Key Points
Ticagrelor acts as an inverse agonist at the P2Y12R, inhibiting basal agonist-independent signaling.
Ticagrelor inhibits the adenosine transporter ENT1 not only on erythrocytes, but on platelets too.
Abstract
Ticagrelor is a potent antagonist of the P2Y12 receptor (P2Y12R) and consequently an inhibitor of platelet activity effective in the treatment of atherothrombosis. Here, we sought to further characterize its molecular mechanism of action. Initial studies showed that ticagrelor promoted a greater inhibition of adenosine 5′-diphosphate (ADP)–induced Ca2+ release in washed platelets vs other P2Y12R antagonists. This additional effect of ticagrelor beyond P2Y12R antagonism was in part as a consequence of ticagrelor inhibiting the equilibrative nucleoside transporter 1 (ENT1) on platelets, leading to accumulation of extracellular adenosine and activation of Gs-coupled adenosine A2A receptors. This contributed to an increase in basal cyclic adenosine monophosphate (cAMP) and vasodilator-stimulated phosphoprotein phosphorylation (VASP-P). In addition, ticagrelor increased platelet cAMP and VASP-P in the absence of ADP in an adenosine receptor–independent manner. We hypothesized that this increase originated from a direct effect on basal agonist-independent P2Y12R signaling, and this was validated in 1321N1 cells stably transfected with human P2Y12R. In these cells, ticagrelor blocked the constitutive agonist-independent activity of the P2Y12R, limiting basal Gi-coupled signaling and thereby increasing cAMP levels. These data suggest that ticagrelor has the pharmacological profile of an inverse agonist. Based on our results showing insurmountable inhibition of ADP-induced Ca2+ release and forskolin-induced cAMP, the mode of antagonism of ticagrelor also appears noncompetitive, at least functionally. In summary, our studies describe 2 novel modes of action of ticagrelor, inhibition of platelet ENT1 and inverse agonism at the P2Y12R that contribute to its effective inhibition of platelet activation.
Introduction
Acute coronary syndrome (ACS) is among the leading causes of death in the world.1 Platelets play a pivotal role in the pathogenesis of ACS. Following atherosclerotic plaque rupture, platelets are exposed to potent agonists, collagen and thrombin, that trigger platelet activation and aggregation. Subsequent release of ADP from activated platelets and its activation of platelet P2Y12 receptors (P2Y12Rs) have a central role in amplifying the response to the initial stimulus. P2Y12R signaling is therefore well established as a major positive feed-forward amplification mechanism to a wide variety of platelet agonists. Pharmacological blockade of the P2Y12R represents an important and clinically well-validated target for the treatment and prevention of thrombosis.2,3
Unlike the thienopyridine P2Y12R antagonists (ticlopidine, clopidogrel, and prasugrel), ticagrelor binds to the P2Y12R in a reversible manner.4,5 Also unlike the thienopyridines, which are all prodrugs requiring metabolic activation to exert an antiplatelet effect, ticagrelor is direct acting. In addition, its main circulating metabolite, AR-C124910XX (present in plasma at 30% to 40% of parent6 ), has similar potency at the P2Y12R as ticagrelor.7 In comparison with clopidogrel, ticagrelor provides higher and more consistent platelet inhibition.8,9 Large clinical trials across 43 countries in patients with ACS have demonstrated a lower fatality rate because of adverse cardiovascular events overall, without an increase in serious bleeding in patients treated with ticagrelor and aspirin compared with clopidogrel and aspirin.2,10 Ticagrelor is also superior to placebo when given on top of aspirin in patients with a prior myocardial infarction.11 Intriguingly, ticagrelor has recently been shown to inhibit the equilibrative nucleoside transporter 1 (ENT1),12 an adenosine transporter, on red blood cells and thereby to increase extracellular adenosine levels in vitro and in the plasma of ticagrelor-treated patients.13-16 Ticagrelor has also been shown to augment a number of physiological responses induced by adenosine, including increased coronary blood flow and adenosine-dependent inhibition of platelet aggregation.15,17,18 Some adverse effects associated with ticagrelor include dyspnoea and ventricular pauses, effects also seen with intracoronary administration of exogenous adenosine.19,20
The aim of this study was to further elucidate the molecular mode of action of ticagrelor on platelets beyond its well-established antagonism of the P2Y12R. Using isolated human platelets, we tested whether ticagrelor’s inhibition of adenosine transport could contribute to changes in downstream signaling. We also verified whether these effects could be explained by the inhibition of platelet-expressed ENT1 by ticagrelor. We also tested the hypothesis that ticagrelor blocks constitutive agonist-independent P2Y12R activity. The aim was to determine whether ticagrelor is an inverse agonist, unlike the active metabolite (R-138727) of the thienopyridine prasugrel, and thus help us gain a better comprehension of ticagrelor’s efficacy.
Materials and methods
Reagents
Membrane-stripping solution and Fura-2 AM were from Thermo Fisher Scientific (Northumberland, United Kingdom). Nonselective adenosine receptor agonist (NECA), xanthine amine congener (XAC), AR-C 66096 tetrasodium salt, PSB 0739, and 6-S-[(4-nitrophenyl)methyl]-6-thioinosine (NBMPR) were procured from Tocris Bioscience (Bristol, United Kingdom). R-138727 was obtained from Eli Lilly Research Laboratories (Indianapolis, IN). Ticagrelor was provided by AstraZeneca (Mölndal, Sweden). Cell culture reagents included Dulbecco's modified Eagle medium, fetal calf serum, penicillin-streptomycin, and G418, which were from Invitrogen (Paisley, United Kingdom). Commercial antibodies used to detect phospho-VASP (Ser157), vasodilator-stimulated phosphoprotein (VASP), phospho-ERK, and extracellular signal-regulated kinase (ERK) were from New England Biolabs (Hertfordshire, United Kingdom); α-tubulin was from Sigma-Aldrich (Dorset, United Kingdom); ENT1 was from Abcam (Cambridge, United Kingdom); and secondary mouse and rabbit horseradish peroxidase–conjugated antibodies were from GE Healthcare (Buckinghamshire, United Kingdom). All other reagents were purchased from Sigma-Aldrich.
Preparation of human platelets
Human blood was collected after informed consent from healthy, drug-free volunteers as described previously.21 Platelets were rested for at least half an hour in the presence of 0.02 U/mL apyrase and 10 µM indomethacin prior to use in the experiments. All platelet preparations were conducted at room temperature, and platelets were stored at 30°C.
Light transmission aggregometry
Platelet rich plasma (PRP) samples stored at 30°C were preheated to 37°C for 2 minutes and stirred at 1000 rpm in an aggregometer (Chronolog Model 700, Labmedics, Manchester, United Kingdom) before adding adenosine 5′-diphosphate (ADP; 10 µM). The change in optical density relative to platelet poor plasma (100% aggregation reference) was recorded for 6 minutes, and maximum aggregation extent was used for data analysis.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
Protein samples were boiled in sodium dodecyl sulfate sample buffer and subjected to western analysis as previously described.22 Where required, blots were stripped using Restore western blot stripping solution (Thermo Scientific) and reprobed for protein of interest. Quantification was performed by densitometry using the ImageJ software (National Institutes of Health, Bethesda, MD). Mean integrated density of bands of interest was used as a quantitative measurement of immunoblot intensity, divided by the mean integrated density of total protein (α-tubulin band), then normalized to the relevant control levels.
Measurement of cytosolic free Ca2+ in platelets
Measurement of intracellular Ca2+ release was adapted from a method used previously.22 PRP was incubated with 4 μM Fura-2 AM (Life Technologies, Paisley, United Kingdom) in the presence of 0.02 U/mL apyrase and 10 µM indomethacin at 30°C for 1 hour. Platelets were pelleted by centrifugation at 550g for 10 minutes and resuspended in a modified Tyrode’s N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (containing 0.02 U/mL apyrase and 10 µM indomethacin) to a final concentration of 4 × 108/mL. Platelets were allowed to rest in the presence of vehicle (0.1% dimethyl sulfoxide [DMSO]) or P2Y12R antagonist for 30 minutes at 30°C. ADP (10 µM)–induced changes in Ca2+ levels were subsequently monitored at 37°C using a Tecan Infinite M200 PRO microplate reader (Labtech International Ltd., East Sussex, United Kingdom), with fluorescence excitation set at 340 and 380 nm, and emission at 510 nm.
Measurement of platelet cAMP levels
Washed platelets were stimulated in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IMBX) (100 µM), and drug-induced changes in platelet cyclic adenosine monophosphate (cAMP) levels were assessed as described before.23
Cell culture
The 1321N1 cells stably expressing either hemagglutinin-tagged P2Y12 or pcNEO vector were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum, 100 U/mL penicillin-streptomycin, and 400 μg/mL G418 (Geneticin) at 37°C in a humidified atmosphere of 95% air and 5% CO2. The cloning and stable expression of P2Y12 tagged at the N terminus with hemagglutinin in 1321N1 cells has previously been described.23
Measurement of P2Y12R activity in 1321N1 cells
P2Y12R-stimulated inhibition of forskolin-stimulated adenylyl cyclase was measured in 1321N1 cells as previously described.23 Changes in cell VASP phosphorylation (VASP-P) levels were assessed by immunoblotting as described previously. Drug-induced changes in cell cAMP levels were determined using a cAMP Enzyme Immunoassay Kit according to the manufacturer’s instructions.
Statistical analysis
Graphs were generated using GraphPad Prism (San Diego, CA). Concentration-response curves were fitted to the data via a variable slope model of nonlinear regression. Data points are displayed as mean ± standard error. One-way analysis of variance (ANOVA), unless otherwise specified, was used to detect statistically significant differences, and Bonferroni post hoc analysis was applied.
Results
Ticagrelor-mediated inhibition of platelet Ca2+ release is partly adenosine receptor dependent
Ticagrelor, AR-C 66096, and the active metabolite of prasugrel, R-138727, all inhibited ADP (10 μM)–induced platelet aggregation in human PRP in a concentration-dependent manner (Figure 1A). Ticagrelor (10 μM) significantly decreased the peak Ca2+ response to ADP (10 μM; Figure 1B) with no evident change in the overall shape of the calcium trace vs that seen with agonist alone (data not shown). The P2Y12R antagonists AR-C 66096 (10 μM) and R-138727 (10 μM) also inhibited Ca2+ release, although to a lesser extent than ticagrelor, as evident by the higher ADP-mediated maximum effect (Emax) values (Table 1).
ADP-induced platelet aggregation and intracellular Ca2+ release. (A) ADP (10 µM)–induced platelet aggregation in human PRP after 30-minute incubation at 30°C with ticagrelor (IC50 = 0.27 μM [0.14-0.51]; n = 3), AR-C 66096 (IC50 = 0.16 μM [0.08-0.33]; n = 4), or R-138727 (IC50 = 3.82 μM [3.03-4.82]; n = 6). The 95% confidence intervals for IC50 values are shown in square brackets. (B) ADP-induced Ca2+ peak responses in Fura-2 AM–loaded platelets in the presence of vehicle (n = 16) or 10 µM ticagrelor (n = 22), AR-C 66096 (n = 12), or R-138727 (n = 12). Baseline readings were recorded over a period of 5 cycles (each cycle ∼7 seconds) before ADP addition, after which recording was continued for a further 15 cycles. The peak Ca2+ response was determined by calculating the change in fluorescence ratio (340 nm/380 nm). (C) ADP (100 µM)–induced platelet peak Ca2+ responses in the presence of vehicle (0.1% DMSO), ticagrelor (10 µM), ticagrelor (10 µM) + XAC (10 µM), AR-C 66096 (10 µM), and R-138727 (10 µM). Data displayed as mean and standard error of the mean (SEM). *P < .05. IC50, 50% inhibitory concentration.
ADP-induced platelet aggregation and intracellular Ca2+ release. (A) ADP (10 µM)–induced platelet aggregation in human PRP after 30-minute incubation at 30°C with ticagrelor (IC50 = 0.27 μM [0.14-0.51]; n = 3), AR-C 66096 (IC50 = 0.16 μM [0.08-0.33]; n = 4), or R-138727 (IC50 = 3.82 μM [3.03-4.82]; n = 6). The 95% confidence intervals for IC50 values are shown in square brackets. (B) ADP-induced Ca2+ peak responses in Fura-2 AM–loaded platelets in the presence of vehicle (n = 16) or 10 µM ticagrelor (n = 22), AR-C 66096 (n = 12), or R-138727 (n = 12). Baseline readings were recorded over a period of 5 cycles (each cycle ∼7 seconds) before ADP addition, after which recording was continued for a further 15 cycles. The peak Ca2+ response was determined by calculating the change in fluorescence ratio (340 nm/380 nm). (C) ADP (100 µM)–induced platelet peak Ca2+ responses in the presence of vehicle (0.1% DMSO), ticagrelor (10 µM), ticagrelor (10 µM) + XAC (10 µM), AR-C 66096 (10 µM), and R-138727 (10 µM). Data displayed as mean and standard error of the mean (SEM). *P < .05. IC50, 50% inhibitory concentration.
Maximal responses (Emax) from ADP concentration vs Ca2+ peak response curves (as calculated in GraphPad Prism) obtained in the presence of different P2Y12R antagonists
| Drug . | Emax (AU) . | P relative to control . | P relative to ticagrelor . | n . |
|---|---|---|---|---|
| Vehicle control | 0.92 [0.88-0.96] | NA | *** | 16 |
| Ticagrelor | 0.41 [0.38-0.45] | *** | NA | 22 |
| AR-C 66096 | 0.61 [0.56-0.66] | *** | *** | 12 |
| R-138727 | 0.61 [0.57-0.66] | *** | *** | 12 |
| Drug . | Emax (AU) . | P relative to control . | P relative to ticagrelor . | n . |
|---|---|---|---|---|
| Vehicle control | 0.92 [0.88-0.96] | NA | *** | 16 |
| Ticagrelor | 0.41 [0.38-0.45] | *** | NA | 22 |
| AR-C 66096 | 0.61 [0.56-0.66] | *** | *** | 12 |
| R-138727 | 0.61 [0.57-0.66] | *** | *** | 12 |
Responses were recorded from washed platelets after incubation with vehicle (0.1% DMSO) or test compound (all at 10 µM; 30 minutes). The 95% confidence for Emax values is shown in square brackets. ***P < .001.
NA, not applicable.
Preincubation with the adenosine receptor antagonist XAC (10 μM) partly reversed the peak Ca2+ response in ticagrelor-treated platelets to levels comparable to Ca2+ responses measured in AR-C 66096–treated and R-138727–treated platelets (Figure 1C). XAC (10 μM) treatment had no further effect on AR-C 66096–treated and R-138727–treated platelets (data not shown).
Ticagrelor induces an adenosine receptor–dependent increase in VASP-P in platelets
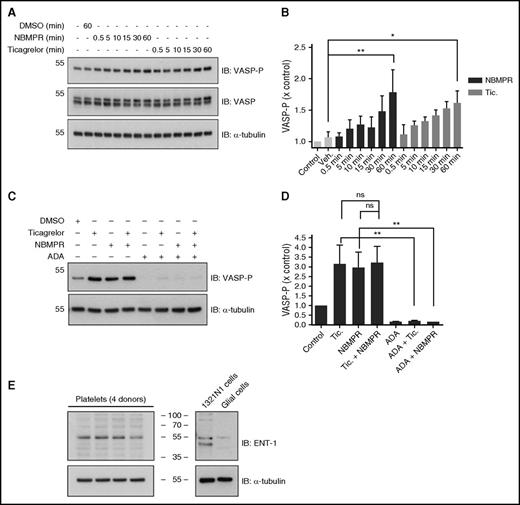
Incubation of washed platelets with ticagrelor induced a time- and concentration-dependent increase in VASP-P in the absence of platelet agonist (Figure 2A-D). Ticagrelor (10 µM) induced a significant increase in VASP-P levels by 30 minutes with an upward trend at earlier time points (Figure 2A-B). Washed platelets incubated for a fixed time period (60 minutes) with a range of ticagrelor concentrations (0.1-10 µM) showed significant increases in VASP-P levels above basal, with concentrations >3 µM (Figure 2C-D). Unlike ticagrelor (10 µM), none of the 3 alternative P2Y12R antagonists, AR-C 66096, PSB 0739, and R-138727 (10 µM), were able to increase VASP-P beyond basal levels after incubation with platelets for 60 minutes (Figure 2E-F).
Time- and concentration-dependence of increases in platelet VASP-P. Representative immunoblots and bar/x-y charts showing VASP-P, VASP, and α-tubulin (internal control) levels in washed platelets after preincubation at 37°C with the following: (A-B) Ticagrelor (10 µM) for 0, 0.5, 5, 10, 15, 30, and 60 minutes; n = 5. (C-D) Vehicle (0.1% DMSO) and 0.1, 0.3, 1, 3, and 10 µM ticagrelor for 60 minutes; n = 5. (E-F) Untreated, vehicle (0.1% DMSO), ticagrelor (10 µM), AR-C 66096 (10 µM), PSB 0739 (10 µM), and R-138727 (10 µM) for 60 minutes; n = 4. (G-H) Untreated, vehicle (0.1% DMSO), ticagrelor (10 µM), and NECA (µM) for 60 minutes following preincubation at 37°C for 30 minutes with untreated, vehicle (0.1% DMSO), or XAC (1 µM); n = 6. Data displayed as mean and SEM. **P < .01; ***P < .001; ns, P > .05. Statistical analysis used was 2-way ANOVA (H). Kilodaltons of molecular size markers are reported on the left.
Time- and concentration-dependence of increases in platelet VASP-P. Representative immunoblots and bar/x-y charts showing VASP-P, VASP, and α-tubulin (internal control) levels in washed platelets after preincubation at 37°C with the following: (A-B) Ticagrelor (10 µM) for 0, 0.5, 5, 10, 15, 30, and 60 minutes; n = 5. (C-D) Vehicle (0.1% DMSO) and 0.1, 0.3, 1, 3, and 10 µM ticagrelor for 60 minutes; n = 5. (E-F) Untreated, vehicle (0.1% DMSO), ticagrelor (10 µM), AR-C 66096 (10 µM), PSB 0739 (10 µM), and R-138727 (10 µM) for 60 minutes; n = 4. (G-H) Untreated, vehicle (0.1% DMSO), ticagrelor (10 µM), and NECA (µM) for 60 minutes following preincubation at 37°C for 30 minutes with untreated, vehicle (0.1% DMSO), or XAC (1 µM); n = 6. Data displayed as mean and SEM. **P < .01; ***P < .001; ns, P > .05. Statistical analysis used was 2-way ANOVA (H). Kilodaltons of molecular size markers are reported on the left.
Similar to ticagrelor, NECA (1 µM; 60 minutes) induced an increase in VASP-P (Figure 2G-H). Preincubating washed platelets with the unselective adenosine receptor antagonist XAC (1 µM; 30 minutes) attenuated, but did not completely abolish, subsequent increases in VASP-P induced by ticagrelor (10 µM; 60 minutes) or NECA (10 µM; 60 minutes) (Figure 2G-H). The inhibitory effect of XAC was numerically but not significantly greater in combination with NECA as compared with ticagrelor. The adenosine receptor–dependent increase in VASP-P by ticagrelor was also observed in the presence of 1 mM extracellular Ca2+ (data not shown). Preincubation with the A2A adenosine receptor–selective antagonist SCH 442416 (1 µM; 30 minutes) significantly attenuated (P < .05; n = 6) subsequent increases in VASP-P induced by ticagrelor (10 µM; 60 minutes), whereas the A2B adenosine receptor–selective antagonist PSB 603 (1 µM; 30 minutes) had no effect. VASP-P levels (fold over basal) were 9.19 ± 2.98, 0.92 ± 0.37, and 8.54 ± 3.97 for ticagrelor alone, ticagrelor + SCH 442416, and ticagrelor + PSB 603, respectively.
Ticagrelor-mediated inhibition of platelet ENT1 likely explains adenosine-related effects
Incubation of washed platelets with the ENT1-specific inhibitor, NBMPR (1 µM), induced a time-dependent increase (P < .01) in VASP-P (Figure 3A-B). This increase was comparable to that seen with ticagrelor (10 µM). NBMPR in combination with ticagrelor did not significantly increase VASP-P compared with ticagrelor or NBMPR alone (Figure 3C-D). Preincubation of platelets with adenosine deaminase (ADA), which degrades adenosine into inosine, together with NBMPR or ticagrelor, prevented both NBMPR-mediated (P < .01) and ticagrelor-mediated (P < .01) increases in VASP-P. Direct evidence of the presence of ENT1 in human platelets was obtained by immunoblotting (Figure 3E) using an ENT1-specific antibody.
Role for platelet-expressed ENT1 in the ticagrelor-induced increase in platelet VASP-P. Representative immunoblots and bar charts showing VASP-P, VASP, and α-tubulin (internal control) levels in washed platelets after incubation at 37°C with the following: (A-B) No drug, vehicle (0.1% DMSO for 60 minutes), NBMPR (1 µM for 0.5, 5, 10, 15, 30, and 60 minutes), or ticagrelor (10 µM for 0.5, 5, 10, 15, 30, and 60 minutes); n = 5. (C-D) Vehicle (0.1% DMSO), ticagrelor (10 µM), NBMPR (1 µM), ticagrelor (10 µM) + NBMPR (1 µM), ADA (10 µg/mL), ADA (10 µg/mL) + ticagrelor (10 µM), and ADA + NBMPR (1 µM); n = 3. (E) Immunoblots showing expression of ENT1 in platelets and 1321N1 cells, with rat glial cells as negative control. Figure is representative of 3 replicates. Data displayed as mean and SEM. *P < .05; **P < .01; ns, P > .05. Statistical test used was 2-way ANOVA (B). Kilodaltons of molecular size markers are reported on the left or in the middle for panel E.
Role for platelet-expressed ENT1 in the ticagrelor-induced increase in platelet VASP-P. Representative immunoblots and bar charts showing VASP-P, VASP, and α-tubulin (internal control) levels in washed platelets after incubation at 37°C with the following: (A-B) No drug, vehicle (0.1% DMSO for 60 minutes), NBMPR (1 µM for 0.5, 5, 10, 15, 30, and 60 minutes), or ticagrelor (10 µM for 0.5, 5, 10, 15, 30, and 60 minutes); n = 5. (C-D) Vehicle (0.1% DMSO), ticagrelor (10 µM), NBMPR (1 µM), ticagrelor (10 µM) + NBMPR (1 µM), ADA (10 µg/mL), ADA (10 µg/mL) + ticagrelor (10 µM), and ADA + NBMPR (1 µM); n = 3. (E) Immunoblots showing expression of ENT1 in platelets and 1321N1 cells, with rat glial cells as negative control. Figure is representative of 3 replicates. Data displayed as mean and SEM. *P < .05; **P < .01; ns, P > .05. Statistical test used was 2-way ANOVA (B). Kilodaltons of molecular size markers are reported on the left or in the middle for panel E.
Ticagrelor induces an adenosine receptor–independent increase in VASP-P and cAMP in platelets
XAC preincubation (10 µM) reduced basal VASP-P (Figure 4A-C), supporting the presence of endogenous adenosine and adenosine receptor signaling in our washed platelet preparations. In the presence of XAC, ticagrelor (10 µM) produced a time-dependent (Figure 4A-B) and concentration-dependent (Figure 4C-D) increase in VASP-P. The adenosine receptor agonist NECA (1 µM; Figure 4E-F) and the P2Y12R antagonists AR-C 66096, PSB 0739, and R-138727 (all at 10 µM), however, did not affect VASP-P levels under the same conditions (Figure 4G-H). Ticagrelor was also still able to increase VASP-P in the presence of AR-C 66096, PSB 0739, or R-138727 (10 μM; Figure 5A-B).
Identification of an adenosine receptor-independent component of the ticagrelor-induced increase in platelet VASP-P and cAMP. Representative immunoblots and bar/x-y charts showing VASP-P, VASP, and α-tubulin (loading control) levels in washed platelets after incubation at 37°C with the following: (A-B) Vehicle (0.1% DMSO for 60 minutes) or 10 µM ticagrelor for 0.5, 5, 10, 15, 30, and 60 minutes following preincubation of all samples (except vehicle control) with XAC (10 µM, at 37°C) for 90 minutes; n = 3. (C-D) Vehicle (0.1% DMSO), 0.1, 0.3, 1, 3, and 10 µM ticagrelor for 60 minutes following preincubation of all samples (except vehicle control) with XAC (10 µM at 37°C) for 90 minutes; n = 5. (E-F) No drug, vehicle (0.1% DMSO), ticagrelor (10 µM), and NECA (1 µM) for 60 minutes following preincubation of all samples (except vehicle control) with XAC (10 µM at 37°C) for 90 minutes; n = 5. (G-H) Vehicle (0.1% DMSO), ticagrelor (10 µM), AR-C 66096 (10 µM), PSB 0739 (10 µM), and R-138727 (10 µM) for 60 minutes. *P < .05; **P < .01; ***P < .001; ns, P > .05. Kilodaltons of molecular size markers are shown on left of blots.
Identification of an adenosine receptor-independent component of the ticagrelor-induced increase in platelet VASP-P and cAMP. Representative immunoblots and bar/x-y charts showing VASP-P, VASP, and α-tubulin (loading control) levels in washed platelets after incubation at 37°C with the following: (A-B) Vehicle (0.1% DMSO for 60 minutes) or 10 µM ticagrelor for 0.5, 5, 10, 15, 30, and 60 minutes following preincubation of all samples (except vehicle control) with XAC (10 µM, at 37°C) for 90 minutes; n = 3. (C-D) Vehicle (0.1% DMSO), 0.1, 0.3, 1, 3, and 10 µM ticagrelor for 60 minutes following preincubation of all samples (except vehicle control) with XAC (10 µM at 37°C) for 90 minutes; n = 5. (E-F) No drug, vehicle (0.1% DMSO), ticagrelor (10 µM), and NECA (1 µM) for 60 minutes following preincubation of all samples (except vehicle control) with XAC (10 µM at 37°C) for 90 minutes; n = 5. (G-H) Vehicle (0.1% DMSO), ticagrelor (10 µM), AR-C 66096 (10 µM), PSB 0739 (10 µM), and R-138727 (10 µM) for 60 minutes. *P < .05; **P < .01; ***P < .001; ns, P > .05. Kilodaltons of molecular size markers are shown on left of blots.
Ticagrelor-mediated, adenosine receptor–independent increases in platelet VASP-P in the presence of other P2Y12 antagonists. (A-B) Representative immunoblots and bar charts showing VASP-P, VASP, and α-tubulin (internal control) levels in washed platelets after incubation (60 minutes) at 37°C with vehicle (0.1% DMSO), ticagrelor (10 µM), or NECA (1 µM). Preincubation conditions were either vehicle (0.1% DMSO) + XAC (10 µM), AR-C 66096 (10 µM) + XAC (10 µM), PSB 0739 (10 µM) + XAC (10 µM), or R-138727 (10 µM) + XAC (10 µM), all at 37°C for 30 minutes; n = 7. (C-D) cAMP accumulation in washed platelets. (C) Platelets were preincubated with either ticagrelor, AR-C 66096, and R-138727 (all at 10 μM; 60 minutes) or vehicle control. Platelets were stimulated with PGE1 (1 μM; 10 minutes) in the absence or presence of ADP (10 μM). (D) Effect of antagonists on basal cAMP levels. Incubation (60 minutes) at 37°C with vehicle (0.1% DMSO), ticagrelor (10 µM), PSB 0739 (10 µM), or R-138727 (10 µM) for 60 minutes and preincubation with XAC (10 μM; 60 minutes). All bar charts display mean and SEM. Statistical test used was 2-way ANOVA (excluding NECA data) (B). *P < .05; **P < .01; ***P < .001. Kilodaltons of molecular size markers are shown on blots.
Ticagrelor-mediated, adenosine receptor–independent increases in platelet VASP-P in the presence of other P2Y12 antagonists. (A-B) Representative immunoblots and bar charts showing VASP-P, VASP, and α-tubulin (internal control) levels in washed platelets after incubation (60 minutes) at 37°C with vehicle (0.1% DMSO), ticagrelor (10 µM), or NECA (1 µM). Preincubation conditions were either vehicle (0.1% DMSO) + XAC (10 µM), AR-C 66096 (10 µM) + XAC (10 µM), PSB 0739 (10 µM) + XAC (10 µM), or R-138727 (10 µM) + XAC (10 µM), all at 37°C for 30 minutes; n = 7. (C-D) cAMP accumulation in washed platelets. (C) Platelets were preincubated with either ticagrelor, AR-C 66096, and R-138727 (all at 10 μM; 60 minutes) or vehicle control. Platelets were stimulated with PGE1 (1 μM; 10 minutes) in the absence or presence of ADP (10 μM). (D) Effect of antagonists on basal cAMP levels. Incubation (60 minutes) at 37°C with vehicle (0.1% DMSO), ticagrelor (10 µM), PSB 0739 (10 µM), or R-138727 (10 µM) for 60 minutes and preincubation with XAC (10 μM; 60 minutes). All bar charts display mean and SEM. Statistical test used was 2-way ANOVA (excluding NECA data) (B). *P < .05; **P < .01; ***P < .001. Kilodaltons of molecular size markers are shown on blots.
Prostaglandin E1 (PGE1) (1 μM), a well-established stimulator of cAMP production through activation of platelet prostanoid receptors, increased cAMP levels, which was attenuated by P2Y12R activation with ADP (10 μM; Figure 5C). As expected, the P2Y12 antagonists ticagrelor, AR-C 66096, and R-138727 (10 μM) attenuated the ADP-mediated reduction in cAMP levels (Figure 5C). However, only ticagrelor (10 μM; 60 minutes) could modulate basal cAMP levels as evident by a twofold increase in cAMP (P < .05). Preincubation with XAC (10 μM; 30 minutes) (Figure 5D) partially attenuated this ticagrelor-induced increase in cAMP. However, even in the presence of XAC, ticagrelor still significantly increased platelet cAMP levels, indicative of an adenosine-independent component in addition to the adenosine-dependent effect.
Ticagrelor also induces an adenosine receptor–independent increase in VASP-P in 1321N1 cells expressing the P2Y12R
Given that ticagrelor increased basal platelet cAMP and VASP-P levels in the absence of adenosine receptor signaling, we hypothesized that ticagrelor was directly modulating agonist-independent P2Y12R activity. In order to test this, we used P2 receptor–null 1321N1 human astrocytoma cells overexpressing the human P2Y12R.24,25
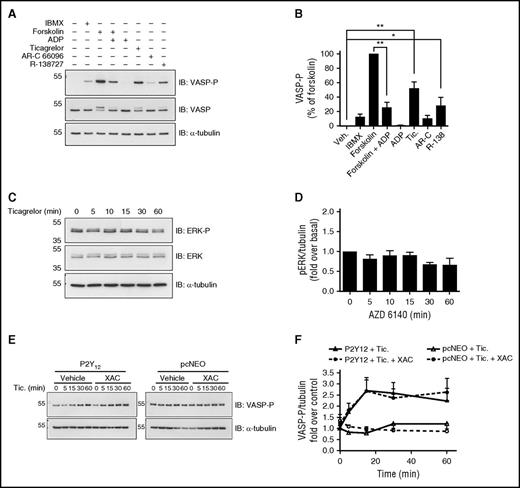
Preincubation with IBMX (100 µM; 15 minutes), a phosphodiesterase inhibitor preventing enzymatic breakdown of cAMP, increased VASP-P as did forskolin (100 nM; 5 minutes), a direct activator of adenylyl cyclase. Forskolin-stimulated increases in VASP-P were, as expected, attenuated by P2Y12R stimulation with ADP (10 μM; 5 minutes). Ticagrelor (10 μM; 5 minutes) increased VASP-P levels above basal whereas the other P2Y12 antagonists, AR-C 66096 and R-138727 (10 μM; 5 minutes), produced more modest effects (Figure 6A-B).
Ticagrelor-mediated adenosine receptor-independent increases in VASP-P in 1321N1 cells expressing P2Y12R. Representative immunoblots and bar/x-y charts showing the following: (A-B) VASP-P, VASP, and α-tubulin (internal control) levels in P2Y12-transfected 1321N1 cells incubated with phosphodiesterase inhibitor IBMX (100 µM), adenylyl cyclase activator forskolin (100 nM), forskolin (100 nM) + ADP (10 µM), ADP (10 µM), ticagrelor (10 µM), AR-C 66096 (10 µM), or R-138727 (10 µM) for 5 minutes at 37°C; n = 5. (C-D) Extracellular signal–regulated kinases (ERK)-P, ERK, and α-tubulin (internal control) levels in P2Y12-transfected 1321N1 cells incubated with ticagrelor for 0, 5, 10, 15, 30, and 60 minutes, at 37°C; n = 5 (E-F) VASP-P and α-tubulin (internal control) levels in P2Y12-transfected (E, left) and vehicle vector pcNEO-transfected (E, right) 1321N1 cells, both preincubated with either XAC (1 µM) or vehicle (0.1% DMSO) for 15 minutes at 37°C before incubation with ticagrelor (10 µM) for 0, 5, 15, 30, and 60 minutes at 37°C. All bar/x-y charts display mean and SEM. Statistical test used was 2-way ANOVA (D). *P < .05; **P < .01. Kilodaltons of molecular size markers are reported on the left.
Ticagrelor-mediated adenosine receptor-independent increases in VASP-P in 1321N1 cells expressing P2Y12R. Representative immunoblots and bar/x-y charts showing the following: (A-B) VASP-P, VASP, and α-tubulin (internal control) levels in P2Y12-transfected 1321N1 cells incubated with phosphodiesterase inhibitor IBMX (100 µM), adenylyl cyclase activator forskolin (100 nM), forskolin (100 nM) + ADP (10 µM), ADP (10 µM), ticagrelor (10 µM), AR-C 66096 (10 µM), or R-138727 (10 µM) for 5 minutes at 37°C; n = 5. (C-D) Extracellular signal–regulated kinases (ERK)-P, ERK, and α-tubulin (internal control) levels in P2Y12-transfected 1321N1 cells incubated with ticagrelor for 0, 5, 10, 15, 30, and 60 minutes, at 37°C; n = 5 (E-F) VASP-P and α-tubulin (internal control) levels in P2Y12-transfected (E, left) and vehicle vector pcNEO-transfected (E, right) 1321N1 cells, both preincubated with either XAC (1 µM) or vehicle (0.1% DMSO) for 15 minutes at 37°C before incubation with ticagrelor (10 µM) for 0, 5, 15, 30, and 60 minutes at 37°C. All bar/x-y charts display mean and SEM. Statistical test used was 2-way ANOVA (D). *P < .05; **P < .01. Kilodaltons of molecular size markers are reported on the left.
The effect of ticagrelor on the phosphorylation state of ERK and Akt/protein kinase B, both shown to be activated downstream of P2Y12R activation,26,27 was explored. Ticagrelor produced no significant changes in ERK (Figure 6C-D) or Akt (data not shown) phosphorylation in P2Y12R-expressing cells.
Ticagrelor had no effect on VASP-P in pcNEO (vehicle vector)–transfected P2Y receptor–null cells, supporting a P2Y12-dependent effect. Further, in P2Y12R-expressing cells, pretreatment with XAC (1 μM; 15 minutes) had no effect on ticagrelor-stimulated changes in VASP-P (Figure 6E-F), supporting an adenosine-independent effect.
Ticagrelor decreases the agonist-independent activity of the P2Y12R
As expected and previously demonstrated, ADP produced a concentration-dependent decrease in forskolin-stimulated adenylyl cyclase activity (Figure 7A-B). Pretreatment with either ticagrelor (Figure 7A) or AR-C 66096 (Figure 7B) both shifted the concentration-response curve of ADP in a parallel manner. However, the highest concentration of ticagrelor (30 nM) reduced the maximal effect of the agonist ADP (Figure 7A). Schild-plot analysis of the shifts in ADP 50% effective concentration produced by increasing concentrations of ticagrelor revealed a slope of 1.73 ± 0.12 and a pA2 of 8.79 ± 0.31. Meanwhile, at all concentrations of AR-C 66096, antagonism was surmountable (Figure 7B). Schild-plot analysis of the respective shifts of in ADP 50% effective concentrations gave a slope of close to unity (1.01 ± 0.10) and a pA2 of 8.2 ± 0.22. Our Schild analysis therefore suggests that ticagrelor and AR-C 66096 are acting as noncompetitive and competitive antagonists, respectively, with pA2 values in broad agreement to those reported.28
Ticagrelor-mediated attenuation of agonist-dependent and agonist-independent P2Y12R activity in 1321N1 cells. Inhibition of forskolin (1 µM; 10 minutes)–induced cAMP production by ADP (0.1 nM-100 µM; 10 minutes) in P2Y12R-transfected 1321N1 cells after preincubation with ticagrelor (3-30 nM; 15 minutes) (A) or AR-C 66096 (AR-C; 3-30 nM; 15 minutes) (B); n = 5. (C) Schild-plot analysis of data from panels A and B. (D) Forskolin (0.1 nM-1 µM; 10 minutes)–induced increases in cAMP levels in pcNEO-transfected and P2Y12R-transfected 1321N1 cells. (E) Forskolin (1 µM; 10 minutes)–induced cAMP production in pcNEO-transfected and P2Y12-transfected cells incubated with vehicle (0.1% DMSO), ticagrelor (10 µM), AR-C 66096 (10 µM), or R-138727 (10 µM) for 30 minutes. (F) Forskolin (1 µM; 10 minutes)–induced cAMP after preincuabtion with vehicle (0.1% DMSO) or ticagrelor (0.01 µM-10 μM; 30 minutes) in P2Y12R-transfected cells; n = 5. All graphs and bar charts display mean ± SEM. All cells were incubated with 0.2 U/mL apyrase (37°C; 60 minutes) prior to drug additions. *P < .05 vs pcNEO controls.
Ticagrelor-mediated attenuation of agonist-dependent and agonist-independent P2Y12R activity in 1321N1 cells. Inhibition of forskolin (1 µM; 10 minutes)–induced cAMP production by ADP (0.1 nM-100 µM; 10 minutes) in P2Y12R-transfected 1321N1 cells after preincubation with ticagrelor (3-30 nM; 15 minutes) (A) or AR-C 66096 (AR-C; 3-30 nM; 15 minutes) (B); n = 5. (C) Schild-plot analysis of data from panels A and B. (D) Forskolin (0.1 nM-1 µM; 10 minutes)–induced increases in cAMP levels in pcNEO-transfected and P2Y12R-transfected 1321N1 cells. (E) Forskolin (1 µM; 10 minutes)–induced cAMP production in pcNEO-transfected and P2Y12-transfected cells incubated with vehicle (0.1% DMSO), ticagrelor (10 µM), AR-C 66096 (10 µM), or R-138727 (10 µM) for 30 minutes. (F) Forskolin (1 µM; 10 minutes)–induced cAMP after preincuabtion with vehicle (0.1% DMSO) or ticagrelor (0.01 µM-10 μM; 30 minutes) in P2Y12R-transfected cells; n = 5. All graphs and bar charts display mean ± SEM. All cells were incubated with 0.2 U/mL apyrase (37°C; 60 minutes) prior to drug additions. *P < .05 vs pcNEO controls.
Upon closer examination of forskolin-stimulated adenylyl cyclase activity, a significant reduction was noted in P2Y12R-expressing cells vs vector control cells (Figure 7D), indicating constitutive (agonist-independent) activity of the P2Y12R. This attenuation of forskolin responsiveness because of P2Y12R expression was reversed by ticagrelor (10 μM; 30 minutes) but not with the other P2Y12R antagonists (Figure 7E). The ability of ticagrelor to modulate this agonist-independent P2Y12R activity was concentration dependent (Figure 7F) and not affected by XAC preincubation (1 μM; 15 minutes; data not shown). In summary, this set of results from 1321N1 cells indicated that ticagrelor was able to reduce agonist-independent P2Y12R activity by acting as an inverse agonist.
