE-7TsQPapwJRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal