Key Points
High-throughput affinity plasma proteomic profiling can identify candidate plasma biomarkers for VTE.
Elevated plasma PDGFB levels are identified as associated with VTE in 2 independent case control studies.
Abstract
There is a clear clinical need for high-specificity plasma biomarkers for predicting risk of venous thromboembolism (VTE), but thus far, such markers have remained elusive. Utilizing affinity reagents from the Human Protein Atlas project and multiplexed immuoassays, we extensively analyzed plasma samples from 2 individual studies to identify candidate protein markers associated with VTE risk. We screened plasma samples from 88 VTE cases and 85 matched controls, collected as part of the Swedish “Venous Thromboembolism Biomarker Study,” using suspension bead arrays composed of 755 antibodies targeting 408 candidate proteins. We identified significant associations between VTE occurrence and plasma levels of human immunodeficiency virus type I enhancer binding protein 1 (HIVEP1), von Willebrand factor (VWF), glutathione peroxidase 3 (GPX3), and platelet-derived growth factor β (PDGFB). For replication, we profiled plasma samples of 580 cases and 589 controls from the French FARIVE study. These results confirmed the association of VWF and PDGFB with VTE after correction for multiple testing, whereas only weak trends were observed for HIVEP1 and GPX3. Although plasma levels of VWF and PDGFB correlated modestly (ρ ∼ 0.30) with each other, they were independently associated with VTE risk in a joint model in FARIVE (VWF P < .001; PDGFB P = .002). PDGFΒ was verified as the target of the capture antibody by immunocapture mass spectrometry and sandwich enzyme-linked immunosorbent assay. In conclusion, we demonstrate that high-throughput affinity plasma proteomic profiling is a valuable research strategy to identify potential candidate biomarkers for thrombosis-related disorders, and our study suggests a novel association of PDGFB plasma levels with VTE.
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most common cardiovascular disease (CVD) and a leading cause of death and disability worldwide.1 Complex interactions between genetic, environmental, and acquired risk factors underlie disease development.2,3 For physicians, VTE risk prediction remains a challenge, and there is a clear need to improve current clinical tools and risk scores.4-7 Continuous treatment of VTE patients with anticoagulants is associated with serious bleeding complications, and so the identification of those at highest risk for recurrent VTE events, and thus most likely to benefit from continuous treatment, holds particular clinical value.8,9 The first step in the process of developing clinically applicable predictive tools is the identification of novel markers that associate with the disease. Genome-wide association (GWA) studies have led to the discovery of novel genetic markers of human diseases and their intermediate phenotypes,10 such as the discovery of 2 unexpected loci (SLC44A2 and TSPAN15) that associate with VTE risk.11 However, identification of novel genes or established VTE susceptibility genes has, thus far, not been translated into useful risk prediction tools, and the clinical value of such genetic information in assessing individual thrombotic risk is a matter of debate.12 In plasma, the integrated effects of genetic, environmental, and acquired factors that influence the risk of thrombosis are reflected in the protein profile. Currently, the only plasma biomarker routinely used for VTE in a clinical context is D-dimer, a split product from the cross-linked fibrin clot, which has low specificity and is elevated in other conditions such as cancer, inflammation, and pregnancy.4,13 Directly studying the human plasma proteome is an alternative, and possibly more efficient, strategy to identify novel biomarkers that associate with VTE.
The development of high-throughput technologies has opened up new possibilities for extensive plasma protein profiling.14 Affinity proteomics, using suspension bead arrays (SBA) built with antibodies from the Human Proteome Atlas (HPA), has been used to successfully identify novel candidate biomarkers for various diseases, such as gastrointestinal cancer, osteoporosis, and amyotrophic lateral sclerosis.15-19 Here, with the aim of identifying plasma protein biomarkers for VTE, we conducted the first affinity plasma proteomic profiling in 2 different VTE case-control studies. We screened samples from a Swedish study of 89 VTE cases and 88 controls for over 400 selected protein candidates, followed by a replication phase in a French study of 603 VTE cases and 597 controls. We identify PDGF as a novel VTE-associated plasma protein and present a panel of other tentative candidates worthy of further investigation.
Materials and methods
Discovery study---Venous thromboEmbolism BIOmarker Study (VEBIOS)
VEBIOS is an on-going case-control study, initiated in January 2011 and conducted by the coagulation unit at Karolinska University Hospital, in collaboration with the Royal Institute of Technology (KTH). Eligible cases were between 18 and 70 years of age at the time of first VTE, confirmed by diagnostic imaging: venous ultrasonography in patients with DVT of the lower limbs, and computed tomography pulmonary angiography or ventilation perfusion scintigraphy (V/Q lung scan) in patients with PE. Cases were recruited from 3 regional hospitals after referral from the emergency clinics following diagnosis. Cases had been treated with anticoagulants (vitamin K antagonist [VKA], direct oral anticoagulant, or low-molecular-weight heparin) for 6 to 12 months and were sampled 1 to 6 months after discontinuation of treatment. Patients with severe thrombophilia, that is, antithrombin, protein S and protein C deficiencies, antiphospholipid syndrome, homozygosity for either factor V Leiden (G1691A) or the G20210A polymorphism in prothrombin gene, or a combined heterozygosity, were excluded. Controls were randomly recruited from the general population of Stockholm County, using the Swedish Tax Agency register20 and matched to cases for age (±2 years) and gender, and sampled within 1 year of the index date (when blood sampling took place) of the matched case. Exclusion criteria were history of VTE, pregnancy in the 3 months prior to index date, or active cancer within the last 5 years.
Data collection.
Participants completed a questionnaire regarding (1) demography; (2) provoking factors within 3 months preceding VTE diagnosis, or the time of sampling for the controls; (3) coexisting cardiovascular risk factors and chronic comorbidities; (4) current health situation: alcohol consumption, smoking habits, physical activity; (5) family history of VTE and other CVD; (6) ongoing medication; and (7) reproductive history and hormonal use (women only).
Blood sampling.
Participants were sampled at the Coagulation Unit, Karolinska Hospital in the morning, following an overnight fast. Whole blood was collected in citrate or EDTA anticoagulant and centrifuged at 2000g for 15 minutes. Plasma aliquots were snap frozen and stored at −80°C. Blood and plasma samples were sent to the Karolinska University Laboratory for measurement of CRP, D-dimer, blood glucose, creatinine, albumin, blood count, lipid profile, and liver enzymes.
Biometry.
On the index date, participant weight, height, blood pressure, and pulse in left arm in sitting position and waist and hip circumference were measured.
Ethics permission.
Informed written consent was obtained from all participants in accordance with the Declaration of Helsinki. VEBIOS was approved by the regional research ethics committee in Stockholm, Sweden (ethical permit: KI2010/636-31/4).
Replication study---The FARIVE study
FARIVE is a French multicenter case-control study carried out between 2003 and 2009 with the aim of studying the interactions of environmental, genetic, and biological risk factors for first VTE and risk of recurrence. The study recruited consecutive inpatients or outpatients from 18 years of age treated for a first episode of DVT and/or PE, confirmed by diagnostic imaging, as previously described.21 Controls were age and sex matched and consisted of inpatients and outpatients, free of history of venous or arterial thrombotic disease. Exclusion criteria included cancer diagnosis, short life expectancy owing to other causes, and renal or liver failure. The replication study was based on a subset of FARIVE with existing genotype data (n = 1200).22
Blood sampling.
Participants were sampled at the respective centers. Whole blood was collected in citrate anticoagulant and centrifuged at 2000g for 20 minutes. Plasma aliquots were snap frozen and stored at −80°C. Whole blood and plasma were collected to the Biobank centralized at Hôpital Européen Georges Pompidou in Paris. A total of 587 cases were sampled at the study entry, within the first week after diagnosis and anticoagulant treatment initiation.
The international normalized ratio (INR) was measured to verify VKA treatment and divided into 3 categories: no measurable effect (INR < 1.2), initiated treatment (INR 1.2-1.99), and therapeutic (≥2.0). The remaining 16 (3%) cases were sampled after withdrawal of anticoagulant treatment.
Ethics permission.
Informed written consent was obtained from all participants in accordance with the Declaration of Helsinki. The study was approved by the Paris Broussais–Hôpital Européen Georges Pompidou ethics committee in Paris (ethical permit: 2002-034).
Selection of candidate proteins and antibody reagents for discovery analysis in VEBIOS
The selection of candidate protein targets was based on (1) previous support or hypothesis of association with VTE or intermediate traits, and (2) the availability of target-specific antibodies in the HPA. The candidate targets were categorized into 4 groups: A-D (ranging from “probable” to “plausible,” see list below) (supplemental Table 1, available on the Blood Web site).
Group A: targets with an established association with VTE, including support from expression analysis and/or functional analysis,for example, von Willebrand factor (VWF),23,24 and Kininogen 1.25
Group B: (1) targets associated with VTE based on genetic data only, such as single-nucleotide polymorphism in gene/locus, for example, BAI3,26 or (2) targets associated with arterial thrombosis and/or cardiovascular events based on genetic and/or functional data, for example, class IA phosphoinositide 3-kinase β.27
Group C: (1) proteins involved in intermediate traits related to thrombosis, for example, protein disulphide isomerase A4,28 or (2) genes with endothelial enriched expression, for example, MYCT129 or ESM1,30 or (3) plasma proteins with significant association to cardiovascular events identified in an in-house proteomics study performed within the Protein Affinity Plasma Profiling group at SciLifeLab (J.O., M.J.I., J.M.S., M.-G.H., and M.U., unpublished data).
Group D: proteins involved in pathways of relevance to thrombosis or intermediate traits, in the absence of evidence for a direct role, for example, integrin α 4 subunit.31
Antibodies corresponding to the proposed targets were obtained from the protein-specific affinity reagents resource generated by the HPA program, which covers ∼17 000 of the human protein-coding genes.15 From 586 proposed targets, a final panel of 408 was selected for the discovery screen in VEBIOS, targeted by 755 individual HPA antibodies. Antibody-binding specificity was assessed by western blotting and immunohistochemistry (www.proteinatlas.org). Multiple antibodies targeted 235 of the protein candidates—raised against the same (“twins”) or a different (“sibling”) recombinant expressed region of the target protein. A list of the selected protein targets and corresponding antibody reagents can be found in supplemental Table 1.
Plasma sample selection
Of the original 190 VEBIOS participants, samples from 177 individuals were used for the discovery stage. Nine individuals (1 case and 8 controls) were excluded due to lack of age-matched controls. Three were excluded due to provoked VTE in combination with estrogen-containing hormonal contraceptives and menopausal replacement treatment, and 1 was removed following detection of a blood-borne infection. In FARIVE, citrate plasma samples were available from 603 cases and 597 controls from 13 centers. Only cases sampled at study entry shortly after diagnosis were included. Cases were paired to controls from the same center, and gender and age matched. Clinical characteristics of the VEBIOS and FARIVE study samples are shown in Table 1 and Table 2, respectively.
Clinical characteristics of the VEBIOS discovery study
| . | 89 Cases . | 88 Controls . | ||
|---|---|---|---|---|
| Variables . | N . | Freq. . | N . | Freq. . |
| Localization of the thrombosis | ||||
| DVT, lower limbs | 47 | 0.53 | ||
| Proximal | 36 | 0.77 | ||
| PE | 45 | 0.51 | ||
| Gender and biometry | ||||
| Gender, women | 35 | 0.39 | 35 | 0.39 |
| Age, y (mean ± SD and range) | 51.1 ± 10.8 | (20-70) | 51.6 ± 10.8 | (21-70) |
| BMI, kg/m2 (mean ± SD and range) | 26.7 ± 4.6 | (17-46) | 25.6 ± 4 | (19-38) |
| Obese, BMI ≥ 30 kg/m2 | 20 | 0.22 | 16 | 0.19 |
| Cardiovascular risk factors | ||||
| Hypertension* | 19 | 0.21 | 10 | 0.11 |
| Hyperlipidemia* | 5 | 0.06 | 5 | 0.06 |
| Diabetes mellitus* | 2 | 0.02 | 5 | 0.06 |
| Current smoking† | 15 | 0.17 | 7 | 0.08 |
| Family history | ||||
| VTE, first-degree relative <60 y old | 15 | 0.17 | 1 | 0.01 |
| Provoked risk factors‡ | 39 | 0.44 | 17 | 0.19 |
| Trauma | 22 | 0.22 | 7 | 0.08 |
| Cast, orthosis, etc | 15 | 0.17 | 1 | 0.01 |
| Surgery | 16 | 0.18 | 2 | 0.02 |
| Pregnancy, postpartum 3 mo | 0 | 0 | 0 | 0 |
| Contraceptives§ | 11 | 0.31 | 9 | 0.26 |
| Menopausal replacement therapy# | 5 | 0.14 | 1 | 0.03 |
| . | 89 Cases . | 88 Controls . | ||
|---|---|---|---|---|
| Variables . | N . | Freq. . | N . | Freq. . |
| Localization of the thrombosis | ||||
| DVT, lower limbs | 47 | 0.53 | ||
| Proximal | 36 | 0.77 | ||
| PE | 45 | 0.51 | ||
| Gender and biometry | ||||
| Gender, women | 35 | 0.39 | 35 | 0.39 |
| Age, y (mean ± SD and range) | 51.1 ± 10.8 | (20-70) | 51.6 ± 10.8 | (21-70) |
| BMI, kg/m2 (mean ± SD and range) | 26.7 ± 4.6 | (17-46) | 25.6 ± 4 | (19-38) |
| Obese, BMI ≥ 30 kg/m2 | 20 | 0.22 | 16 | 0.19 |
| Cardiovascular risk factors | ||||
| Hypertension* | 19 | 0.21 | 10 | 0.11 |
| Hyperlipidemia* | 5 | 0.06 | 5 | 0.06 |
| Diabetes mellitus* | 2 | 0.02 | 5 | 0.06 |
| Current smoking† | 15 | 0.17 | 7 | 0.08 |
| Family history | ||||
| VTE, first-degree relative <60 y old | 15 | 0.17 | 1 | 0.01 |
| Provoked risk factors‡ | 39 | 0.44 | 17 | 0.19 |
| Trauma | 22 | 0.22 | 7 | 0.08 |
| Cast, orthosis, etc | 15 | 0.17 | 1 | 0.01 |
| Surgery | 16 | 0.18 | 2 | 0.02 |
| Pregnancy, postpartum 3 mo | 0 | 0 | 0 | 0 |
| Contraceptives§ | 11 | 0.31 | 9 | 0.26 |
| Menopausal replacement therapy# | 5 | 0.14 | 1 | 0.03 |
freq., frequency; N, numbers; SD, standard deviation.
Medical treatment.
Daily within the last year.
Within 3 mo from diagnose or index date.
Estrogen containing (oral, patch, vaginal devices).
Estrogen containing (oral only).
Clinical characteristics of the FARIVE verification study
| . | 603 Cases . | 597 Controls . | ||
|---|---|---|---|---|
| Variables . | N . | Freq. . | N . | Freq. . |
| Localization of the thrombosis | ||||
| DVT, lower limbs | 102 | 0.28 | ||
| PE | 147 | 0.41 | ||
| DVT and PE | 105 | 0.3 | ||
| Gender and biometry | ||||
| Gender, women | 363 | 0.60 | 340 | 0.57 |
| Age, y (mean ± SD and range) | 53.1 ± 19.7 | (17-91) | 51.2 ± 18.4 | (18-89) |
| Height, m (mean ± SD and range) | 1.68 ± 0.9 | (1.48-2) | 1.68 ± 0.9 | (1.48-2.00) |
| BMI, kg/m2 (mean ± SD and range) | 26.5 ± 5.6 | (15-55) | 25.9 ± 6 | (15-47) |
| Obese, BMI ≥30 kg/m2 | 135 | 0.22 | 114 | 0.19 |
| Cardiovascular risk factors | ||||
| Hypertension* | 194 | 0.32 | 247 | 0.42 |
| Hyperlipidemia* | 156 | 0.26 | 134 | 0.22 |
| Diabetes mellitus* | 40 | 0.07 | 103 | 0.17 |
| Current smoking† | 100 | 0.17 | 138 | 0.24 |
| Family history | ||||
| VTE, first-degree relative | 145 | 0.24 | NA | |
| Provoked risk factors‡ | ||||
| Trauma | 24 | 0.04 | NA | |
| Cast, orthosis | 22 | 0.04 | NA | |
| Surgery | 109 | 0.18 | NA | |
| Pregnancy, postpartum 3 mo | 21 | 0.03 | NA | |
| Hormonal use (women only) | ||||
| Contraceptives§ | 146 | 0.40 | NA | |
| Menopausal replacement therapy§ | 28 | 0.08 | NA | |
| Sampled at study entry# | 587 | 0.97 | ||
| Warfarin treatment effect | ||||
| PK INR < 1.2 | 253 | 0.43 | ||
| PK INR 1.2-1.99 | 176 | 0.30 | ||
| PK INR 2.0-5.0 | 145 | 0.25 | ||
| Missing value | 13 | 0.02 | ||
| . | 603 Cases . | 597 Controls . | ||
|---|---|---|---|---|
| Variables . | N . | Freq. . | N . | Freq. . |
| Localization of the thrombosis | ||||
| DVT, lower limbs | 102 | 0.28 | ||
| PE | 147 | 0.41 | ||
| DVT and PE | 105 | 0.3 | ||
| Gender and biometry | ||||
| Gender, women | 363 | 0.60 | 340 | 0.57 |
| Age, y (mean ± SD and range) | 53.1 ± 19.7 | (17-91) | 51.2 ± 18.4 | (18-89) |
| Height, m (mean ± SD and range) | 1.68 ± 0.9 | (1.48-2) | 1.68 ± 0.9 | (1.48-2.00) |
| BMI, kg/m2 (mean ± SD and range) | 26.5 ± 5.6 | (15-55) | 25.9 ± 6 | (15-47) |
| Obese, BMI ≥30 kg/m2 | 135 | 0.22 | 114 | 0.19 |
| Cardiovascular risk factors | ||||
| Hypertension* | 194 | 0.32 | 247 | 0.42 |
| Hyperlipidemia* | 156 | 0.26 | 134 | 0.22 |
| Diabetes mellitus* | 40 | 0.07 | 103 | 0.17 |
| Current smoking† | 100 | 0.17 | 138 | 0.24 |
| Family history | ||||
| VTE, first-degree relative | 145 | 0.24 | NA | |
| Provoked risk factors‡ | ||||
| Trauma | 24 | 0.04 | NA | |
| Cast, orthosis | 22 | 0.04 | NA | |
| Surgery | 109 | 0.18 | NA | |
| Pregnancy, postpartum 3 mo | 21 | 0.03 | NA | |
| Hormonal use (women only) | ||||
| Contraceptives§ | 146 | 0.40 | NA | |
| Menopausal replacement therapy§ | 28 | 0.08 | NA | |
| Sampled at study entry# | 587 | 0.97 | ||
| Warfarin treatment effect | ||||
| PK INR < 1.2 | 253 | 0.43 | ||
| PK INR 1.2-1.99 | 176 | 0.30 | ||
| PK INR 2.0-5.0 | 145 | 0.25 | ||
| Missing value | 13 | 0.02 | ||
freq., frequency; N, numbers; SD, standard deviation.
According to medical records or treatment.
Four participants smoked <1 cigarette per day, 10 missing information.
Within 3 mo from diagnosis or index date.
Not defined.
Sampled shortly after diagnosis of DVT or PE.
Protein plasma profiling
Protein plasma profiles were generated using HPA antibodies coupled to magnetic color-coded beads and analyzed on a Luminex system, as described.18,32 Each SBA was composed of 380 antibodies and 4 controls to sequentially generate profiles of 384 samples in parallel.18,32 This method allowed for protein profiling in the absence of purification, fractionation, or depletion steps.33 Protein profiling was performed in citrate plasma in VEBIOS and FARIVE, and in VEBIOS also in EDTA plasma from the same individuals. Paired samples were randomly distributed within the same 96-well area subsets used to build 384-well plates.
Statistical analyses
Median fluorescence intensity (MFI) values were obtained from the SBA assay by detecting at least 32 beads per infected dose and sample with the FlexMap 3D instrument (Luminex Corp). Samples with lower bead counts were excluded (4 from VEBIOS and 4 from FARIVE). Principal components analysis identified study-specific outliers, leading to additional exclusion of 14 individuals in FARIVE.34 In FARIVE, 13 further samples were excluded from the cases, as these were sampled after withdrawal of VKA. In total, 88 cases and 85 controls in VEBIOS and 580 cases and 589 controls in FARIVE remained for analysis. Probabilistic quotient normalization was used to normalize the data for sample-by-sample variation.35 No detectable heterogeneity was observed across the 13 FARIVE centers. Association of proteomic markers with VTE was tested using linear regression analysis while adjusting for age, gender, and center (FARIVE). Log-transformation was applied to reduce any skewness in the proteomic data distribution. The analyses were performed using the R statistical computing software.36
Immunocapture mass spectrometry (IC-MS)
IC-MS was performed as described by Neiman et al with some modifications.32 In brief, 100 µL of pooled citrate plasma from a VEBIOS sample set was diluted, heated at 56°C, and incubated overnight with beads covalently coupled to the HPA011972 antibody, or with normal rabbit immunoglobulin G as a negative control. Each IC experiment was performed in duplicate. After digestion, samples were analyzed using a Q-Exactive HF (Thermo) equipped with an Ultimate 3000 RSLC nanosystem (Dionex). Raw data were queried against the Uniprot complete human proteome (updated 21 May 2015) using the Sequest engine under the platform Proteome Discoverer (v1.4.0.339; Thermo Scientific). Detailed information about the MS analysis conditions and data analysis is provided in supplemental Methods. For each IC, proteins identified were considered potential antibody targets if they fulfilled the following criteria: a peptide-spectrum match >2; identification in both duplicates; frequency of identification <50% in an internal database containing the most frequent proteins identified by IC-MS in control plasma due to nonspecific interaction. This database was also used to assign a z score to each protein. A z score of ≥4 was considered as cutoff to claim a protein as specifically enriched.
PDGFB immunoassays
From the VEBIOS discovery screen, 11 samples were selected with different PDGFB levels, as measured in MFI by the HPA011972 single-binder Luminex assay. The samples were measured in parallel on the Luminex system with single binder assay and sandwich assay, and in a commercially available immunoassay kit system (ELLA; ProteinSimple; catalog number SPCKA-PS-000179), which allows for absolute quantification. For the bead-based sandwich assay, the HPA011972 capture antibody was coupled to magnetic beads and a biotinylated goat anti-human PDGF-BB was used as a detection antibody (RnD; catalog number DY220, part 840926). For the ELLA assay, all reagents were provided by the manufacturer. In brief, samples were thawed on ice and centrifuged for 2 minutes at 2000 rpm. Plasma samples were diluted 1:1 or 1:10 with SD13 buffer and applied into the ELLA cartridge or incubated with Luminex beads, respectively.
Results
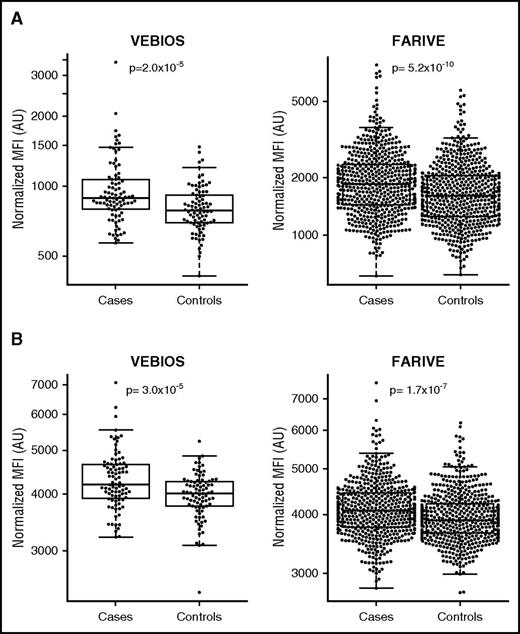
In the VEBIOS case-control cohort, we measured 408 plasma proteins, using 755 polyclonal antibody reagents, to identify associations with VTE. Target selection was based on indications from the literature of a probable or plausible link to VTE (supplemental Table 1). Four candidate proteins were significantly associated with VTE in VEBIOS after Bonferroni correction (P < 6.6 × 10−5) (Table 3, supplemental Table 1). Human immunodeficiency virus type I enhancer binding protein 1 (HIVEP1), VWF, and platelet-derived growth factor β (PDGFB) levels were significantly higher in cases, compared with controls (P = 9.5 × 10−6, 2.0 × 10−5, 3.0 × 10−5, respectively) (Figure 1A, Table 3). Conversely, glutathione peroxidase 3 (GPX3) levels were significantly lower in cases compared with controls (P = 1.4 × 10−5) (Table 3). Concordance was observed between protein measurements in citrate and EDTA anticoagulated samples (Pearson’s correlation 0.65, 0.69, 0.83, and 0.64 for HIVEP1, GPX3, VWF, and PDGFB, respectively). VTE-association P values derived from EDTA measurements were 5.9 × 10−6, 1.9 × 10−3, 2.2 × 10−7, and 1.1 × 10−3 for HIVEP1, GPX3, VWF, and PDGFB, respectively (Table 3). With a reduced statistical cutoff (P < .01 in both citrate and EDTA plasma), we identified 24 further provisional VTE-associated candidate proteins in VEBIOS Discovery (supplemental Table 1).
| Binder name . | Gene . | VEBIOS-Citrate . | VEBIOS-EDTA . | FARIVE-Citrate . | |||
|---|---|---|---|---|---|---|---|
| Controls . | Cases . | Controls . | Cases . | Controls . | Cases . | ||
| (N = 85) . | (N = 88) . | (N = 86) . | (N = 87) . | (N = 589) . | (N = 580) . | ||
| HPA050724 | HIVEP1 | 6.734 (0.012) | 6.820 (0.015) | 7.066 (0.015) | 7.172 (0.018) | 6.370 (0.006) | 6.384 (0.006) |
| β = +0.089 (0.019); P = 9.5 × 10−6 | β = +0.107 (0.023); P = 5.9 × 10−6 | β = +0.012 (0.009); P = .160 | |||||
| HPA059686 | GPX3 | 5.872 (0.016) | 5.788 (0.012) | 5.791 (0.021) | 5.713 (0.016) | 6.699 (0.006) | 6.679 (0.007) |
| β = −0.086 (0.019); P = 1.4 × 10−5 | β = −0.080 (0.025); P = 1.9 × 10−3 | β = −0.018 (0.009); P = .036 | |||||
| HPA002082 | VWF | 6.678 (0.025) | 6.845 (0.033) | 6.586 (0.030) | 6.841 (0.043) | 7.396 (0.015) | 7.529 (0.016) |
| β = +0.170 (0.040); P = 2.0 × 10−5 | β = −0.260 (0.048); P = 2.2 × 10−7 | β = +0.130 (0.021); P = 5.2 × 10−10 | |||||
| HPA011972 | PDGFB | 8.285 (0.012) | 8.363 (0.016) | 8.418 (0.013) | 8.480 (0.015) | 8.280 (0.005) | 8.317 (0.006) |
| β = +0.082 (0.019); P = 3.0 × 10−5 | β = +0.063 (0.019); P = 1.1 × 10−3 | β = +0.037 (0.007); P = 1.7 × 10−7 | |||||
| Binder name . | Gene . | VEBIOS-Citrate . | VEBIOS-EDTA . | FARIVE-Citrate . | |||
|---|---|---|---|---|---|---|---|
| Controls . | Cases . | Controls . | Cases . | Controls . | Cases . | ||
| (N = 85) . | (N = 88) . | (N = 86) . | (N = 87) . | (N = 589) . | (N = 580) . | ||
| HPA050724 | HIVEP1 | 6.734 (0.012) | 6.820 (0.015) | 7.066 (0.015) | 7.172 (0.018) | 6.370 (0.006) | 6.384 (0.006) |
| β = +0.089 (0.019); P = 9.5 × 10−6 | β = +0.107 (0.023); P = 5.9 × 10−6 | β = +0.012 (0.009); P = .160 | |||||
| HPA059686 | GPX3 | 5.872 (0.016) | 5.788 (0.012) | 5.791 (0.021) | 5.713 (0.016) | 6.699 (0.006) | 6.679 (0.007) |
| β = −0.086 (0.019); P = 1.4 × 10−5 | β = −0.080 (0.025); P = 1.9 × 10−3 | β = −0.018 (0.009); P = .036 | |||||
| HPA002082 | VWF | 6.678 (0.025) | 6.845 (0.033) | 6.586 (0.030) | 6.841 (0.043) | 7.396 (0.015) | 7.529 (0.016) |
| β = +0.170 (0.040); P = 2.0 × 10−5 | β = −0.260 (0.048); P = 2.2 × 10−7 | β = +0.130 (0.021); P = 5.2 × 10−10 | |||||
| HPA011972 | PDGFB | 8.285 (0.012) | 8.363 (0.016) | 8.418 (0.013) | 8.480 (0.015) | 8.280 (0.005) | 8.317 (0.006) |
| β = +0.082 (0.019); P = 3.0 × 10−5 | β = +0.063 (0.019); P = 1.1 × 10−3 | β = +0.037 (0.007); P = 1.7 × 10−7 | |||||
Shown values correspond to log MFI relative to levels of the protein targeted by the HPA antibodies separately in controls and cases with the standard error. The β coefficient (standard error) corresponds to the log level’s increase in cases compared with controls, adjusted for age, sex and additionally center in FARIVE.
The statistical thresholds used for declaring statistical association were P = 6.6 × 10−5 (∼0.05/755) in VEBIOS and P = .0125 (=.05/4) in FARIVE.
Relative levels of VWF and PDGFB in VTE in the discovery phase and replication phase. Dot box plots of relative levels of VWF (A) and PDGFB (B) in VEBIOS and in FARIVE. Values are in expressed as MFI as measured in the single binder bead assay on the Luminex system using HPA002082 antibody for VWF (A) or HPA11972 antibody for PDGFB (B).
Relative levels of VWF and PDGFB in VTE in the discovery phase and replication phase. Dot box plots of relative levels of VWF (A) and PDGFB (B) in VEBIOS and in FARIVE. Values are in expressed as MFI as measured in the single binder bead assay on the Luminex system using HPA002082 antibody for VWF (A) or HPA11972 antibody for PDGFB (B).
For replication of the targets identified in VEBIOS as significantly associated with VTE following Bonferroni correction, we used citrated plasma samples from 580 VTE cases and 589 controls from the FARIVE study. VWF and PDGFB levels were significantly associated with VTE in FARIVE (P = 5.2 × 10−10 and 1.7 × 10−7, respectively) (Table 3, Figure 1). GPX3 levels were lower in cases compared with controls (P = .036), consistent with VEBIOS findings, but the results did not reach Bonferroni corrected statistical significance (P = .0125). HIVEP1 levels tended to be higher in cases than controls, but the association was not statistically significant (P = .16) (Table 3).
HIVEP1 was originally selected as a target candidate as a polymorphism in its structural gene, rs169713, had been found to associate with VTE in a combined analysis with 4 case-control studies, including FARIVE.37 Interestingly, although there was no association between HIVEP1 levels and VTE in rs169713-TT carriers (P = .61), HIVEP1 levels were slightly higher in cases compared with controls, carrying the VTE-associated C allele (difference in log-MFI β = 0.032 ± 0.014, P = .022) (Table 4). Nevertheless, the test for a genotype-specific association of HIVEP1 and VTE was not significant (P = .13).
Associations of HIVEP1 rs169713, HIVEP1 levels, and VTE risk in FARIVE
| HIVEP1 rs169713 . | Controls . | Cases . | β coefficient . |
|---|---|---|---|
| TT | 6.375 (0.150) | 6.381 (0.142) | β = +0.0059 (0.011) |
| N = 341 | N = 319 | P = .6 | |
| TC | 6.357 (0.169) | 6.392 (0.129) | β = +0.033 (0.016) |
| N = 184 | N = 183 | P = .04 | |
| CC | 6.361 (0.102) | 6.390 (0.138) | β = +0.030 (0.032) |
| N = 25 | N = 38 | P = .3 |
| HIVEP1 rs169713 . | Controls . | Cases . | β coefficient . |
|---|---|---|---|
| TT | 6.375 (0.150) | 6.381 (0.142) | β = +0.0059 (0.011) |
| N = 341 | N = 319 | P = .6 | |
| TC | 6.357 (0.169) | 6.392 (0.129) | β = +0.033 (0.016) |
| N = 184 | N = 183 | P = .04 | |
| CC | 6.361 (0.102) | 6.390 (0.138) | β = +0.030 (0.032) |
| N = 25 | N = 38 | P = .3 |
HIVEP1 was targeted by HPA050724 and association results are presented in MFI levels (log) with standard error. Reported β coefficient (with its standard error) corresponds to the relative levels (log) increase in cases compared with controls, adjusted for age and sex.
We performed IC-MS to verify that PDGFB was specifically bound by HPA011972. A total of 244 bound proteins were identified (data not shown), and these were filtered using an internal database of the proteins most frequently found in plasma by IC-MS, and z scores were calculated. The highest z score of 4.9 was obtained for PDGFB (supplemental Table 2), with 3 peptides from the protein identified (supplemental Figures 1 and 2A-C). To further confirm this result, we performed a comparative analysis of data collected from the analysis of a subset of VEBIOS plasma samples using the ELLA microfluidics immunoassay system and the bead-based Luminex assays using the HPA011972 as capture antibody in the single binder assay or sandwich assay. The absolute concentration of plasma PDGFB measured using the ELLA microfluidic immunoassay system ranged from 47 to 1050 pg/mL (mean 398 ± 302 pg/mL) levels in the same order of magnitude as previously reported in healthy controls (range 100-690 pg/mL, mean 320 ± 140 pg/mL).38 MFI values from the Luminex single binder and sandwich assay correlated well with these data (Pearson’s correlation 0.6 [P = .04] and 0.68 [P = .01], respectively). Together these data confirm that the HPA011972 antibody specifically bound and measured PDGFB from plasma.
PDGFB and VWF moderately correlated in VEBIOS and FARIVE (Pearson’s correlation 0.42 and 0.26, respectively) and remained significantly associated with VTE when both were entered in a joint logistic regression model. In a joint model adjusted for age, sex, and center in FARIVE, the significance levels for association of VWF and PDGFB with VTE risk were P < .001 and P = .002, respectively. There was no influence of VKA treatment of PDGFB, VWF, HIVEP1, or GPX3 levels, when comparing cases stratified by INR with controls (data not shown).
Discussion
Here, we present an extensive screening for plasma protein biomarker candidates for VTE risk, in a discovery and independent replication study. VWF, PDGFB, HIVEP1, and GPX3 were identified as VTE associated in the VEBIOS cohort, and the VWF and PDGFB associations were replicated in FARIVE.
Elevated plasma VWF levels are linked to thrombosis,23,24 and thus, the association of VWF with VTE in our study serves as a proof of principle and positive control for the methodological approach. VWF plays an important role in the regulation of factor VIII (FVIII) levels, a coagulation factor which has been touted as a predictor of first and recurrent VTE.39,40 FVIII was included among the 408 candidate proteins in our study, and we observed increased plasma levels in VTE patients compared with controls in VEBIOS (supplemental Table 1). However, this did not reach our prespecified statistical significance threshold for being forwarded to the replication phase (FVIII; P = .0005). It is difficult to dissect the relative effect of VWF versus FVIII plasma levels in VTE. Indeed, the potential role of protein-protein interactions, such as VWF-FVIII, should be considered for all targets when investigating their specific role, beyond their validity as a disease marker per se.
PDGFB was included as a discovery target based on its enhanced expression in endothelium29,41 and platelets,41 key cell types in thrombosis development. PDGFs have been reported to induce pathological mesenchymal responses in a variety of vascular disorders, including atherosclerosis, pulmonary hypertension, and restenosis,42 but, to our knowledge, PDGFB has not previously been linked to VTE.
GPX3 and HIVEP1 were identified as VTE associated in VEBIOS. The same pattern of association was also observed in FARIVE, but it did not reach the prespecified level of statistical significance. GPX3 was included as a discovery target as it has previously been associated with arterial thrombosis,43 and there is a proposed overlap between arterial CVD and VTE.44,45 Levels of GPX3 were lower in cases than controls in VEBIOS (the same trend was observed in FARIVE [P = .036]), a pattern of association also previously observed in stroke.43 HIVEP1 was included as a discovery target due to its identification as a susceptibility gene for VTE in a GWA study.37 Although HIVEP1 was not significantly elevated in cases compared with controls in FARIVE replication study, a trend dependent on the VTE-associated rs169713 polymorphism identified in the context of GWAS37 was observed. The biological function of HIVEP1 is unknown, but it is suggested to play a role in inflammation,37 providing a link to a thrombosis-related mechanism.37
The strength of our study is the design, involving a discovery study followed by a replication of tentative targets in an independent study adopting a stringent statistical approach. As in all omics research, proteomics data are multidimensional and at risk of overfitting, thereby leading to false positives. To reduce the risk of pursuing chance findings, we ran the discovery analysis in 2 independent analyses using citrate and EDTA plasma from the same patients, verifying a good concordance for our 4 tentative targets in the 2 sample types before proceeding to replication in FARIVE. Finally, VEBIOS and FARIVE have used similar protocols for collection, handling, and storage of blood samples for cases and controls. Important confounders, for example, age and body mass index (BMI), which influence protein profile, are similar in the 2 studies. Several limitations should also be acknowledged. The Bonferroni correction used in the discovery study could be too stringent and lead to elimination of interesting candidates prior to validation. With a reduced statistical cutoff (P < .01 in both citrate and EDTA plasma), we identify 24 further provisional VTE-associated candidate proteins in VEBIOS Discovery (supplemental Table 1). This list contains both proteins previously linked to VTE, such as FVIII,39,40 and those with no current established VTE association, such as Coartactin. Further exploration of this candidate selection in other studies is warranted. VEBIOS and FARIVE are observational studies that can be prone to different biases. Recruitment of the controls was random and population based in VEBIOS, but hospital based in FARIVE. This recruitment is reflected by the high frequency of cardiovascular risk factors in FARIVE compared with VEBIOS (Tables 1 and 2). However, in FARIVE, we found no association between PDGFB levels and the cardiovascular risk factors of smoking and obesity (BMI > 30), and also no association with family history (data not shown). Another bias could be related to the difference in time of sampling of the VTE patients in relation to diagnosis. In FARIVE, cases were sampled in the acute phase when anticoagulant treatment was initiated while sampling in VEBIOS took place after withdrawal of treatment (≥7 months after diagnosis). An inflammatory response to the VTE event as well as effects of anticoagulant treatment could affect the plasma protein profile in the acute phase. Nevertheless, we were able to replicate 2 of the 4 candidates, regardless of influence of anticoagulant treatment with VKA (data not shown). There are also technological limitations to be acknowledged. In the single binder assay, all proteins are labeled with the same detection molecule (biotin). The detection limit of the current single binder assay technology is in the subnanogram range (0.1-1 ng/mL), and differences in plasma concentrations for protein targets of low abundance, such as many interleukins, will remain undetected.46 To exclude the possibility of off-target antibody binding to other proteins, verification is mandatory.32 In our case, PDGFB was verified as a target protein of the SBA assay by both IC-MS and a sandwich (dual binder) assay, using the same bead-coupled capture antibody and compared with absolute quantification using a commercial immunoassay platform. Due to assay-dependent differences in sensitivity at different plasma concentrations, strict linearity between SBA measurements in MFI (shown in Figure 1) and absolute quantifications in pictogram per milliliter may not exist over the full concentration range of PDGFB in plasma. Thus, significant associations determined by single binder analysis require further validation, for example, development of clinical sandwich assays to enable absolute quantification and thus more precise risk estimation and assessment of potential to improve current risk scores for prediction.4-7,47-49 VTE is a multigene disease, and a combined analysis of several candidate proteins in plasma could be more useful for predication than any individual marker. To be able to properly evaluate multiprotein VTE-associated signatures, which may incorporate both increased and decreased protein levels concurrently, absolute protein concentrations would also be required.
To conclude, we present the first application of affinity proteomics for large-scale VTE plasma biomarker screening, identify PDGFB as a novel VTE-associated plasma protein, and present a panel of 26 additional proteins of interest worthy of further exploration. Considering the recent availability of affinity reagents of near-proteome coverage in the HPA, our pioneering work demonstrates the feasibility of proteome-wide screenings for novel biomarkers for VTE.
This article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
On behalf of the VEBIOS group, the authors thank Anna Fahlén for recruiting controls and handling the blood samples and Biobank and Doris Näslin for advice regarding the case report form and sampling protocol. Eva-Lotta Hempel, Anna Ågren, Ammar Majeed, and other colleagues at Coagulation Unit at Karolinska University Hospital are thanked for recruiting patients to the study. On behalf of the FARIVE group, Anne Roche is acknowledged for providing information on blood sampling. The authors particularly thank the Biobank Profiling group at SciLifeLab and the staff of the HPA for their efforts enabling this study.
M.B., M.J.I., and J.O. were funded by Stockholm County Council (SLL20150924 and SLL20130741). The KTH Center for Applied Proteomics, funded by the Erling-Persson Family Foundation, is acknowledged for financial support. This study was further supported by grants from Science for Life Laboratory, the Swedish Heart Lung Foundation (20140691), and the Knut and Alice Wallenberg Foundation. D.A.T. and B.G. were supported by the ICAN Institute for Cardiometabolism and Nutrition (ANR-10-IHU-05). This research was also partially supported by the French Clinical Research Infrastructure Network on Venous Thrombo-Embolism.
Authorship
Contribution: M.B., M.J.I., J.M.S., D.-A.T., P.-E.M., and J.O. designed the analysis protocols; M.B., M.J.I., J.C.S., A.S., J.M.S., R.J.S., L.M.B., D.-A.T., P.-E.M., and J.O. selected target candidate panels; M.B., M.-G.H., A.H., M.H., and J.O. designed VEBIOS; M.U. and J.M.S. provided the HPA antibodies and SBA technology; M.B., A.S., and M.J.I. coordinated sample handling for VEBIOS; and M.J.I., D.M.S., and P.-E.M. coordinated sample handling for FARIVE. M.F. constructed the VEBIOS clinical database; D.M.S. and M.B. constructed the FARIVE clinical database; M.J.I. ran the affinity proteomic experiments and verified the SBA assay; L.S.-R. and C.F. performed the IC-MS analysis; M.-G.H. and D.-A.T. performed the statistical association analyses with assistance of M.B., B.G., and M.J.I.; M.B., M.J.I., L.S.-R., P.-E.M., D.-A.T., L.M.B., and J.O. participated in the writing of the manuscript. All authors were involved in the editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jacob Odeberg, Department of Proteomics, School of Biotechnology, Royal Institute of Technology, Science for Life Laboratory, Tomtebodavägen 23, Box 1031, Solna, Stockholm, SE17121 Sweden; e-mail: jacob.odeberg@scilifelab.se.
References
Author notes
M.B. and M.J.I. contributed equally to this study.