Discussion
The P2Y12R plays a central role in platelet activation and thrombosis and has been by far the most successful target for antiplatelet therapy.2,10,17,29-31 Ticagrelor is the first nonthienopyridine P2Y12 antagonist approved for the prevention of thrombotic events in ACS patients. Unlike the thienopyridines, ticagrelor is direct acting and binds to P2Y12 in a reversible manner to block platelet activity. Clinical studies provide evidence that ticagrelor treatment can offer superior efficacy compared with clopidogrel, with a lower mortality rate resulting from vascular causes, myocardial infarction, or stroke. The reasons for this are unclear but have recently been reviewed.7 This study aimed to fully characterize how ticagrelor modulates human platelet function and demonstrates 2 novel antiplatelet mechanisms. First, ticagrelor is able to directly block platelet ENT1 activity, leading to a rise in local extracellular adenosine levels, which in turn activates platelet A2A adenosine receptors, increasing platelet cAMP levels and reducing platelet activity. Second, ticagrelor inhibits agonist-independent platelet P2Y12R activity, which indicates that ticagrelor has the pharmacological profile of an inverse agonist. These effects are in addition to ticagrelor’s primary antiplatelet mechanism of action, P2Y12 antagonism.
A number of recent studies have shown that ticagrelor can increase extracellular adenosine levels.15,16 Experiments in whole blood demonstrated that ticagrelor blocked ENT1 adenosine transporters on red blood cells, resulting in increased plasma adenosine levels and inhibition of platelet aggregation.15 However, a direct effect on platelet adenosine transport has not yet been demonstrated. In order to investigate potential effects on platelet signaling, we examined changes in platelet ADP-induced Ca2+ release, basal cAMP, and basal VASP-P levels. We show that ticagrelor was more effective at reducing ADP-induced platelet Ca2+ release than other P2Y12 antagonists with an evident decrease in agonist-stimulated peak Ca2+ concentrations but no overall change in the shape of the Ca2+ trace. The antagonism of the Ca2+ responses by ticagrelor, R-138727, and AR-C 66096 was insurmountable. For R-138727, this could be considered as consistent with it being an irreversibly binding antagonist. Ticagrelor32 and the adenosine triphosphate (ATP) analog, AR-C 66096,33 however, reversibly bind to the P2Y12R and yet display insurmountable antagonism.
In addition, ticagrelor treatment, unlike other P2Y12 antagonists, produced a time- and concentration-dependent increase in basal VASP-P in human washed platelets. Importantly, pretreatment of platelets with the adenosine receptor antagonist XAC attenuated but did not abolish the ticagrelor-induced increase in VASP-P. This effect was independent of the presence of extracellular Ca2+. Although platelets express the A2A adenosine receptor subtype and, at lower levels, the A2B subtype,34,35 only the A2A adenosine receptor–selective antagonist SCH 442416 attenuated the ticagrelor-induced increase in platelet VASP-P.
Our study is the first to demonstrate that ticagrelor is able to inhibit platelet-expressed ENT1. Previously, it has been shown that ticagrelor selectively inhibits cellular adenosine uptake via inhibition of ENT1 at clinically relevant concentrations12 and that this inhibition translates into an increase in extracellular adenosine concentration.15,16 ENT1 on red blood cells is believed to play a key role because these cells are thought to be the main sink of adenosine in the circulation. However, this could not be applicable to our study since our assays were carried out using washed platelets in the absence of erythrocytes. Proteomics databases (eg, Platelet Web,36 ProteomicsDB37 ) suggest that platelets do express ENT1, and our studies used western blotting to confirm for the first time that platelets express ENT1. The identified protein was ∼50 kDa in size, similar to that observed by other groups.38,39 Importantly, this band was not found in a mixed culture of rat glial cells, which are reported to have little or no ENT1 expression.40 Further, the ENT1-selective inhibitor NBMPR, like ticagrelor, caused a time-dependent increase in VASP-P in washed platelets. Likewise, VASP-P peaked after a 60-minute incubation period with NBMPR or ticagrelor, indicative of a gradual buildup of adenosine after the adenosine transporter ENT1 was blocked. Hence, following ticagrelor treatment, it would appear that inhibition of platelet and red blood cell ENT1 contributes to an increase in extracellular adenosine levels in blood.
Our results prompt us to speculate on the source of adenosine in our studies. Adenosine may be generated following ATP or ADP breakdown during preparation of washed platelets, which is undertaken in the presence of apyrase to preserve P2Y1R and P2Y12R responsiveness.23 It is well established that platelets express CD39/nucleoside triphosphate diphosphohydrolase 1 (NTPDase1), an ecto-nucleosidase enzyme that hydrolyses both ADP and ATP to AMP.41,42 Another platelet membrane–anchored enzyme, CD73, then converts AMP into adenosine.43,44 Critically, addition of ADA, which degrades adenosine into inosine, effectively blocked any adenosine-dependent effects of ticagrelor. Therefore, as in previous studies, our data support the fact that that ticagrelor does not directly bind to or stimulate platelet adenosine receptors but induces adenosine effects by increasing extracellular adenosine levels.12,45
Our studies also show for the first time that ticagrelor affects agonist-independent platelet cAMP and VASP-P, likely via P2Y12R. This effect was adenosine receptor independent as ticagrelor could still increase levels of platelet cAMP and VASP-P in the presence of adenosine receptor antagonism with XAC. The kinetics of the rise in VASP-P following XAC-induced adenosine receptor antagonism was notably faster compared with that in the absence of XAC. We hypothesize that the slow rise of VASP-P can be attributed to the gradual buildup of adenosine. In contrast, the rapid change in VASP-P apparent following adenosine receptor antagonism is suggestive of an effect consequent to direct receptor-ligand interaction. We suggest that ticagrelor is acting as an inverse agonist at the P2Y12R, therefore having the opposite effect to ADP. This property again appears unique to ticagrelor vs the other P2Y12R antagonists tested (Figure 4G-H).
Our hypothesis was validated in 1321N1 cells stably expressing the human P2Y12R. Ticagrelor increased VASP-P only in 1321N1 cells expressing the P2Y12R and not in P2Y-null vector controls. In addition, we showed that XAC preincubation had no effect on ticagrelor-induced increase in VASP-P, indirectly supporting a selective effect on the P2Y12R. Intriguingly, unlike in platelets, R-138727 but not AR-C 66096 also marginally increased VASP-P. Therefore, R-138727 may potentially also effect agonist-independent P2Y12R activity. However, as we did not see any measurable effect on VASP-P in human platelets, or changes in forskolin responsiveness (see subsequent discussion), we did not explore this further.
