Leukemia is a developmental disease of the hematopoietic system. In this issue of Blood, Dagklis et al1 identified hyperactivation of the hedgehog developmental pathway in a subgroup of T-cell acute lymphoblastic leukemias (T-ALLs). Given the availability of US Food and Drug Administration (FDA)–approved hedgehog-inhibiting drugs and the extremely poor prognosis of relapsed T-ALL, this discovery may present a new therapeutic opportunity.
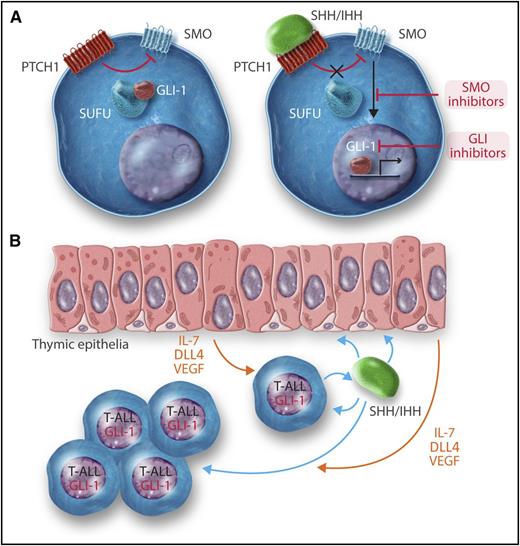
(A) A very simplified scheme of the hedgehog signaling pathway. (Left) In the absence of ligand the Patched (PTCH1) receptor inhibits the activity of the smoothened (SMO) transmembrane protein. The GLI-1 (and GLI-2) transcription factor is kept in the cytosol by Suppressor of Fused (SUFU) and other proteins. (Right) Upon binding of hedgehog ligands, either SHH or IHH, to PTCH1, the inhibitory activity on SMO is relieved. Signaling downstream to SMO releases GLI-1 that translocases into the nucleus, binds to DNA, and regulates the expression of hedgehog target genes. (B) Proposed mechanisms of the positive effects of hedgehog ligands on T-ALL. Approximately 20% of T-ALLs secrete either SHH and IHH. These cytokines have both autocrine and paracrine effects. They bind to T-ALL cells and activate the hedgehog pathway, resulting in activation of GLI-1 and consequent upregulation of prosurvival and growth genes. In addition, SHH/IHH bind to thymic epithelium cells and induce the expression of T-ALL–promoting proteins such as the Notch ligand DLL4, interleukin 7 (IL-7), and vascular endothelial growth factor (VEGF).
(A) A very simplified scheme of the hedgehog signaling pathway. (Left) In the absence of ligand the Patched (PTCH1) receptor inhibits the activity of the smoothened (SMO) transmembrane protein. The GLI-1 (and GLI-2) transcription factor is kept in the cytosol by Suppressor of Fused (SUFU) and other proteins. (Right) Upon binding of hedgehog ligands, either SHH or IHH, to PTCH1, the inhibitory activity on SMO is relieved. Signaling downstream to SMO releases GLI-1 that translocases into the nucleus, binds to DNA, and regulates the expression of hedgehog target genes. (B) Proposed mechanisms of the positive effects of hedgehog ligands on T-ALL. Approximately 20% of T-ALLs secrete either SHH and IHH. These cytokines have both autocrine and paracrine effects. They bind to T-ALL cells and activate the hedgehog pathway, resulting in activation of GLI-1 and consequent upregulation of prosurvival and growth genes. In addition, SHH/IHH bind to thymic epithelium cells and induce the expression of T-ALL–promoting proteins such as the Notch ligand DLL4, interleukin 7 (IL-7), and vascular endothelial growth factor (VEGF).
The hedgehogs are a group of secreted proteins that regulate the development of many tissues.2 Their mode of action is depicted in a very simplified scheme in the figure (panel A). Hedgehog ligands, Sonic or Indian hedgehog (SHH or IHH, respectively), bind and block the activity of their receptor, Patched (PTCH1 or PTCH2). Patched is an unusual receptor because it is a tonic repressor of another transmembrane protein, Smoothened (SMO). Once Patched is repressed by hedgehog ligands, Smoothened is activated. This activation results in translocation of the transcription factors GLI-1 or GLI-2 from the cytosol into the nucleus and transcription of hedgehog-responsive genes. Activation of the hedgehog pathway caused by germline mutations in PTCH1 causes the hereditary Gorlin basal cell nevus syndrome (OMIM #109400), characterized by developmental abnormalities, irradiation hypersensitivity, and an increased incidence of basal cell carcinomas, medulloblastoma, and soft tissue sarcomas. Somatic hyperactivation of the hedgehog pathway by increased expressed of its ligands or mutations in its signaling components is also observed in many cancers. SMO inhibitors were recently approved by the FDA for treatment of advanced basal cell carcinomas and are in clinical trials for multiple cancers including some hematopoietic malignancies.3
The hedgehog pathway has been previously implicated in hematopoietic and T-cell development.4,5 This and the finding of rare mutations in SMO in T-ALL, prompted Dakglis et al to examine gene-expression data of T-ALL for evidence of perturbation in hedgehog signaling. They identified high expression of the hedgehog ligands SHH or IHH and of the downstream GLI-1 transcription factor in ∼20% of T-ALLs. These observations were followed by detailed in vitro and in vivo functional studies involving both human and mouse T-ALLs. What emerged is that abnormal high expression of hedgehog ligands by some T-ALLs promotes their growth by a combination of autocrine and paracrine mechanisms (see figure, panel B). In addition to direct stimulation of leukemic cells, the secreted hedgehog ligands also bind to the thymic epithelial cells and stimulate them to express 3 T-ALL–promoting factors: Notch ligand Delta4 (DLL4), the T-cell promoting cytokine interleukin-7, and the vascular endothelial growth factor. The elucidation of these mechanisms beautifully illustrates the usefulness of studying both human and mouse leukemias. Only syngeneic mouse models enable detailed studies of the interaction between cancer cells and their native microenvironment.
These discoveries raise at least 2 outstanding questions: What are the biological characteristics of T-ALLs that are sensitive to hedgehog inhibitors and what is the potential relevance for patients?
The investigators provide some experimental evidence suggesting that expression of GATA1 and GATA2 transcription factors in the T-ALL cells induces the abnormal expression of hedgehog ligands. This finding is a bit surprising because GATA1/2 are myeloid transcription factors that are not directly involved in T-cell development. Interestingly, mutations in GATA3, the major GATA factor regulating T-cell differentiation, have been reported in T-ALL.6 It is thus tempting to speculate that the subgroup of T-ALLs with activated hedgehog signaling is characterized by developmental, genetic, or epigenetic perturbations of the GATA2 to GATA3 transition during T-cell development.
The therapeutic question is the most compelling, especially in light of the few current options for therapy of relapsed T-ALL. The investigators show that hedgehog inhibitors partially block the growth of human T-ALL cells in vitro and in vivo. It is interesting that a novel compound (GANT61) that binds directly to GLI-1 was much more cytotoxic to T-ALLs than the SMO inhibitors. One explanation could be off-target effects of this compound, and another is that the activation of GLI1 in some T-ALLs may be caused not only by hedgehog but also by other signaling pathway such as RAS.7,8 Because of the modest activity of the FDA-approved SMO inhibitors against xenografts of human T-ALL and the known fast emergence of resistance mutations observed in other cancer clinical trials,9 I believe that any future clinical trials with SMO inhibitors in T-ALL should incorporate combination chemotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal