Key Points
A subset of T-ALL cases show high expression of hedgehog pathway genes including the SHH ligand and the GLI1 transcription factor.
T-ALL samples with high GLI1 expression levels respond to hedgehog inhibitor treatment in vitro and in vivo.
Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive childhood leukemia that is caused by the accumulation of multiple genomic lesions resulting in transcriptional deregulation and increased cell proliferation and survival. Through analysis of gene expression data, we provide evidence that the hedgehog pathway is activated in 20% of T-ALL samples. Hedgehog pathway activation is associated with ectopic expression of the hedgehog ligands Sonic hedgehog (SHH) or Indian hedgehog (IHH), and with upregulation of the transcription factor GLI1. Ectopic expression of SHH or IHH in mouse T cells in vivo caused hedgehog pathway activation in both lymphoid and epithelial cells in the thymus and resulted in increased expression of important T-cell stimulatory ligands (Dll4, Il7, and Vegf) by thymic epithelial cells. In T-ALL cell lines, pharmacological inhibition or short interfering RNA–mediated knockdown of SMO or GLI1 led to decreased cell proliferation. Moreover, primary T-ALL cases with high GLI1 messenger RNA levels, but not those with low or undetectable GLI1 expression, were sensitive to hedgehog pathway inhibition by GANT61 or GDC-0449 (vismodegib) using ex vivo cultures and in vivo xenograft models. We identify the hedgehog pathway as a novel therapeutic target in T-ALL and demonstrate that hedgehog inhibitors approved by the US Food and Drug Administration could be used for the treatment of this rare leukemia.
Introduction
The hedgehog signaling pathway contributes to embryonic pattern formation and adult tissue homeostasis as it is important to regulate cell proliferation, survival, and differentiation.1,2 In mammalian cells, the hedgehog pathway is negatively regulated by one of the 2 transmembrane receptors Patched 1 (PTCH1) or Patched 2 (PTCH2). This negative regulation is released by binding of 1 of the 3 ligands: the Sonic hedgehog (SHH), the Indian hedgehog (IHH), or the Desert hedgehog.1,3 Binding of the ligand to PTCH1 results in activation of another transmembrane protein smoothened (SMO) and subsequent activation of GLI transcription factors (GLI1, GLI2, and GLI3), which act as transcriptional activators or repressors.3 GLI activity can also be regulated by sequestration into a multiprotein complex that includes the suppressor of fused (SUFU), preventing the nuclear accumulation of the GLI proteins.1,3,4
Several lines of evidence suggest that the hedgehog signaling pathway is important for normal T-cell development.5,6 Thymic epithelial cells (TECs) express hedgehog ligands, whereas T-cell progenitors express SMO and PTCH1 and can react on hedgehog ligand stimulation, which is important for the survival and the proliferation of T cells and also regulates their differentiation.7-9 This has been illustrated by Ptch1 knockout mice in which T-cell development is severely affected resulting in early thymic atrophy5 and the observation that Shh knockout mice have a 10-fold reduction of thymocytes with a particular reduction of the DN2 T cells already early in development.9 Studies have pointed out that SHH is important for the differentiation and proliferation of early thymocyte progenitors, T-cell receptor formation, CD4 vs CD8 lineage commitment, and negative selection of autoreactive cells.9-11 In contrast to SHH, which is only expressed by the TECs, IHH is expressed by CD4CD8 double-positive T cells and promotes early T-cell differentiation and restricts late T-cell development.10-13
Because the hedgehog pathway plays a major role in key developmental processes, it is not surprising that it is aberrantly activated in cancer and is firmly correlated to the etiology of basal cell carcinoma and medulloblastoma.14 Activation of the hedgehog pathway is also associated with increased tumor proliferation, chemoresistance, metastasis, and cancer stem cell maintenance/proliferation in various solid tumors.15-18 Oncogenic activation of the hedgehog pathway can be the result of mutations in different hedgehog components or can be the result of ectopic ligand expression.14
Despite the importance of the hedgehog pathway in T-cell development and in several hematological malignancies,19-27 the role of the hedgehog pathway in T-cell acute lymphoblastic leukemia (T-ALL) is unclear with conflicting data reported. One study has shown that hedgehog signaling is dispensable for T-ALL development,28 whereas other studies have documented sensitivity of T-ALL cell lines to hedgehog inhibitors suggesting this pathway could be important in T-ALL development and/or maintenance.29,30 We have recently identified activating mutations in the hedgehog pathway in T-ALL,31,32 further supporting the notion that deregulation of the hedgehog pathway plays a role in T-ALL development. In this current report, we show that hedgehog pathway activation occurs in 20% of T-ALL cases and that such cases are sensitive to hedgehog inhibitors both in vitro and in vivo.
Methods
Cell culture and leukemia samples
T-ALL cell lines were purchased from DSMZ (www.dsmz.de). Karyotype and sequence analysis was performed and matched with public data confirming T-ALL cell line identity. Primary childhood leukemia samples used in this study were provided by University Hospital Leuven (Belgium) and the Bloodwise (formerly Leukaemia and Lymphoma Research) Childhood Leukaemia Cell Bank working with the laboratory teams in the Bristol Genetics Laboratory, Southmead Hospital, Bristol; Molecular Biology Laboratory, Royal Hospital for Sick Children, Glasgow; Molecular Haematology Laboratory, Royal London Hospital, London; and Molecular Genetics Service, Sheffield Children’s Hospital, Sheffield.
Messenger RNA expression analysis
RNA was isolated using the Maxwell 16 LEV simplyRNA Cells Kit (Promega) followed by complementary DNA synthesis with the Superscript III kit (Invitrogen). LC480 SYBR Green I Master Mix (Roche Applied Science) was used for quantitative real-time polymerase chain reaction (PCR) with the LightCycler 480 Real-Time PCR System (Roche Applied Science). HPRT1, TBP, or GUSB1 genes were used as control genes. Data were analyzed by the LC480 software (Roche Applied Science) and subsequently with the comparative ddCT method or the qBase+ software.
Cell proliferation, siRNA, and drug treatment assays
For drug treatment assays, cells were plated in triplicate at a density of 5 × 105 cells/mL in a 96-well plate and treated with 1 or 5 µM of cyclopamine, GDC-0449 (ChemieTek), or GANT61 (MedChem Express). Cells were counted daily for up to 14 days to determine the effect on cell proliferation. For short interfering (siRNA) treatment and/or ligand stimulation, cells were plated at a density of 3 × 105 cells/mL in a 12-well plate and stimulated with 1 μg/mL SHH (Peprotech) or 1.5 μg/mL IHH (Neuromics) for 30 minutes. siRNAs targeting SMO, GLI1, SUFU, or control siRNA were transfected in cell lines by electroporation (400 nM siRNA, 4-mm cuvette (Bio-rad), electroporation 300 V/1000 µF), using the GenePulser XCell (Bio-rad). Cells were counted with the flow cytometer FACSCanto (BD).
Immunofluorescence
Cells were fixed with cold 4% formaldehyde and incubated for 20 minutes at room temperature. Cells were permeabilized in Dulbecco's modified phosphate buffered saline, 0.2% Triton buffer, blocked in Dulbecco's modified phosphate buffered saline 10% fetal bovine serum, and finally incubated with the anti-rabbit polyclonal GLI1 antibody (Santa Cruz Technologies). Fluor 488 goat anti-mouse immunoglobulin G (Molecular Probes) and TO-PRO were used as secondary antibody and nuclear staining, respectively.
Immunohistochemistry
Spleen and lymph nodes were collected and fixed in 10% neutral buffered formalin (Sigma) for 48 hours and then processed for paraffin embedding (Thermo Scientific Excelsior AS Tissue Processor and HistoStar Embedding Workstation). Sections of 5 µm were mounted on Superfrost Plus Adhesion slides (Thermo Scientific) and stained with hematoxylin and eosin (Diapath). Immunoflorescence and/or immunopreoxidase stain were performed on paraffin sections using the following antibodies: CD3ε (Santa Cruz), cleaved caspase-3 (Cell Signaling), green fluorescent protein (GFP), Ly75 (Abcam), UEA1 lectin (Vector Laboratories), Ki67 (ThermoScientific), Alexa Fluor 568 donkey anti-rabbit or Alexa Fluor 488 donkey anti-goat (Molecular Probes), goat anti-rabbit EnVision+/HRP reagent (Dako), and biotinilated rabbit anti-goat (Abcam). The degree of splenic colonization by leukemic cells was determined by staining with human HLA-A (Abcam) as described33 with a digital image analysis algorithm created on the ImageJ software platform. Proliferation and apoptosis were measured via cleaved caspase-3 and phospho-histone H3 (Cell Signaling).
Bone marrow transplantation assay
Retroviral vectors containing the complementary DNAs of Shh, Ihh, or JAK3(M511I) were used to transduce lineage negative mouse bone marrow cells as previously described.34 Transduced cells were injected into irradiated BALB/c female mice 6 to 8 weeks old. Transduced cells were tracked by the expression of GFP/mCherry fluorescence. Blood samples were drawn every 2 weeks and were measured with the Automatic Blood Counter (ABC counter).
TEC and lymphocyte isolation
TECs were isolated and purified as previously described.35 Purification efficiency was evaluated by using CD45 and EpCAM markers in flow cytometry (Miltenyi Biotec). RNA was extracted from isolated TECs with the illustra RNAspin Mini kit (GE HealthCare).
Ex vivo culture of primary T-ALL cells and in vivo drug treatment
T-ALL samples were injected intravenously into immunodeficient NSG mice. Human cells were identified in blood samples by anti-CD45 (eBioscience) staining by flow cytometry. Human cells were harvested from spleen, bone marrow, and, when possible, thymus and lymph nodes. For ex vivo cultures, cells were plated into 24-well plates treated with Dll4 as previously described36 and maintained in culture for 5 to 7 days in RPMI 1640 medium supplemented with 20% fetal bovine serum, interleukin-4 (IL-4), IL-7, stem cell factor, and SDF1a (PeproTech). NSG mice were treated daily for 14 days with DMSO, GDC-0449 (50 mg/kg), or GANT61 (50 mg/kg) when the leukemic clone was detectable in the blood.
Gene expression data and statistical analysis
Gene expression data37-39 were analyzed with MEV4.8.1 and MATLAB R2014A. Further statistical analysis was performed with PRISM6 and SPSS statistics programs. The Kyoto Encyclopedia of Genes and Genomes pathways gene set enrichment analysis was performed with the gene set enrichment analysis (GSEA) software of the Broad Institute.35 The Student t test was performed to determine significant differences between 2 groups. Normality tests were applied to test the normal distribution. The graphs represent mean values ± standard error of the mean (SEM). For the statistical analysis of the in vivo xenograft T-ALL experiment, 2-way analysis of variance (repeated measurements) was used.
Ethical approval
Human T-ALL samples were collected at the University Hospital Leuven for genomic analysis and to inject in immune-deficient mice to establish xenografts. The ethical committee of the University of Leuven approved these experiments. Mouse bone marrow transplants were performed under an animal project approved the ethical committee of the University of Leuven and according to all guidelines. Mouse xenograft experiments were approved by the ethical committee of the University of Leuven.
Results
Gene expression profiling reveals a hedgehog pathway activation signature in a subset of T-ALL cases
In solid tumors, activation of the hedgehog pathway is because of mutations in PTCH1 or SMO, or ectopic expression of the ligands SHH, IHH, or Desert hedgehog. These aberrations lead to activation of the hedgehog pathway that is associated with upregulation of the transcription factor GLI1, as GLI1 stimulates its own transcription.
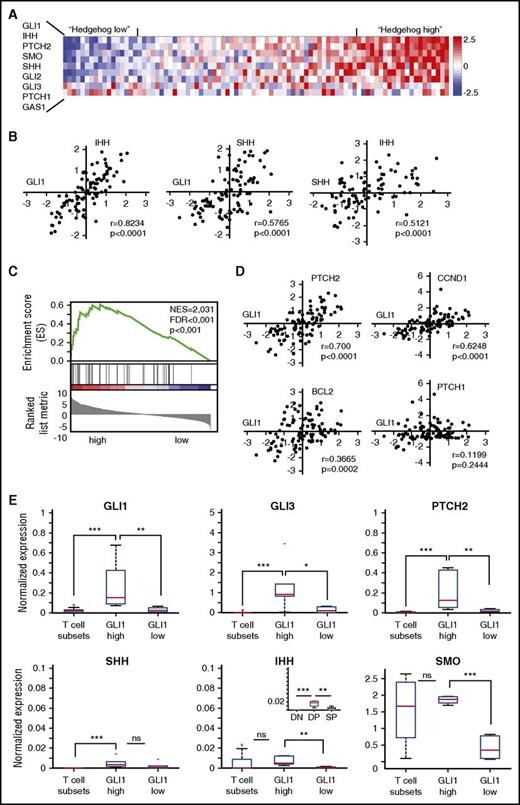
To investigate the possibility of hedgehog pathway activation in T-ALL development, we analyzed available gene expression profiles of primary T-ALL cases from our own cohort (51 T-ALL cases profiled by RNA-sequencing) and from a second independent cohort (88 T-ALL cases profiled by Affymetrix arrays).37,38 Analysis of the expression data from T-ALL samples compared with normal T-cell progenitors39 revealed ectopic expression of SHH, IHH, and GLI1 in 30 of 139 (22%) T-ALL samples (Figure 1A). Moreover, we observed in both studies a strong positive correlation of GLI1 expression levels with the expression of the hedgehog ligands SHH and IHH (Figure 1B; supplemental Figure 1, available on the Blood Web site), suggesting the presence of an autocrine activation loop. GSEA confirmed that transcripts linked to the hedgehog pathway were enriched in T-ALL cases with high SHH/IHH/GLI1 expression compared with the other T-ALL cases (Figure 1C). In addition, all known GLI1 target genes, except PTCH1, were upregulated in the same samples, indicative for hedgehog pathway activation (Figure 1D). Expression of hedgehog pathway genes in normal T-cell subsets was significantly lower compared with the subgroup of T-ALL patients with high SHH/IHH/GLI1 expression (Figure 1E). Especially the expression of SHH is remarkable, as this gene is not expressed in any of the normal T-cell subsets and is thus clearly ectopically expressed in some of the T-ALL cases. Together, these data provide strong evidence for hedgehog pathway activation in 22% of T-ALL cases, which we could not link to any specific subtype of T-ALL (supplemental Figure 2).40
Hedgehog components are ectopically expressed in a subgroup of T-ALL patients. (A) Heat map showing the relative expression of genes encoding hedgehog components in a cohort of 88 T-ALL patients. Patients were ordered based on the expression of the 9 hedgehog pathway genes. (B) GLI1 expression levels correlate with expression of the hedgehog ligands SHH and IHH. (C) The hedgehog pathway signature is enriched in T-ALL patients with high GLI1 levels. Patients were categorized in “High,” “Intermediate,” or “Low” based on the expression levels of the GLI1 gene. Subsequent GSEA between “High” and “Low” patients identified hedgehog pathway as significantly upregulated. (D) Correlation between expression levels of GLI1 and known GLI1 target genes. A significant correlation was found with PTCH2, CCND1, and BCL2, but not with PTCH1. (E) Box plots showing the relative expression of 6 genes of the hedgehog pathway during normal mouse T-cell development and in T-ALL patients with high or low GLI1 expression. *P < .05; **P < .01; ***P < .001. Error bars indicate SEM.
Hedgehog components are ectopically expressed in a subgroup of T-ALL patients. (A) Heat map showing the relative expression of genes encoding hedgehog components in a cohort of 88 T-ALL patients. Patients were ordered based on the expression of the 9 hedgehog pathway genes. (B) GLI1 expression levels correlate with expression of the hedgehog ligands SHH and IHH. (C) The hedgehog pathway signature is enriched in T-ALL patients with high GLI1 levels. Patients were categorized in “High,” “Intermediate,” or “Low” based on the expression levels of the GLI1 gene. Subsequent GSEA between “High” and “Low” patients identified hedgehog pathway as significantly upregulated. (D) Correlation between expression levels of GLI1 and known GLI1 target genes. A significant correlation was found with PTCH2, CCND1, and BCL2, but not with PTCH1. (E) Box plots showing the relative expression of 6 genes of the hedgehog pathway during normal mouse T-cell development and in T-ALL patients with high or low GLI1 expression. *P < .05; **P < .01; ***P < .001. Error bars indicate SEM.
Transcriptional deregulation and hedgehog pathway expression in T-ALL
Several mechanisms could be responsible for hedgehog pathway activation in T-ALL. We have previously identified mutations in hedgehog pathway components in T-ALL, including 2 truncating mutations in SMO and a missense mutation in GLI3, but these mutations are very rare.31 It is plausible to expect that the general transcriptional deregulation present in T-ALL could lead to increased expression of hedgehog pathway genes and/or ectopic SHH expression.
We used iRegulon, a computational method to reverse-engineer transcriptional regulatory networks, to gain insight in the spectrum of transcription factors that could drive the expression of hedgehog pathway genes in T-ALL.41 iRegulon uses information from gene expression data and curated information on DNA binding motifs of hundreds of transcription factors and implements a genome-wide ranking-and-recovery approach to detect enriched transcription factor motifs and their optimal sets of direct targets. This approach identified 32 transcriptional factors predicted to bind the promoters of at least 1 hedgehog gene. Moreover, we observed that several of these transcriptional factors share a similar expression profile with most hedgehog genes in the T-ALL cohorts (supplemental Figure 3). Of particular interest, we confirmed a strong positive correlation between GLI1 expression levels and levels of GATA1 and GATA2, but a negative correlation with GATA6 in T-ALL cases. GATA1/2 transcription factors have known binding sites in the GLI1 and SHH promoters,42 and GATA6 is known to suppress the SHH gene in limb development.43,44 Using chromatin immunoprecipitation, we confirmed binding of GATA1 to 2 predicted regions of the GLI1 promoter in HSB2, a T-ALL cell line that expresses GATA1 (supplemental Figure 4). In addition, NF-κB2 was found to be positively correlated with SHH, IHH, and GLI1 expression (supplemental Figure 3). NF-κB has recently been shown to upregulate the expression of SHH in pancreatic cancer.45,46
Ectopic expression of Shh or Ihh drives clonal selection in a JAK3 mutant T-ALL mouse model
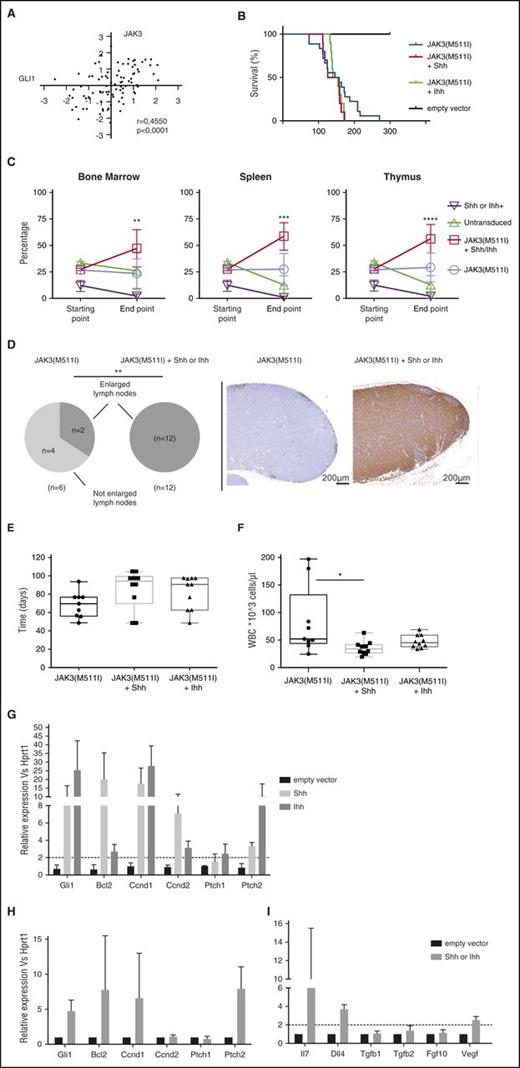
To determine whether Shh or Ihh expression could contribute to leukemia development or progression in a leukemia T-cell model, we ectopically expressed the ligands Shh or Ihh together with a JAK3(M511I) mutant in bone marrow cells of Balb/C mice. We have previously shown that the JAK3(M511I) mutant causes a long latency T-ALL development, characterized by high white blood cell count and accumulation of immature CD8 single positive leukemia cells.34 We selected this model because of the strong correlation between GLI1 and JAK3 expression in human T-ALL samples (Figure 2A), suggesting that hedgehog pathway activation could synergize with activated JAK3 signaling in leukemia development.
Overexpression of the Shh/Ihh ligands in a JAK3-dependent T-ALL mouse model results in a competitive growth advantage and modification of the thymic niche. (A) GLI1 expression levels are strongly correlated with JAK3 levels in T-ALL patients. (B) Kaplan-Meier survival plot showing disease latency in mice transplanted with bone marrow progenitor cells expressing JAK3(M511I), JAK3(M511I)+Shh, or JAK3(M511I)+Ihh. (C) Leukemic cells expressing JAK3(M511I)+Shh or JAK3(M511I)+Ihh show a competitive growth advantage compared with cells expressing either gene alone. The percentage of each subpopulation was measured before injection (starting point) and at leukemia development (end point). (D) Lymph node infiltration in leukemic mice with JAK3(M511I), JAK3(M511I)+Shh, or JAK3(M511I)+Ihh. Pie charts show the number of mice with enlarged inguinal lymph nodes. The images show a representative example of the mesenteric lymph nodes with almost no infiltration in a JAK3(M511I) mouse, but high infiltration for a JAK3(M511I)+Shh mouse. Staining shown for anti-GFP. (E-F) Leukemic T lymphocytes are significantly reduced in blood of JAK3(M511I)+Shh or Ihh mice. (E) Time point (in days) at which the white blood cell count (WBC) exceeds 10 000 cells per µL. (F) Absolute white blood cell count at end point. (G) Gli1 and Gli1 target genes are significantly upregulated in mice expressing the hedgehog ligands. Relative expression of Gli1 target genes in thymic lymphocytes isolated from mice expressing the empty vector or the hedgehog ligands are shown. (H-I) Activation of TECs by hedgehog ligands results in upregulation of Gli1, Gli1 target genes, and ligands correlated with T-cell development. Relative expression of Gli1 target genes (H) and ligands (I), which are physiologically expressed by TECs are shown. **P < .01.
Overexpression of the Shh/Ihh ligands in a JAK3-dependent T-ALL mouse model results in a competitive growth advantage and modification of the thymic niche. (A) GLI1 expression levels are strongly correlated with JAK3 levels in T-ALL patients. (B) Kaplan-Meier survival plot showing disease latency in mice transplanted with bone marrow progenitor cells expressing JAK3(M511I), JAK3(M511I)+Shh, or JAK3(M511I)+Ihh. (C) Leukemic cells expressing JAK3(M511I)+Shh or JAK3(M511I)+Ihh show a competitive growth advantage compared with cells expressing either gene alone. The percentage of each subpopulation was measured before injection (starting point) and at leukemia development (end point). (D) Lymph node infiltration in leukemic mice with JAK3(M511I), JAK3(M511I)+Shh, or JAK3(M511I)+Ihh. Pie charts show the number of mice with enlarged inguinal lymph nodes. The images show a representative example of the mesenteric lymph nodes with almost no infiltration in a JAK3(M511I) mouse, but high infiltration for a JAK3(M511I)+Shh mouse. Staining shown for anti-GFP. (E-F) Leukemic T lymphocytes are significantly reduced in blood of JAK3(M511I)+Shh or Ihh mice. (E) Time point (in days) at which the white blood cell count (WBC) exceeds 10 000 cells per µL. (F) Absolute white blood cell count at end point. (G) Gli1 and Gli1 target genes are significantly upregulated in mice expressing the hedgehog ligands. Relative expression of Gli1 target genes in thymic lymphocytes isolated from mice expressing the empty vector or the hedgehog ligands are shown. (H-I) Activation of TECs by hedgehog ligands results in upregulation of Gli1, Gli1 target genes, and ligands correlated with T-cell development. Relative expression of Gli1 target genes (H) and ligands (I), which are physiologically expressed by TECs are shown. **P < .01.
Coexpression of Shh or Ihh with JAK3(M511I) did not significantly reduce disease latency (Figure 2B), but the leukemia clone expressing both the ligand and the JAK3 mutant had a clear clonal advantage over the clones expressing Shh or Ihh or JAK3(M511I) alone (Figure 2C). In all animals, we observed that the leukemia cells expressing both the hedgehog ligand and JAK3(M511I) became the dominant clone in all organs. In addition, we observed more infiltration of the leukemia cells in lymph nodes and a lower white blood cell count than with JAK3(M511I) alone, in agreement with increased homing of the cells to hematopoietic organs (Figure 2D-F).
Because the leukemia cells express the secreted proteins Shh or Ihh that could also bind to and affect nonhematopoietic cells, we determined if TECs were altered in thymi from mice expressing Shh or Ihh in the T cells. As expected, T cells showed increased Gli1 levels, as well as increased expression of Gli1 target genes, indicative of hedgehog pathway activation by autocrine activation (Figure 2G). Similarly, also TECs showed increased hedgehog pathway activation as compared with the same cells isolated from wild-type animals (Figure 2H). Interestingly, the TECs also showed increased expression of Dll4, Il7, and Vegf, all ligands that affect T-cell development and stimulate T-cell proliferation and survival (Figure 2I). These data indicate that Shh or Ihh expressed by the T-ALL cells can affect normal TECs, thereby stimulating these cells to produce more ligands that are required for the survival and proliferation of the developing T-ALL cells.
Inhibition or downregulation of the hedgehog pathway affects the growth of T-ALL cell lines in vitro
To investigate whether the activated hedgehog signaling is important for human T-ALL cells, we initially investigated T-ALL cell lines. We treated 9 T-ALL cell lines with either cyclopamine, GDC-0449 (vismodegib), or GANT61. Cyclopamine and GDC-0449 (both SMO inhibitors) caused a dose-dependent inhibition of proliferation in 3/9 T-ALL cell lines (Figure 3A; supplemental Figure 5), and GANT61 (inhibitor of GLI1/GLI2) caused a dose-dependent inhibition in 6/9 T-ALL cell lines (Figure 3B). The inhibitory effect on proliferation strongly correlated with the direct effect of the drugs on the hedgehog pathway, as measured by changes in GLI1 expression levels after drug treatment (Figure 3C-D). Cell lines in which the drugs showed the strongest downregulation of GLI1 also showed the strongest inhibition of cell proliferation.
Inhibition of the hedgehog pathway reduces the proliferation of T-ALL cell lines. (A-B) Pharmacological inhibition of the hedgehog pathway by treatment with SMO inhibitor GDC-0449 (A) or GLI1 inhibitor GANT61 (B) reduces the proliferation of T-ALL cell lines. Cell lines in which the proliferation is significantly inhibited are shown in red. (C-D) Response of the same T-ALL cell lines to the inhibitor treatment as measured by effects on GLI1 expression. Red color corresponds to the cell lines with significant inhibitory effects on proliferation as shown in panels A and B.
Inhibition of the hedgehog pathway reduces the proliferation of T-ALL cell lines. (A-B) Pharmacological inhibition of the hedgehog pathway by treatment with SMO inhibitor GDC-0449 (A) or GLI1 inhibitor GANT61 (B) reduces the proliferation of T-ALL cell lines. Cell lines in which the proliferation is significantly inhibited are shown in red. (C-D) Response of the same T-ALL cell lines to the inhibitor treatment as measured by effects on GLI1 expression. Red color corresponds to the cell lines with significant inhibitory effects on proliferation as shown in panels A and B.
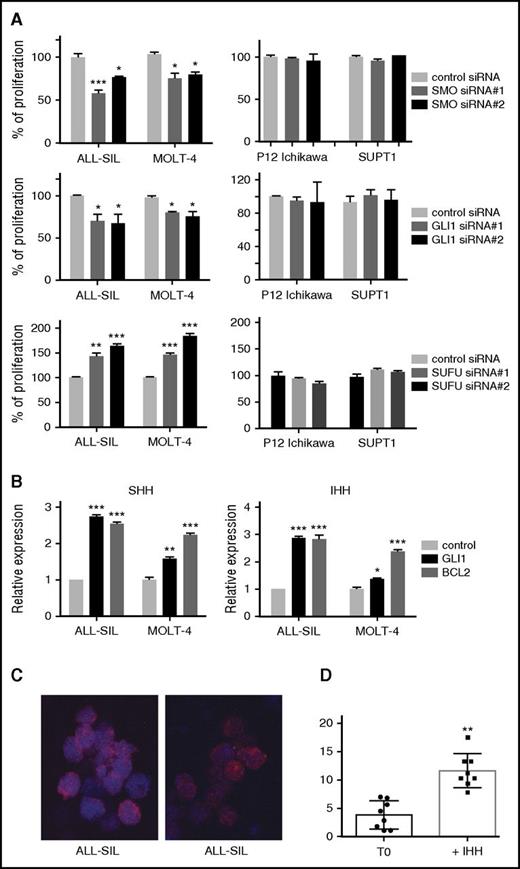
To confirm that these were on-target effects on the hedgehog pathway, we compared the effects obtained with inhibitors to siRNA-mediated knockdown of SMO or GLI1. ALL-SIL and MOLT4 cells showed sensitivity to all hedgehog inhibitors, and we selected these 2 cell lines as well as P12-Ichikawa and SUPT-1, 2 cell lines with low GLI1 expression and nonresponders to hedgehog inhibitor treatment. siRNA-mediated knockdown of the positive regulators SMO or GLI1 resulted in a significant reduction of proliferation of ALL-SIL and MOLT4 cells, whereas this had no effect on the control cells P12-Ichikawa and SUPT1 (Figure 4A). In contrast, knockdown of SUFU, an important negative regulator of the hedgehog pathway, led to a significant increase in proliferation of ALL-SIL and MOLT4, indicating that further stimulation of the hedgehog pathway in these cells could further increase cell proliferation (Figure 4A). Knockdown efficiencies are shown in supplemental Figure 6.
The hedgehog pathway is functional in T-ALL cell lines. (A) Relative proliferation of ALL-SIL, MOLT4 (hedgehog inhibitor sensitive cell lines) and P12-Ichikawa, SUPT1 (hedgehog inhibitor insensitive cell lines) after knockdown by siRNA targeting SMO, GLI1, and SUFU messenger RNA. (B) Stimulation of ALL-SIL and MOLT4 cells with hedgehog ligands results in upregulation of the GLI1 target genes BCL2 and GLI1. (C-D) Stimulation of ALL-SIL with hedgehog ligand results in GLI1 nuclear relocation. GLI1 (red) localization in ALL-SIL cells before stimulation (T0) and 30 minutes after stimulation with the IHH ligand (C). Statistical representation of GLI1 localization as ratio between nucleus and cytoplasm (D). **P < .01; ***P < .001. Error bars indicate SEM.
The hedgehog pathway is functional in T-ALL cell lines. (A) Relative proliferation of ALL-SIL, MOLT4 (hedgehog inhibitor sensitive cell lines) and P12-Ichikawa, SUPT1 (hedgehog inhibitor insensitive cell lines) after knockdown by siRNA targeting SMO, GLI1, and SUFU messenger RNA. (B) Stimulation of ALL-SIL and MOLT4 cells with hedgehog ligands results in upregulation of the GLI1 target genes BCL2 and GLI1. (C-D) Stimulation of ALL-SIL with hedgehog ligand results in GLI1 nuclear relocation. GLI1 (red) localization in ALL-SIL cells before stimulation (T0) and 30 minutes after stimulation with the IHH ligand (C). Statistical representation of GLI1 localization as ratio between nucleus and cytoplasm (D). **P < .01; ***P < .001. Error bars indicate SEM.
Therefore, in a complimentary manner, and to confirm that the hedgehog pathway is completely functional in these 2 T-ALL cell lines, we treated ALL-SIL and MOLT4 cell lines with the ligands SHH or IHH. After 30 minutes of stimulation, a significant increase in expression of GLI1 and/or BCL2, 2 well-established hedgehog target genes, was observed (Figure 4B). SHH or IHH treatment also stimulated the proliferation of the T-ALL cells (supplemental Figure 7). Using immunofluorescence, we confirmed that GLI1 relocated from the cytoplasm to the nucleus, indicative for hedgehog pathway activation (Figure 4C-D). Taken together, these data strongly indicate that all components of the hedgehog pathway are present in these T-ALL cells and that hedgehog pathway stimulation by the ligands SHH or IHH results in activation of GLI1, as shown by nuclear relocalization and increased expression of target genes.
Inhibition of the hedgehog pathway sensitizes T-ALL cell lines to chemotherapy treatment.
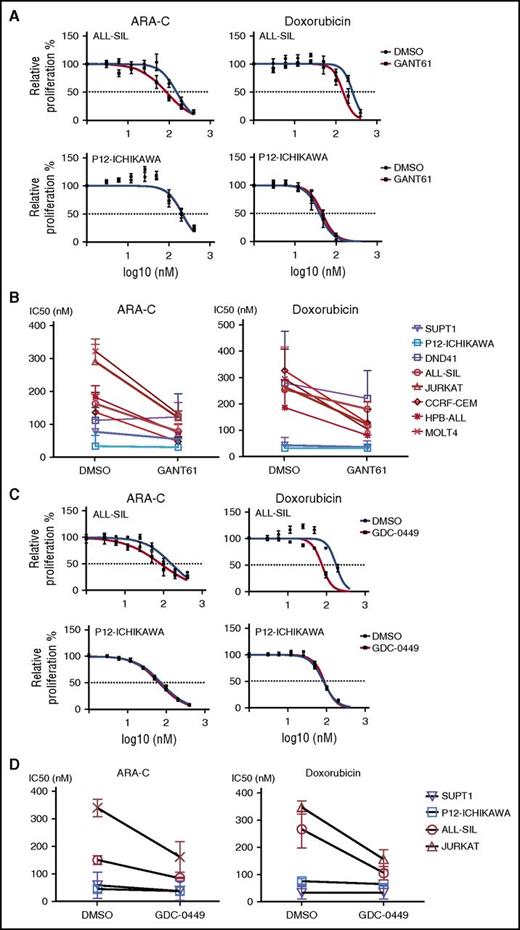
In a subsequent experiment, T-ALL cell lines were treated with ARA-C or doxorubicin, 2 chemotherapeutic agents that are used for the treatment of ALL. As expected, all T-ALL cell lines were sensitive to both drugs. Interestingly, T-ALL cell lines that showed sensitivity to GANT61 were sensitized to ARA-C or doxorubicin inhibition in the presence of GANT61 (Figure 5A-B). For cells that were insensitive to GANT61, a combined GANT61/ARA-C treatment was not different than ARA-C alone. Similar results were obtained with GDC-0449 in combination with ARA-C or doxorubicin (Figure 5C-D). These data confirm that hedgehog inhibitors can increase the sensitivity of T-ALL cells to chemotherapy.
Pharmacological inhibition of the hedgehog pathway decreases the chemoresistance in T-ALL cell lines that are sensitive to hedgehog inhibitors. (A) Relative proliferation of a sensitive cell line (ALL-SIL, top) and of an insensitive cell line (P12-Ichikawa, bottom) to GANT61. (B) IC50 values of T-ALL cell lines treated with ARA-C, ARA-C and 5 μM of GANT61, doxorubicin, or doxorubicin and 5 μM of GANT61. Cell lines sensitive to GANT61 treatment are shown in red; insensitive cell lines are shown in blue. (C) Diagrams showing the relative proliferation of a sensitive cell line (ALL-SIL, top) and of an insensitive cell line (P12-Ichikawa, bottom) to GDC-0449. (D) IC50 values of T-ALL cell lines treated with ARA-C, ARA-C and 5 μM of GDC-0449, doxorubicin, or doxorubicin and 5 μM of GDC-0449. Cell lines sensitive to GDC-0449 treatment are shown in red, insensitive cell lines are shown in blue.
Pharmacological inhibition of the hedgehog pathway decreases the chemoresistance in T-ALL cell lines that are sensitive to hedgehog inhibitors. (A) Relative proliferation of a sensitive cell line (ALL-SIL, top) and of an insensitive cell line (P12-Ichikawa, bottom) to GANT61. (B) IC50 values of T-ALL cell lines treated with ARA-C, ARA-C and 5 μM of GANT61, doxorubicin, or doxorubicin and 5 μM of GANT61. Cell lines sensitive to GANT61 treatment are shown in red; insensitive cell lines are shown in blue. (C) Diagrams showing the relative proliferation of a sensitive cell line (ALL-SIL, top) and of an insensitive cell line (P12-Ichikawa, bottom) to GDC-0449. (D) IC50 values of T-ALL cell lines treated with ARA-C, ARA-C and 5 μM of GDC-0449, doxorubicin, or doxorubicin and 5 μM of GDC-0449. Cell lines sensitive to GDC-0449 treatment are shown in red, insensitive cell lines are shown in blue.
Pharmacological inhibition of the hedgehog pathway inhibits the growth of patient-derived T-ALL xenograft samples ex vivo and in vivo
We next tested if hedgehog pathway inhibition could inhibit the growth of primary T-ALL cells. We treated patient-derived leukemic cells from xenotransplanted NSG mice with GDC-0449 or GANT61 ex vivo or in vivo. To determine which patients have activated hedgehog signaling, we measured the expression levels of GLI1, which is an essential transcription factor of the hedgehog pathway and is upregulated when the pathway is activated. Four of 17 primary T-ALL samples (24%) expressed GLI1 and also expressed most hedgehog components (Figure 6A). Treatment of T-ALL samples with GLI1 expression (X09, X10, X15, and X753) ex vivo with GDC-0449 or GANT61 led to a significant decrease in proliferation. In contrast, T-ALL samples with low to undetectable GLI1 expression levels (X14, X37, and X567) were not sensitive to hedgehog pathway inhibition as they did not show any significant decrease in cell proliferation (Figure 6B). We also confirmed that in samples X09 and X10, both sensitive to hedgehog inhibitors, the expression of GLI1 and 2 target genes BCL2 and PTCH2 was downregulated after treatment with GANT61 or GDC-0449 (Figure 6C), confirming direct activity on GLI1 transcriptional activity.
T-ALL human xenograft samples with GLI1 expression are sensitive to hedgehog inhibitors ex vivo. (A) Quantitative reverse transcription polymerase chain reaction data showing the relative expression level of GLI1 in 17 patient-derived T-ALL xenograft samples. (B) T-ALL samples with detectable GLI1 expression (left) or very low to undetectable GLI1 expression (right) were cultured ex vivo and treated with GDC-0449, GANT61, or vehicle (DMSO). Diagrams depict the survival rate of the T-ALL samples relative to DMSO-treated cells. (C) Relative expression of GLI1, BCL2, and PTCH2 in untreated cells vs cells treated with GDC0449 or GANT61.
T-ALL human xenograft samples with GLI1 expression are sensitive to hedgehog inhibitors ex vivo. (A) Quantitative reverse transcription polymerase chain reaction data showing the relative expression level of GLI1 in 17 patient-derived T-ALL xenograft samples. (B) T-ALL samples with detectable GLI1 expression (left) or very low to undetectable GLI1 expression (right) were cultured ex vivo and treated with GDC-0449, GANT61, or vehicle (DMSO). Diagrams depict the survival rate of the T-ALL samples relative to DMSO-treated cells. (C) Relative expression of GLI1, BCL2, and PTCH2 in untreated cells vs cells treated with GDC0449 or GANT61.
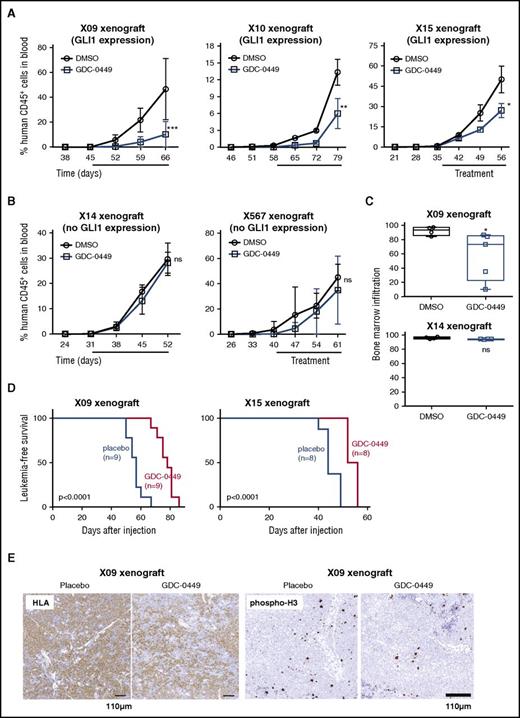
To investigate the in vivo response of T-ALL cells to hedgehog inhibitors, we injected 3 primary T-ALL samples with high GLI1 expression (Figure 7A) and 2 samples with low GLI1 expression (Figure 7B) into immune-deficient NSG mice. Mice were treated by oral gavage with GDC-0449, GANT61, or vehicle once the leukemic clone was detectable in the peripheral blood. In the 3 xenografts with high GLI1 expression, the percentage of the leukemic cells in the blood of mice treated with hedgehog inhibitors was significantly lower compared with placebo-treated animals (Figure 7A; supplemental Figure 8). Furthermore, we noticed that the leukemic infiltration in the bone marrow was significantly reduced compared with placebo-treated animals (Figure 7C), and animals treated with GDC-0449 showed a prolonged duration of leukemia-free survival (Figure 7D). Furthermore, a 20% to 50% reduction in proliferative cells was observed by phospho-histone H3 staining, but no increase in apoptosis was detected (Figure 7E; supplemental Figure 8). In contrast, NSG mice transplanted with T-ALL samples with low GLI1 expression did not show any significant benefit from hedgehog inhibitor treatment (Figure 7A-C). Taken together, the ex vivo and in vivo data demonstrate that T-ALL samples with high GLI1 expression are sensitive to hedgehog pathway inhibition, whereas cases with undetectable or low GLI1 expression do not show sensitivity to hedgehog inhibitor treatment.
T-ALL human xenograft samples with GLI1 expression are sensitive to hedgehog inhibitors in vivo. (A-B) NSG mice injected with patient-derived T-ALL xenograft samples were treated with GDC-0449 or vehicle for 3 weeks (treatment period indicated by solid bar). Graphs show the percentage of human CD45 positive cells in the peripheral blood of NSG mice over time. (C) NSG mice injected with T-ALL xenograft cells with high GLI1 expression levels exhibit lower leukemic infiltration in bone marrow after treatment with hedgehog inhibitors. (D) Leukemia-free survival of mice injected with samples X09 or X15 treated with GDC-0449 or placebo. (E) Representative example of human leukemic cells and cells that proliferate, as defined by HLA and phospho-histone H3 staining, respectively, in the spleen of mice treated with placebo or GDC-0449. *P < .05; **P < .01; ***P < .001. Error bars indicate SEM.
T-ALL human xenograft samples with GLI1 expression are sensitive to hedgehog inhibitors in vivo. (A-B) NSG mice injected with patient-derived T-ALL xenograft samples were treated with GDC-0449 or vehicle for 3 weeks (treatment period indicated by solid bar). Graphs show the percentage of human CD45 positive cells in the peripheral blood of NSG mice over time. (C) NSG mice injected with T-ALL xenograft cells with high GLI1 expression levels exhibit lower leukemic infiltration in bone marrow after treatment with hedgehog inhibitors. (D) Leukemia-free survival of mice injected with samples X09 or X15 treated with GDC-0449 or placebo. (E) Representative example of human leukemic cells and cells that proliferate, as defined by HLA and phospho-histone H3 staining, respectively, in the spleen of mice treated with placebo or GDC-0449. *P < .05; **P < .01; ***P < .001. Error bars indicate SEM.
Discussion
The hedgehog signaling pathway is important for normal T-cell development. Developing T cells can express hedgehog pathway genes at specific stages of development in the thymus and also after exit from the thymus.5,6 It has been clearly illustrated that IHH can be expressed by T cells and was confirmed by gene expression profiling of normal T-cell subsets, whereas SHH is normally expressed by the TECs and not by the T cells (Figure 1).8 Here, we provide evidence that ectopic expression of SHH in leukemic T cells can be detected in a subset of T-ALL cases, and that in these cells, other hedgehog components are also highly expressed. This hedgehog signature defines a clear subset of T-ALL cases that is sensitive to hedgehog pathway inhibitors, as shown by the treatment of patient-derived xenograft models.
Our findings that hedgehog pathway inhibitors show activity as single agent to inhibit the proliferation of T-ALL cells in vitro and in vivo warrant the clinical investigation of SMO and GLI1 inhibitors for the treatment of T-ALL. By using GLI1 expression as a biomarker for hedgehog activation, we identified 4 out of 17 T-ALL patient-derived xenografts with high GLI1 expression, and all 4 showed exquisite sensitivity to hedgehog inhibition in an ex vivo treatment study. Moreover, 3 of those cases were also tested in vivo and showed sensitivity to GANT61 and GDC-0449 as single-agent therapy. Interestingly, the T-ALL cases that showed sensitivity to hedgehog inhibitors were independent from any previous T-ALL subgroup classification based on expression of TAL1, TLX1/3, LMO2, or HOXA genes. Importantly, T-ALL cases with low or undetectable GLI1 expression did not show sensitivity to hedgehog inhibitors, clearly establishing the link between GLI1 expression (as a biomarker for hedgehog pathway activation) and sensitivity to SMO or GLI1 inhibitors. With 2 hedgehog pathway inhibitors being approved by the US Food and Drug Administration for the treatment of basal cell carcinoma,47 our data warrant further investigation of these drugs for the treatment of T-ALL if our data are confirmed and further extend toward studies with combination therapy.
Hedgehog pathway activation has been described in various leukemias, but its potential importance during T-cell leukemia development has remained controversial. It was previously reported that the activity of the hedgehog pathway was dispensable for T-ALL development in a NOTCH1-ICN-dependent mouse model.28 Our results do not necessarily contradict these findings. NOTCH1-ICN is an extremely potent oncogene, and mice expressing high levels of ICN develop T-ALL disease in a very short time. It is likely that the hedgehog pathway is not essential in that model; however, this does not exclude the possibility that it could contribute to T-ALL development in other models or in human disease. In our mouse model, we used a JAK3(M511I) mutant as the main oncogenic driver of T-ALL. Although we do not see a decreased latency in disease progression, the activation of the hedgehog pathway does provide a clear clonal advantage providing evidence that the hedgehog pathway can contribute to the survival and maintenance of T-ALL cells.
Moreover, in our mouse model, we used ectopic expression of the hedgehog ligands that are able to alter the microenvironment, an effect not possible to study after Smo inactivation as reported for the NOTCH1-ICN T-ALL model.28 Our data indicate that ectopic Shh or Ihh expression in T cells has both autocrine and paracrine effects, which may contribute to leukemia development by direct effects on the leukemia cells and by stimulating epithelial cells to produce more factors that contribute to the proliferation and survival of the leukemia cells. Despite the fact that human T-ALL cells harbor mutations that activate their proliferation and survival, it has been shown that they remain partially dependent on the presence of stimulatory ligands such as DLL4 and IL-7.48,49 Thus, the stimulation of the TECs to produce such ligands, as observed in our study, would provide a benefit to the leukemia cell proliferation and survival.
Our data provide evidence that a subgroup of T-ALL patients show activation of the hedgehog pathway and could potentially benefit from the treatment with SMO or GLI1 inhibitors. Further studies are needed to confirm our data and to study hedgehog inhibitors in combination with chemotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christine Harrison for help with the selection of T-ALL samples for this study.
This work was supported by a grant from European Research Council grant (J.C.), FWO-Vlaanderen (grant G068312N) (J.C.), a fellowship grant from the European Hematology Association (C.V.), a postdoctoral fellowship from Kom op Tegen Kanker (A.D.), and grants from Kom op Tegen Kanker and the Stichting Tegen Kanker (J.C.). S. Degryse is supported by the Agentschap voor Innovatie door Wetenschap en Technologie, and J.D.B. is supported by Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Authorship
Contribution: A.D., S. Demeyer, E.R., D.P., and J.C. designed the research; A.D. and S. Demeyer performed expression data analysis; A.D., J.D.B., E.R., D.P., S. Degryse, O.G., R.V., and C.E.d.B. performed the experiments; E.G., A.U., and N.B. collected, characterized, and provided the T-ALL patient samples; A.D., S. Demeyer, E.R., C.V., C.E.d.B., and J.C. wrote the manuscript; and A.D. and J.C. designed the experiments and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Cools, Center for Human Genetics, Herestraat 49 (Box 602), B-3000 Leuven, Belgium; e-mail: jan.cools@kuleuven.be.