It is also worth noting that ticagrelor did not affect ERK (Figure 6C-D) or Akt activation (data not shown), which are both phosphorylated following P2Y12R activation.46 Closer examination of P2Y12R signaling in 1321N1 cells showed that the receptor exhibited a high degree of constitutive activity visualized by an attenuation of forskolin responsiveness only in P2Y12R-expressing cells.47 Preincubation with apyrase to remove residual ADP or preincubation with other P2Y12R antagonists had no effect on forskolin responsiveness, providing further evidence that the receptor is able to initiate signaling in the absence of an agonist. Only ticagrelor was able to suppress agonist-independent activity of the P2Y12R, as indicated by increased forskolin-induced cAMP levels in receptor-expressing cells to a level comparable with non-receptor-expressing cells. Our data provide compelling evidence that ticagrelor is an inverse agonist, stabilizing the receptor in an inactive conformation upon binding and preventing agonist-independent coupling to Gi.48,49 We do recognize that these studies were undertaken in cell lines overexpressing the P2Y12R (∼10 times greater than what we find in human platelets50 ). Although receptor overexpression can affect the degree of agonist-independent activity, it is reassuring that we find comparable changes in the ability of ticagrelor to modulate basal P2Y12R activity when the receptor is endogenously expressed on human platelets. Importantly, the “ionic lock” between the bottom of transmembrane domains III and VI seen in some G protein–coupled receptors, which is thought to stabilize the inactive conformation of a G protein–coupled receptor in the absence of agonist ligand, is not present in the P2Y12R.51
Another noteworthy finding from our studies in platelets was that pretreatment with other P2Y12R antagonists failed to attenuate adenosine receptor–independent, ticagrelor-stimulated changes in basal VASP-P (Figure 5A-B). This suggests that AR-C 66096, PSB 0739, and R-138727, reported to bind to or close to the orthosteric/ADP binding site,51-53 did not prevent ticagrelor from binding to the P2Y12R. A possible explanation is that ticagrelor binds to different residues than where the other antagonists bind. Closer examination of the 2-methylthioadenosine diphosphate (2MeSADP) (agonist)–bound state crystal structure of the P2Y12R revealed that ticagrelor cannot dock inside the 2MeSADP-binding cavity, whereas ADP and some P2Y12R antagonists, including AR-C 66096, do have access.51 Ticagrelor’s bulky side chains on N6 have been suggested to cause a steric clash, with docking likely requiring P2Y12R helical rearrangements.5 A study assessing P2Y12R function in CHO cells indicated that ticagrelor acted in a competitive manner based on functional readouts although only low concentrations of ticagrelor (<30 nM) were included in the analysis.28 In conflicting studies, ticagrelor has been shown to display characteristics of a noncompetitive antagonist, decreasing the maximum response (Emax) as well as right-shifting the ADP concentration-response curve.4 Ticagrelor did not displace [3H]ADP from the receptor (Ki >10 μM) but displaced 2MeS-ADP from membranes prepared from human washed platelets.4 However, docking analysis would suggest that different binding sites for ADP and 2MeSADP are unlikely.51 Relating to our platelet studies (Figure 1B) and Schild analysis in cell lines (Figure 7C), the data indicate that ticagrelor is acting in a noncompetitive manner vs ADP. Because of the complex pharmacology of ticagrelor, it appears in our studies to behave as a noncompetitive antagonist at a functional level, although it may well bind to the orthosteric/ADP-binding site.
Only few inverse agonists are documented to act at the P2Y12R.54 Ding et al reported that AR-C78511 increased cAMP generation in cells expressing a chimeric P2Y12R that displayed a high degree of constitutive activity and is thus a potent inverse agonist.55 A follow-up study also revealed that AR-C78511 had superior antiplatelet activity compared with a neutral antagonist (cangrelor) in transgenic mice expressing the same chimeric receptor.56 Interestingly, AR-C78511, like ticagrelor, could not be docked to the 2MeSADP-bound conformation of the P2Y12R, seemingly because of its own bulky N6 substituents.51
In summary, ticagrelor has a unique mode of action on human platelets, through blockade of agonist-dependent and independent P2Y12R activity and through adenosine uptake inhibition. Notably, these studies were carried out after blood drawing, with platelets isolated by centrifugation in the presence of anticoagulant. Most assays were carried out in the absence of erythrocytes and plasma. With these limitations of experimental design in mind, the relative contribution of agonist-dependent and independent P2Y12R activity to the clinical efficacy of ticagrelor in vivo remains to be determined.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Bristol Platelet Group and other University of Bristol staff for kindly donating their blood and time, and Jonathan Hanley’s laboratory for providing the rat glial cells.
Work supported by Senior British Heart Foundation Research Fellowship FS/11/49/28751 (S.J.M.).
Authorship
Contribution: R.A., S.J.M., and A.C. designed and performed the research and wrote the manuscript; M.B. performed some of the research; E.K., A.M., and S.J.M. designed and supervised the research; and S.N. contributed to the manuscript.
Conflict-of-interest disclosure: S.N. is an employee of AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Stuart J. Mundell, School of Physiology, Pharmacology and Neuroscience, Faculty of Biomedical Sciences, University of Bristol, Bristol BS8 1TD, United Kingdom; e-mail: s.j.mundell@bristol.ac.uk.

![Figure 1. ADP-induced platelet aggregation and intracellular Ca2+ release. (A) ADP (10 µM)–induced platelet aggregation in human PRP after 30-minute incubation at 30°C with ticagrelor (IC50 = 0.27 μM [0.14-0.51]; n = 3), AR-C 66096 (IC50 = 0.16 μM [0.08-0.33]; n = 4), or R-138727 (IC50 = 3.82 μM [3.03-4.82]; n = 6). The 95% confidence intervals for IC50 values are shown in square brackets. (B) ADP-induced Ca2+ peak responses in Fura-2 AM–loaded platelets in the presence of vehicle (n = 16) or 10 µM ticagrelor (n = 22), AR-C 66096 (n = 12), or R-138727 (n = 12). Baseline readings were recorded over a period of 5 cycles (each cycle ∼7 seconds) before ADP addition, after which recording was continued for a further 15 cycles. The peak Ca2+ response was determined by calculating the change in fluorescence ratio (340 nm/380 nm). (C) ADP (100 µM)–induced platelet peak Ca2+ responses in the presence of vehicle (0.1% DMSO), ticagrelor (10 µM), ticagrelor (10 µM) + XAC (10 µM), AR-C 66096 (10 µM), and R-138727 (10 µM). Data displayed as mean and standard error of the mean (SEM). *P < .05. IC50, 50% inhibitory concentration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2016-03-707844/4/m_blood707844f1.jpeg?Expires=1767705431&Signature=DkYmB7TjrZ4XoHmwb5STn49iG1Ob9nuGHm0fdC4~Z7DYghUduaidrdO5F46eAM8oJX1qaGmVdvpB13KBNm4rW8a~jg1S2DTnjsn6dYskwIDc0JPAkzHsNCsbnihya9hgmVApqi16K5lHt12B0b~VKuaLZ~EaCz6MVOE~ici-wM58Msh2O6b5qh4j-ZDA5crcxBfAW-Pb81QwnibjpDn5n-cky0I11w0j3hiSWikn5sq3hh9RqZQZE0id2csjyi4t23t4ZWIxA~QbihMw1OgiBPj-F~gh7bXvpXp1vZiHldtIZtJU7QrWhGCFsQyUD9rllJeA3u8kZzd7icuJeVg9nA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal