Key Points
Genetically heterogeneous subclones with varying leukemia-initiating potential exist in neonatal transient abnormal myelopoiesis.
This novel xenograft model of transient abnormal myelopoiesis may provide unique insight into the evolutionary process of leukemia.
Abstract
Transient abnormal myelopoiesis (TAM) is a clonal preleukemic disorder that progresses to myeloid leukemia of Down syndrome (ML-DS) through the accumulation of genetic alterations. To investigate the mechanism of leukemogenesis in this disorder, a xenograft model of TAM was established using NOD/Shi-scid, interleukin (IL)-2Rγnull mice. Serial engraftment after transplantation of cells from a TAM patient who developed ML-DS a year later demonstrated their self-renewal capacity. A GATA1 mutation and no copy number alterations (CNAs) were detected in the primary patient sample by conventional genomic sequencing and CNA profiling. However, in serial transplantations, engrafted TAM-derived cells showed the emergence of divergent subclones with another GATA1 mutation and various CNAs, including a 16q deletion and 1q gain, which are clinically associated with ML-DS. Detailed genomic analysis identified minor subclones with a 16q deletion or this distinct GATA1 mutation in the primary patient sample. These results suggest that genetically heterogeneous subclones with varying leukemia-initiating potential already exist in the neonatal TAM phase, and ML-DS may develop from a pool of such minor clones through clonal selection. Our xenograft model of TAM may provide unique insight into the evolutionary process of leukemia.
Introduction
Neonates with Down syndrome (DS) are at high risk of developing a unique hematologic disorder referred to as transient abnormal myelopoiesis (TAM), transient myeloproliferative disorder, or transient leukemia. In most cases, TAM resolves spontaneously within 3 months.1,2 However, after spontaneous remission, 20% of TAM patients develop myelodysplastic syndrome and acute megakaryocytic leukemia referred to as myeloid leukemia of DS (ML-DS) within 4 years.3,4 Blast cells in most patients with TAM and ML-DS have mutations in exon 2 of the gene coding for the transcription factor GATA1,5-8 which is essential for the normal development of erythroid and megakaryocytic cells.9,10 Although blast cells in most TAM and ML-DS patients share the identical GATA1 mutation, recurrent additional cytogenetic abnormalities are commonly observed during disease progression.2,5,11,12 In fact, a ML-DS case derived from a minor clone with a distinct GATA1 mutation in the TAM phase was previously reported by our group.13 These clinical findings suggest that although most TAM cells disappear in the early neonatal phase, a few clones persist during apparent remission to develop ML-DS later. Because only one fifth of TAM cases progress to ML-DS, additional genetic events besides GATA1 mutation are likely to be involved in the progression of TAM to ML-DS.14 As mentioned above, the development of ML-DS is significantly correlated with karyotypic abnormalities such as duplication (dup)(1q), deletion (del)(6q), del(7p), dup(7q), +8, +11, and del(16q),2,11,12 which are rarely observed in the TAM phase. These clinical findings have led many physicians to consider TAM as preleukemia and the progression of TAM to ML-DS as an attractive model to investigate multistep leukemogenesis.
Animal models have contributed to our understanding of the pathogenesis of TAM/ML-DS and other leukemias.15-21 Mice models in which primary human leukemic cells were transplanted into immunodeficient hosts provided significant clues to advance our understanding of the pathogenesis of human leukemia.19-22 However, xenograft models of primary patient samples from the preleukemic phase have been rarely reported, and the TAM xenograft model would be an attractive method to investigate leukemogenesis.
We previously described the development of novel immunodeficient NOD/Shi-scid, interleukin (IL)-2Rγnull (NOG) mice with a superior capacity for the engraftment of human hematopoietic and neoplastic cells.23-26 In contrast to a previous study in which TAM cells showed a limited ability to expand in immunodeficient mice,27 we established a xenograft model where TAM cells were transplanted into NOG mice to recapitulate the pathophysiology of TAM/ML-DS. This xenograft model in combination with high-throughput genomic technology was used to show that genetically heterogeneous minor subclones with leukemia-initiating potential already exist in the neonatal TAM phase and could serve as initiating clones evolving to ML-DS in a patient. Our TAM xenograft model may be of value to gain insight into the evolutionary process of leukemia.
Materials and methods
Patients and sample collection
Peripheral blood (PB) samples were obtained from patients diagnosed with TAM associated with DS in acute and complete remission phases. Mononuclear cells were separated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation, as previously described.23 Informed consent was obtained from the patients’ parents in accordance with the Declaration of Helsinki, and the research was approved by the institutional ethics committee of Kyoto University Hospital.
Mice
NOG mice were developed at the Central Institute of Experimental Animals (Kawasaki, Japan) as previously described28 and were maintained in our pathogen-free facility and cared for in accordance with the institutional guidelines for animal welfare.
Primary and serial xenogeneic transplantation into NOG mice
Xenotransplantation and analysis of TAM cells were performed using a previously reported method with some modifications.26 In brief, PB mononuclear cells (PBMCs) obtained from TAM patients (1–3 × 106 cells) were injected into 2.4 Gy–irradiated 8- to 12-week-old NOG mice through the tail vein. To screen for the proliferation of TAM-derived cells, bone marrow (BM) cells were aspirated from the tibia every 4 weeks. Engraftment was defined as >1% of cells staining positive for human CD7 (hCD7), hCD33, hCD41a, hCD45, and hCD117 at 12 weeks after transplantation. For serial transplantation, recipient BM cells were collected 12 to 18 weeks after transplantation; the equivalent of 1 × 106 hCD45+ cells was intravenously transplanted into new mice. For a detailed determination of chromosomal and genetic alterations in TAM-derived cells, serial transplantation experiments using preserved PBMC samples were performed.
Flow cytometric analysis of transplanted TAM-derived cells
For analysis of TAM-derived cells in murine BM, mice were euthanized, and the BM was removed and mechanically dispersed. Mononuclear cells were purified from the BM and stained with antibodies. Dead cells were excluded according to 4′,6-diamidino-2-phenylindole staining. Blast cells were identified by classical CD45/SSC blast gating.29 See supplemental Methods on the Blood Web site for details.
Human cell sorting
Colony assay
Leukemic colony formation was assessed according to a previously described method with some modifications.30 See supplemental Methods for details.
GATA1 genomic sequencing analysis
The GATA1 gene was amplified using polymerase chain reaction (PCR) as previously described8 and sequenced by an ABI 3130xl Genetic analyzer (Applied Biosystems, Foster City, CA).
DNA copy number analysis
DNA copy number analysis was performed using GeneChip Human Mapping 250K Nsp arrays (Affymetrix, Inc., Santa Clara, CA) according to the manufacturer's standard protocols. Genomic copy numbers including allele-specific copy numbers were calculated using CNAG/AsCNAR software (http://www.genome.umin.jp), and genomic DNA obtained from PB of patients in the remission phase was used as a control. Copy number abnormalities and other allelic imbalances were detected using a hidden Marcov model–based algorithm.
Statistical analysis
Data are presented as the mean ± standard deviation. The 2-sided P value was determined by testing the null hypothesis that the 2 population medians are equal. P values <0.05 were considered to be significant.
Results
Establishment of a TAM xenograft model using NOG mice
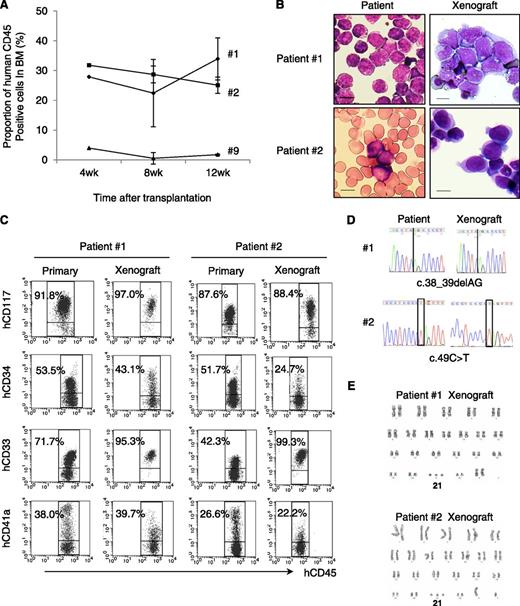
To determine whether NOG mice provide a TAM xenograft model, Ficoll-purified PB samples from 11 TAM patients were transplanted into irradiated NOG mice. Patient characteristics are shown in Table 1. Patients’ ages at sample collection, percentage of blast cells, number of cells injected, and number of engrafted recipients for each PB sample are shown in supplemental Table 1. Of 11 patient samples, 3 (patients 1, 2, and 9) were engrafted successfully in the recipient mice. Engraftment was maintained ≥12 weeks in all cases (Figure 1A). The spleen and liver of the recipients were also infiltrated with hCD45+ blast cells (data not shown). These TAM-derived cells were morphologically similar to the primary TAM cells obtained from the patients (Figure 1B). Flow cytometric analysis of surface antigens detected the expression of CD117, CD34, CD33, and CD41a on hCD45+ cells, which was consistent with the pattern observed in primary cells of TAM patients (Figure 1C). The presence of the same GATA1 mutation was confirmed in the primary TAM cells and the engrafted cells in NOG mice (Figure 1D; supplemental Table 1). Chromosomal analysis of engrafted cells showed no abnormalities other than trisomy 21 (Figure 1E). These TAM-derived cells were detectable in the recipient’s BM for >24 weeks (data not shown).
Clinical characteristics of 11 TAM patients
| Patient no. . | Gender . | Period of gestation, wk . | Weight at birth, g . | PB at diagnosis of TAM . | Cytogenetics International System for Human Cytogenetic Nomenclature (2009) . | GATA1 mutation . | Treatment . | Clinical outcome . | Onset of ML-DS (m of age) . | Follow-up interval . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC, ×103/µL . | Blast, % . | Hb, g/dL . | Plt, ×103/µL . | Exchange transfusion . | Low-dose Ara-C . | |||||||||
| 1 | F | 36 | 3050 | 159 | 91 | 9.1 | 247 | 47,XX,+21 [20] | c.38_39delAG | No | Yes | Alive | Yes (14) | 27 |
| 2 | M | 37 | 1868 | 45.0 | 65 | 19.0 | 80 | 47,XY,+21 [20] | c.49C>T | No | Yes | Alive | No | 24 |
| 3 | F | 39 | 3102 | 40.3 | 37 | 15.1 | 304 | 47,XX,+21 [20] | c.59_174del116 | No | No | Alive | No | 23 |
| 4 | M | 37 | 2780 | 15.6 | 24 | 17.0 | 50 | 47,XY,+21 [20] | c.163_169del | No | No | Alive | No | 22 |
| 5 | M | 39 | 3052 | 60.9 | 48 | 20.7 | 258 | 47,XY,+21 [19] | c.37G>T | No | No | Alive | No | 21 |
| 6 | F | 37 | 2050 | 13.6 | 12 | 20.9 | 291 | 47,XX,+21 [20] | N/A | No | No | Alive | No | 19 |
| 7 | M | 38 | 2694 | 280 | 87 | 13.6 | 26 | 47,XY,+21 [20] | c.186C>G | Yes | Yes | DOD | No | 1 |
| 8 | M | 35 | 2070 | 174 | 80 | 19.2 | 117 | 47,XY,+21 [20] | c.-19-1G>A, c.1A>G | Yes | No | Alive | No | 17 |
| 9 | M | 39 | 3380 | 56.2 | 65 | 20.2 | 156 | 47,XY,+21 [20] | c.35C>G | No | Yes | Alive | No | 14 |
| 10 | M | 36 | 2131 | 199 | 84 | 12.2 | 73 | 47,XY,+21 [20] | c.19_20insCCTGA | Yes | Yes | Alive | No | 14 |
| 11 | F | 33 | 2032 | 254 | 90 | 14.3 | 178 | 47,XX,+21 [20] | c.-19-62_-5delinsA | Yes | Yes | Alive | No | 10 |
| Patient no. . | Gender . | Period of gestation, wk . | Weight at birth, g . | PB at diagnosis of TAM . | Cytogenetics International System for Human Cytogenetic Nomenclature (2009) . | GATA1 mutation . | Treatment . | Clinical outcome . | Onset of ML-DS (m of age) . | Follow-up interval . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC, ×103/µL . | Blast, % . | Hb, g/dL . | Plt, ×103/µL . | Exchange transfusion . | Low-dose Ara-C . | |||||||||
| 1 | F | 36 | 3050 | 159 | 91 | 9.1 | 247 | 47,XX,+21 [20] | c.38_39delAG | No | Yes | Alive | Yes (14) | 27 |
| 2 | M | 37 | 1868 | 45.0 | 65 | 19.0 | 80 | 47,XY,+21 [20] | c.49C>T | No | Yes | Alive | No | 24 |
| 3 | F | 39 | 3102 | 40.3 | 37 | 15.1 | 304 | 47,XX,+21 [20] | c.59_174del116 | No | No | Alive | No | 23 |
| 4 | M | 37 | 2780 | 15.6 | 24 | 17.0 | 50 | 47,XY,+21 [20] | c.163_169del | No | No | Alive | No | 22 |
| 5 | M | 39 | 3052 | 60.9 | 48 | 20.7 | 258 | 47,XY,+21 [19] | c.37G>T | No | No | Alive | No | 21 |
| 6 | F | 37 | 2050 | 13.6 | 12 | 20.9 | 291 | 47,XX,+21 [20] | N/A | No | No | Alive | No | 19 |
| 7 | M | 38 | 2694 | 280 | 87 | 13.6 | 26 | 47,XY,+21 [20] | c.186C>G | Yes | Yes | DOD | No | 1 |
| 8 | M | 35 | 2070 | 174 | 80 | 19.2 | 117 | 47,XY,+21 [20] | c.-19-1G>A, c.1A>G | Yes | No | Alive | No | 17 |
| 9 | M | 39 | 3380 | 56.2 | 65 | 20.2 | 156 | 47,XY,+21 [20] | c.35C>G | No | Yes | Alive | No | 14 |
| 10 | M | 36 | 2131 | 199 | 84 | 12.2 | 73 | 47,XY,+21 [20] | c.19_20insCCTGA | Yes | Yes | Alive | No | 14 |
| 11 | F | 33 | 2032 | 254 | 90 | 14.3 | 178 | 47,XX,+21 [20] | c.-19-62_-5delinsA | Yes | Yes | Alive | No | 10 |
Brackets under International System for Human Cytogenetic Nomenclature indicate the number of analyzed cells in metaphase.
Ara-C, cytosine arabinoside; DOD, died of disease; Hb, hemoglobin; N/A, not assessed; Plt, platelet; WBC, white blood cell.
TAM cells engrafted in NOG mice. (A) Proportion of human CD45+ cells in the BM of NOG mice at 4, 8, and 12 weeks after transplantation (n = 3–5 per group). (B) May-Giemsa staining of the BM smear of patients and cytospin preparation of human CD45+ cells in the recipient NOG mice. Blast cells with cytoplasmic blebbing consistent with megakaryocytic differentiation were present in the BM of recipient mice. (C) Surface marker analysis of engrafted TAM cells. Human CD45+ TAM-derived cells expressing hCD117, hCD34, hCD33, and hCD41a are detected in the recipient’s BM. Blast cells were identified by CD45/SSC gating, and debris (low forward scatter) and dead cells (4′,6-diamidino-2-phenylindole positive) were excluded from the analysis. A representative result of >3 experiments is shown. (D) Genomic direct sequencing shows the presence of concordant GATA1 mutation in xenograft and original patients (1 and 2). (E) G-band karyotyping of TAM-derived cells in recipient murine BM shows no additional chromosome abnormality apart from constitutional trisomy 21, consistent with the findings in the original patients. The GATA1 mutation and the karyotype of engrafted cells from patient 9 were not assessed because of a low cell number.
TAM cells engrafted in NOG mice. (A) Proportion of human CD45+ cells in the BM of NOG mice at 4, 8, and 12 weeks after transplantation (n = 3–5 per group). (B) May-Giemsa staining of the BM smear of patients and cytospin preparation of human CD45+ cells in the recipient NOG mice. Blast cells with cytoplasmic blebbing consistent with megakaryocytic differentiation were present in the BM of recipient mice. (C) Surface marker analysis of engrafted TAM cells. Human CD45+ TAM-derived cells expressing hCD117, hCD34, hCD33, and hCD41a are detected in the recipient’s BM. Blast cells were identified by CD45/SSC gating, and debris (low forward scatter) and dead cells (4′,6-diamidino-2-phenylindole positive) were excluded from the analysis. A representative result of >3 experiments is shown. (D) Genomic direct sequencing shows the presence of concordant GATA1 mutation in xenograft and original patients (1 and 2). (E) G-band karyotyping of TAM-derived cells in recipient murine BM shows no additional chromosome abnormality apart from constitutional trisomy 21, consistent with the findings in the original patients. The GATA1 mutation and the karyotype of engrafted cells from patient 9 were not assessed because of a low cell number.
NOG mice can support self-renewal of TAM-derived cells
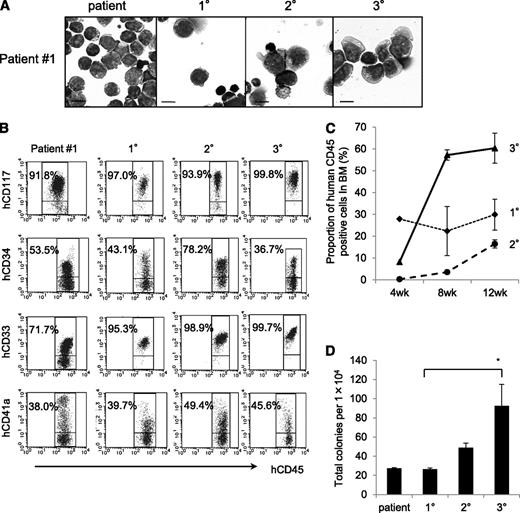
To examine the self-renewal capacity of TAM-derived cells, we performed serial transplantation of engrafted cells in the BM of recipient mice. Only the TAM-derived cells from patient 1 were successfully engrafted into the secondary (2°) and tertiary (3°) recipients. The morphology and surface antigen expression of these engrafted cells remained unchanged throughout the serial transplantation (Figure 2A-B). Interestingly, the TAM-derived cells expanded rapidly in the 3° recipients (Figure 2C). The colony-forming ability of the engrafted cells also increased in subsequent generations (Figure 2D). These cells could be grown by serial transplantation for >1 year and ≥8° recipients, indicating that some TAM clones had long-term self-renewal capacity, a characteristic of leukemia. Indeed, patient 1 developed ML-DS at the age of 1 year, whereas the other patients did not (Table 1).
The NOG mouse model can support self-renewal of TAM-derived cells. (A) May-Giemsa staining of TAM-derived cells in recipients of patient 1. (B) Surface marker analysis of TAM-derived cells in recipients by flow cytometry. Viable cells were gated according to their forward scatter (FSC) and 4′,6-diamidino-2-phenylindole staining, blast cells were identified by CD45/SSC gating, and hCD45+-gated cells were tested for the expression of hCD117, hCD34, hCD33, and hCD41a. (C) Proportion of hCD45+ cells in BM of 1°, 2°, and 3° recipient mice at 4, 8, and 12 weeks after transplantation. (D) Colony assay of hCD45+ cells in BM of 1°, 2°, and 3° recipient mice. hCD45+ cells were seeded at 1.0 × 104 cells per 35-mm dish in triplicate, and the number of colonies in each dish was counted. Bars represent the standard deviation of the mean of 3 independent experiments. *Significant difference (P < .05).
The NOG mouse model can support self-renewal of TAM-derived cells. (A) May-Giemsa staining of TAM-derived cells in recipients of patient 1. (B) Surface marker analysis of TAM-derived cells in recipients by flow cytometry. Viable cells were gated according to their forward scatter (FSC) and 4′,6-diamidino-2-phenylindole staining, blast cells were identified by CD45/SSC gating, and hCD45+-gated cells were tested for the expression of hCD117, hCD34, hCD33, and hCD41a. (C) Proportion of hCD45+ cells in BM of 1°, 2°, and 3° recipient mice at 4, 8, and 12 weeks after transplantation. (D) Colony assay of hCD45+ cells in BM of 1°, 2°, and 3° recipient mice. hCD45+ cells were seeded at 1.0 × 104 cells per 35-mm dish in triplicate, and the number of colonies in each dish was counted. Bars represent the standard deviation of the mean of 3 independent experiments. *Significant difference (P < .05).
TAM-NOG xenograft model recapitulates leukemic evolution from TAM
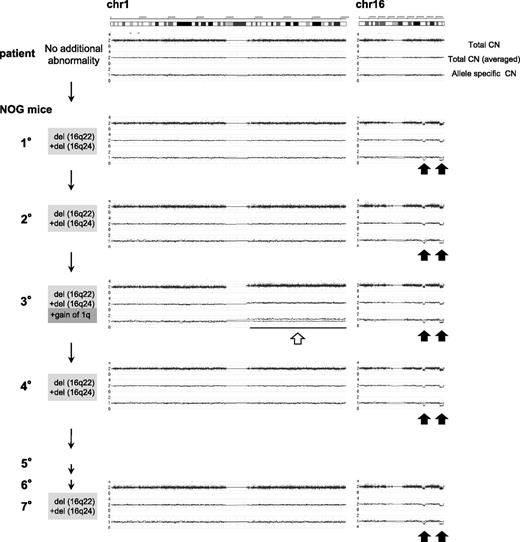
Additional chromosomal alterations are frequently observed in ML-DS in comparison with TAM, suggesting that these alterations in genomic structure could be related to the evolution of ML-DS from TAM.2,11,12 Therefore, we first investigated whether the serially engrafted TAM-derived cells (from patient 1) had DNA copy number alterations (CNAs) using Affymetrix GeneChip Mapping 250K arrays. Primary samples from patient 1 had no CNAs other than the gain of chromosome 21. However, the TAM-derived cells in the 1° recipients showed heterozygous deletion of 16q22 and 16q24 (Figure 3). To determine whether these deletions were present in the same cell, we calculated the signal intensities of each deletion using array data. Nearly 100% of TAM-derived cells harbored each deletion, indicating that these 2 deletions exist in a single TAM-derived cell. Although 2° recipients showed the same CNAs, 3° recipients showed additional CNAs, namely the gain of the entire chromosome 1q (Figure 3; supplemental Figure 1A). Interestingly, the 1q gain was not detected in the 4° to 7° recipients, whereas deletions of 16q22 and 16q24 were present (Figure 3). In this series of transplantations, the original GATA1 mutation found in the primary patient sample (patient 1) remained unchanged (supplemental Figure 1B).
Sequential CNA analysis of TAM-derived cells in the recipients of patient 1. DNA obtained from the original patient sample and sorted hCD45+ recipient BM cells were analyzed by Affymetrix GeneChip Mapping 250K arrays and compared with the PB sample of the original patient in complete remission phase. The primary sample of the patient in TAM phase (blast 92%) had no CNA. hCD45+ BM cells of 1° to 7° recipients had a hemi-allelic deletion in regions 16q22 and 16q24 (black arrows). The 3° recipient had a gain of the entire arm of chromosome 1q (white arrow) in addition to deletion of 16q22 and 16q24. Arrowhead indicates abnormal CNA.
Sequential CNA analysis of TAM-derived cells in the recipients of patient 1. DNA obtained from the original patient sample and sorted hCD45+ recipient BM cells were analyzed by Affymetrix GeneChip Mapping 250K arrays and compared with the PB sample of the original patient in complete remission phase. The primary sample of the patient in TAM phase (blast 92%) had no CNA. hCD45+ BM cells of 1° to 7° recipients had a hemi-allelic deletion in regions 16q22 and 16q24 (black arrows). The 3° recipient had a gain of the entire arm of chromosome 1q (white arrow) in addition to deletion of 16q22 and 16q24. Arrowhead indicates abnormal CNA.
Gain of 1q and deletions in 16q are recurrent chromosomal abnormalities in ML-DS.11,12,31 The result of G-band karyotyping of TAM-derived cells in 3° recipients was 47,XX,+1, der (1;15)(q10;q10),+21 in 20/20 metaphase cells (supplemental Figure 1C), confirming genomic structural change, which is a hallmark of ML-DS. These data suggest that leukemic evolution of TAM-derived cells was observed in our NOG mouse model.
Genetically heterogeneous subclones with varying repopulating capacity expanded in the TAM-NOG xenograft model
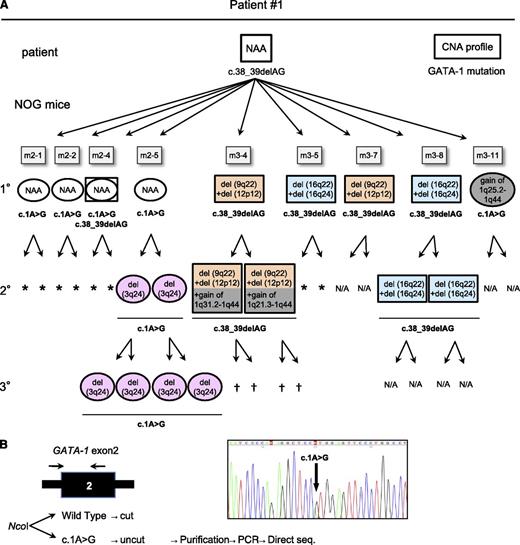
To examine the kinetics of the leukemic evolution of TAM cells, another 2 sets of serial transplantations were performed using the preserved patient 1 sample (Figure 4A). Four of 5 mice in the second group (m2-1–m2-5) and 5 of 11 mice in the third group (m3-1–m3-11) harbored TAM cells from the patient. Of the total of 9 engrafted mice, 2 had the same CNAs detected in the first series of serial transplantations: deletion of 16q22 and 16q24 (m3-5 and m3-8; Figure 3). Moreover, 2 combinations of new CNAs were detected in the 1° recipients: deletion of 9q22 +12p12 (m3-4 and m3-7) and gain of 1q25.2-1q44 (m3-11). No CNAs other than the gain of chromosome 21 were detected in the other recipients (m2-1, m2-2, m2-4, and m2-5).
TAM-derived cells show genetic and functional diversity. (A) Summary of the serial transplantation of TAM cells of patient 1 and the results of CNA profiling and GATA1 mutation analysis. The original patient sample had a single GATA1 mutation, c.38G_39delAG, and no additional CNAs. Diverse subpopulations with or without additional CNAs expanded in each recipient. GATA1 mutation analysis showed 2 distinct mutations in recipients: one identical to that of the original patient (c.38_39delAG) and a different mutation (c.1A>G). The mice harboring cells with the original mutation (c.38_39delAG) are shown in rectangles, and the mice with cells harboring the other mutation (c.1A>G) are shown in ovals, with a CNA profile note inside. The GATA1 mutation is indicated below the symbol. NAA, no additional alteration; N/A, not assessed because of low blast cell count. †Death of recipient before analysis. *No engraftment. (B) Detection of a minor clone with the c.1A>G mutation in the original sample of patient 1. Ncol digestion of a DNA fragment obtained by PCR of GATA1 exon 2 yielded 2 fragments in the wild type, whereas the mutant allele (c.1A>G) remained undigested. PCR of the undigested band and direct sequence analysis identified the same GATA1 mutation (c.1A>G mutation) in the patient sample. Black arrow indicates the primer set.
TAM-derived cells show genetic and functional diversity. (A) Summary of the serial transplantation of TAM cells of patient 1 and the results of CNA profiling and GATA1 mutation analysis. The original patient sample had a single GATA1 mutation, c.38G_39delAG, and no additional CNAs. Diverse subpopulations with or without additional CNAs expanded in each recipient. GATA1 mutation analysis showed 2 distinct mutations in recipients: one identical to that of the original patient (c.38_39delAG) and a different mutation (c.1A>G). The mice harboring cells with the original mutation (c.38_39delAG) are shown in rectangles, and the mice with cells harboring the other mutation (c.1A>G) are shown in ovals, with a CNA profile note inside. The GATA1 mutation is indicated below the symbol. NAA, no additional alteration; N/A, not assessed because of low blast cell count. †Death of recipient before analysis. *No engraftment. (B) Detection of a minor clone with the c.1A>G mutation in the original sample of patient 1. Ncol digestion of a DNA fragment obtained by PCR of GATA1 exon 2 yielded 2 fragments in the wild type, whereas the mutant allele (c.1A>G) remained undigested. PCR of the undigested band and direct sequence analysis identified the same GATA1 mutation (c.1A>G mutation) in the patient sample. Black arrow indicates the primer set.
Each 1° engrafted mouse was subjected to 2° transplantation, and 5 of 9 series (m2-5, m3-4, m3-7, m3-8, and m3-11) successfully gave rise to the xenografts in the 2° recipients. It is noteworthy that the TAM-derived cells of the 2° recipients in 2 of the 3 analyzed series (m2-5 and m3-4) acquired additional CNAs, whereas the CNAs in 2 descendent 2° recipients of m3-8 remained unchanged. The additional CNA of gain of 1q was detected in the 2° recipients of m3-4, similar to that observed in the 3° recipient in Figure 3. Although gain of 1q was recurrently observed in this series, the duplicated regions were diverse: 1q25.2-1q44 (1°, m3-11), 1q21.3-1q44 (2°, m3-4), 1q31.2-1q44 (2°, m3-4), and the whole arm of chromosome 1q (3° in Figures 3 and 4A; supplemental Figure 2). In m2-5, a deletion of 3q24 appeared in the 2° and 3° recipients. These results demonstrated that TAM cells derived from patient 1 acquired various CNAs and showed divergent repopulating capacity in our xenograft model.
TAM-NOG xenograft model revealed the presence of a minor clone with a distinct GATA1 mutation
ML-DS can arise from a minor TAM clone with a GATA1 mutation that is distinct from that of the major TAM clone in a patient.13 To determine whether the GATA1 mutation in the primary patient’s TAM cells was preserved in engrafted TAM-derived cells, GATA1 mutation analysis was performed. TAM-derived cells in the series m3-4, m3-5, m3-7, and m3-8 had the same GATA1 mutation (c.38_39delAG) as that of patient 1 (Figure 4A). Surprisingly, this mutation was not detected in TAM-derived cells in m2-1, m2-2, m2-5, and m3-11; instead, these samples showed a distinct GATA1 mutation (c.1A>G) that was not detectable in the primary patient sample by direct sequencing. One of the 1° recipients (m2-4) showed both GATA1 mutations. These results suggested that a minor clone with a distinct GATA1 mutation (c.1A>G) was present in the primary patient sample and that this minor clone coexisted with, or predominated over, other clones in some 1° recipients. Therefore, a mutation-specific restriction enzyme digestion assay was performed using the primary sample from patient 1, which confirmed the presence of cells with the GATA1 mutation (c.1A>G) as a minor clone (Figure 4B). Moreover, this minor clone propagated and acquired CNAs in NOG mice independently of the major clones (Figure 4A), further demonstrating the genetic heterogeneity of TAM cells. Interestingly, the major clone in the original patient 1 sample with a c.38_39delAG GATA1 mutation and no CNAs did not become dominant in any of the recipients.
Minor subclone with additional CNAs was present in the primary TAM patient sample
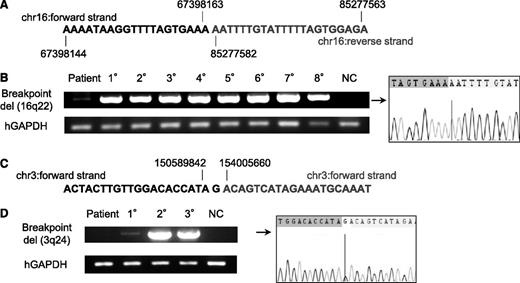
TAM-derived cells in multiple 1° recipients derived from patient 1 had various CNAs including deletions of 16q22 and 16q24 (Figure 4A). To determine whether these subclones were present at low levels in the primary sample of patient 1, specific PCR for the 16q22 deletion was performed using primer pairs designed to bookend the deletion site. CNA analysis and genome sequencing data in these deletion sites (16q22 and 16q24) revealed the presence of genomic breakage and inversion (Figure 5A; supplemental Figures 3 and 4; see supplemental Methods for details). A primer set was designed to detect the deduced breakpoint and used to perform PCR on TAM-derived cells from patient 1 in the recipients with 16q22 and 16q24 deletions. PCR using genomic DNA from TAM-derived cells in the 1° to 8° recipients of the first series of transplantations (Figure 3) produced a uniformly bright DNA fragment of the same size, consistent with the results of CNA profiling (Figure 5B). A faint fragment was detected by applying this PCR method to genomic DNA from the primary patient sample (patient 1), which was confirmed to contain the deletion breakpoint in 16q22 by Sanger sequencing. These results demonstrated that TAM cells with the 16q22 and 16q24 deletions already resided as a minor population in the original sample of patient 1. The frequency of the mutant cells was estimated to be ∼1.0% to 0.2% of the patient’s PBMCs by a serial dilution assay (supplemental Figure 5).
A minor subclone with additional CNAs was present in the primary TAM patient sample, whereas a new clone emerged in a 1° recipient. (A) Contig of del(16q22) breakpoint deduced by whole genome sequencing of the clone containing del(16q22) and del(16q24) in patient 1. Details are shown in supplemental Figure 6. (B) Breakpoint-specific PCR for the del(16q22) clone using genomic DNA from the original patient sample (1; PB in TAM phase), 1° to 8° xenografts (hCD45+ BM cells; Figure 3), and NC (negative control; PBMCs from a healthy adult). Cells from 1° to 8° recipients showed a bright band. The original patient sample showed a faint band, and direct sequencing revealed the presence of the deduced breakpoint for del(16q22). Human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) was used as an internal control. (C) Contig of del(3q24) breakpoint deduced by whole-genome sequencing. Details are shown in supplemental Figure 9. (D) Breakpoint-specific PCR for the del(3q24) clone using genomic DNA from the original patient sample (1; PB in TAM phase), 1° to 3° xenografts (hCD45+ BM cells, m2–5; Figure 4A), and NC. Cells from 2° and 3° recipients showed a bright band. No band was detected in the original patient sample, but a faint band was detected in the 1° recipient sample. hGAPDH was used as an internal control. Direct sequencing confirmed the presence of cells with the deduced breakpoint for del(3q24) in the 1° recipient.
A minor subclone with additional CNAs was present in the primary TAM patient sample, whereas a new clone emerged in a 1° recipient. (A) Contig of del(16q22) breakpoint deduced by whole genome sequencing of the clone containing del(16q22) and del(16q24) in patient 1. Details are shown in supplemental Figure 6. (B) Breakpoint-specific PCR for the del(16q22) clone using genomic DNA from the original patient sample (1; PB in TAM phase), 1° to 8° xenografts (hCD45+ BM cells; Figure 3), and NC (negative control; PBMCs from a healthy adult). Cells from 1° to 8° recipients showed a bright band. The original patient sample showed a faint band, and direct sequencing revealed the presence of the deduced breakpoint for del(16q22). Human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) was used as an internal control. (C) Contig of del(3q24) breakpoint deduced by whole-genome sequencing. Details are shown in supplemental Figure 9. (D) Breakpoint-specific PCR for the del(3q24) clone using genomic DNA from the original patient sample (1; PB in TAM phase), 1° to 3° xenografts (hCD45+ BM cells, m2–5; Figure 4A), and NC. Cells from 2° and 3° recipients showed a bright band. No band was detected in the original patient sample, but a faint band was detected in the 1° recipient sample. hGAPDH was used as an internal control. Direct sequencing confirmed the presence of cells with the deduced breakpoint for del(3q24) in the 1° recipient.
The same method was used to detect a subclone with a 3q24 deletion in the primary patient sample (m2-5; Figure 4A; supplemental Figure 6). At the site of the deletion, genomic breakage was confirmed, and the ends were bound by insertion of a G-nucleotide (Figure 5C; supplemental Figure 7). Consistent with the results of CNA profiling, PCR using DNA from engrafted cells in the 2° and 3° mice (m2-5; Figure 4A) produced a bright DNA fragment, which was confirmed to contain the deletion breakpoint in 3q24 by Sanger sequencing (Figure 5D). Engrafted cells from the BM of the 1° recipient produced a faint DNA fragment, although CNAs were not detected in these cells by array-based methods. We could not detect the corresponding DNA fragment in the primary sample of patient 1. These results suggest the subclone with the 3q24 deletion arose in the 1° recipient mouse as a minor population, emerged as a major population in the 2° recipient, and subsequently engrafted into the 3° recipients. However, because the sensitivity of the specific PCR targeting of the 3q24 deletion was ∼0.1% as determined by the dilution assay (data not shown), it is also possible that this minor clone already existed in the primary patient sample at a frequency below the sensitivity limit. Collectively, our results provide evidence that subclones with additional genetic alterations already exist in the TAM phase and suggest that clonal selection occurs continuously in this xenograft model.
TAM cells derived from patients who did not develop ML-DS had limited self-renewal capacity and fewer additional CNAs than those from the patient who developed ML-DS
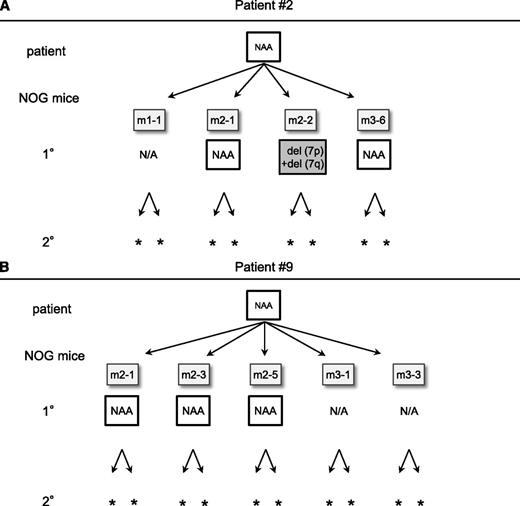
To assess whether TAM cells derived from the patients who did not develop ML-DS had similar self-renewal capacity and genetic instability to those from patient 1, CNA analysis of TAM-derived cells was performed by transplanting the preserved PBMC samples of patients 2 and 9. In patient 2, 4 1° transplantation attempts resulted in successful engraftment. The primary sample of patient 2 had no CNAs (Figure 6A). However, TAM-derived cells in 1 of the 1° recipients (m2-2) showed 7p and 7q deletions, suggesting that a subclone with these CNAs may exist in the primary patient sample. The other 2 1° recipients had no additional CNAs (m2-1 and m3-6). In patient 9, engraftment succeeded in 5 1° recipients, and no additional CNAs were detected in either primary patient sample or engrafted TAM-derived cells (Figure 6B). The engrafted cells in all of the recipient mice harbored the same GATA1 mutation as that of the primary samples of patients 2 and 9. In these 2 cases, our xenograft assay did not detect potent TAM clones with self-renewal capacity in serial transplantation assays (Figure 6A-B; supplemental Table 1), which may reflect the favorable clinical outcome of these patients.
Serial transplantation and CNA profiling of TAM-derived cells from patients that did not develop ML-DS. (A) Serial transplantation assay using TAM cells from patient 2. Four attempts resulted in successful analysis in 1° recipients. CNAs with del(7p) and del(7q) were observed in 1 recipient (m2-2). No additional CNAs were observed in any other recipients. No engraftment was observed in 2° recipients. (B) Serial transplantation assay of TAM cells from patient 9. Five attempts resulted in successful analysis in 1° recipients. No additional CNAs were observed in any analyzed recipients, and no engraftment was observed in 2° recipients. NAA, no additional alteration; N/A, not assessed because of low cell count. *No engraftment.
Serial transplantation and CNA profiling of TAM-derived cells from patients that did not develop ML-DS. (A) Serial transplantation assay using TAM cells from patient 2. Four attempts resulted in successful analysis in 1° recipients. CNAs with del(7p) and del(7q) were observed in 1 recipient (m2-2). No additional CNAs were observed in any other recipients. No engraftment was observed in 2° recipients. (B) Serial transplantation assay of TAM cells from patient 9. Five attempts resulted in successful analysis in 1° recipients. No additional CNAs were observed in any analyzed recipients, and no engraftment was observed in 2° recipients. NAA, no additional alteration; N/A, not assessed because of low cell count. *No engraftment.
Taken together, the results show that only the TAM cells derived from patients who subsequently developed ML-DS had long-term self-renewal capacity with additional CNAs in our serial transplantation assay.
Discussion
New genomic technologies have led to a better understanding of the complex clonal architecture of leukemia and have shown that disease progression occurs through clonal evolution.20-22,32 However, most studies have been based on the retrospective analysis of frank leukemia samples, and data on the evolutionary process occurring in the preleukemic phase are limited because primary preleukemia samples are rarely available and are difficult to maintain in vitro or in vivo. TAM is a unique hematologic condition associated with DS that is mostly self-limited but leads to ML-DS in 20% of cases after spontaneous remission. Therefore, TAM has been considered a preleukemic state and is a suitable pathological condition to analyze the evolutionary process of leukemia.
Because mice models in which primary human leukemic cells were transplanted into immunodeficient hosts provided significant clues to advance our understanding of the pathogenesis of human leukemia,19-22 we hypothesized that xenograft models of TAM cells would be an attractive method to investigate leukemogenesis. In this report, we demonstrated the long-term engraftment of primary TAM cells in NOG mice and showed that TAM cells from a patient that subsequently developed ML-DS had the potential to gain diverse additional genomic alterations and self-renewal capacity. Although we were unable to determine whether the clonal evolution of TAM cells observed in our model reflected the clinical phenotype of the original patient because of insufficient sample from the ML-DS phase, our model is likely to enable the prospective evaluation of leukemic evolution and can be a powerful tool to study the pathophysiology of leukemogenesis. Our model using NOG mice contrasts somewhat with the study by Chen et al,27 who reported that TAM cells resided only in the BM after intra-BM infusion into NOD/SCID mice. We speculate that a severe and unique immunodeficient microenvironment may have contributed to the successful engraftment of TAM cells in NOG mice.
In the present study, primary TAM cell samples from 3 of 11 patients engrafted in NOG mice (Figure 1), but serial engraftment was successful only with cells obtained from the patient who developed ML-DS at the age of 1 year (Figures 2, 3, and 6). The results of extensive serial transplantation revealed the emergence of subclones with various additional CNAs characteristic of ML-DS (Figures 3 and 4). Furthermore, we showed that minor subclones with various CNAs and a distinct GATA1 mutation were already present in the ML-DS patient during the early TAM phase (Figures 4 and 5), as previously described for polyclonality of TAM.2,33 These findings suggest that several preleukemic clones with high leukemia-initiating potential may already reside as minor clones in TAM cells of patients fated to develop ML-DS and show high repopulating capacity in the special microenvironment of NOG mice. Our findings support the hypothesis that ML-DS develops from a pool of heterogeneous minor clones through clonal selection, illustrating the early evolutionary process of leukemia.34
Long-term engraftment of TAM-derived cells was observed for only a minority of TAM patients. This finding suggests that factors other than the properties of the TAM-derived cells, such as technical issues, affected engraftment efficiency. In this regard, increasing the number of transplanted cells resulted in a higher rate of engraftment in recipients of samples from patient 9 (supplemental Table 1). However, there was no clear association between the percentage of TAM blast cells in transplanted samples and successful engraftment (supplemental Table 1). Likewise, frozen samples from 3 patient samples (patients 1, 2, and 9) were as efficient for engraftment as fresh samples from these patients. Therefore, although the number of injected TAM cells and technical issues may affect engraftment, we speculate that engraftment efficiency is an intrinsic property of each TAM-derived cell population.
In addition to trisomy 21, somatic GATA1 mutation is considered an early essential event of TAM and ML-DS occurring in utero.35,36 Interestingly, our TAM-NOG mice model enhanced the emergence of a minor clone with a distinct GATA1 mutation that was not detectable in the original patient sample by conventional sequencing methods. In our model, a minor GATA1 mutant clone expanded predominantly in some recipients and acquired CNAs independently of clones with the original GATA1 mutation, raising the possibility that leukemic evolution occurred from this minor clone, similar to the clinical observation in our previous report.13 In this scenario, a common founder clone of TAM/ML-DS may be established before the acquisition of the GATA1 mutation, or TAM clones with distinct GATA1 mutations may arise independently in the fetal period.
It has long been considered that the linear sequential acquisition of genetic alterations induces disease progression in TAM/ML-DS.37 By contrast, recent studies using high-throughput genomic technology indicate that evolutionary trajectories are more complex and branching in other cancers and leukemias, as previously proposed by Nowell.38 In this theory, genomic instability in founder cells gives rise to heterogeneous mutant subclones, and under selective pressure, some subclones expand to result in disease progression, whereas others become extinct or remain dormant. Thus, leukemic clones may evolve and emerge through the complex interaction of selectively advantageous “driver” mutations, additional advantageous “cooperating” mutations, neutral “passenger” mutations, and deleterious mutations.32,38 It is clinically true that genomic alterations are more frequently observed in ML-DS than in TAM.2,11,39 In this paper, we showed that diverse subclones with various CNAs can be generated in TAM, and these events occurred preferentially in a patient who later developed ML-DS. These findings suggest the presence of leukemic driver mutations in the early phase of TAM in this patient, which may have induced genomic instability. We were unable to find any candidate tumor-associated genes on the deletion sites (3q24, 9q22, 12p12, 16q22, and 16q24) of TAM-derived cells using the The National Center for Biotechnology Information database, suggesting that other genetic mutations and epigenetic events may contribute to the progression to ML-DS, including a few candidate mutations identified previously.40-42 It is also noteworthy that subclones in each recipient mouse showed different repopulating capacities in this study. The dominant clones in each recipient were not always identical in the 1° generations, and the dominant clone in a certain recipient did not always propagate dominantly in the next generation recipients (Figure 4A). Differences between the recipient mice or technical problems may have caused variations in engraftment outcome, which is a potential weakness of this xenograft model; however, it is more likely that cooperating genetic event(s) important for leukemogenesis led to the cells of a specific TAM clone becoming the dominant population in each recipient. Such cooperating event(s) could have a considerable impact on a preleukemic TAM clone, and clonal selection might occur in a somewhat random manner. Thus, leukemic evolution may depend on random chance to an extent. Our TAM xenograft model may help demonstrate the branching architecture of clonal evolution in a preleukemic phase, which contrasts with a linear and deterministic pattern of evolution.34,38 Further genomewide analysis is needed to elucidate the true driver or cooperating mutation(s) and unravel the evolutionary process of leukemia.
In conclusion, we established a xenograft model of TAM using highly immunodeficient NOG mice. Our model enabled the observation of clonal selection and expansion of minor mutant TAM clones and is likely to mimic the early phase of the leukemic evolutionary process, demonstrating the striking genetic heterogeneity and the propagating potential of minor clones in a preleukemic phase. Our xenograft model could be valuable tool for gaining insight into the leukemogenesis of ML-DS and for evaluating the prognosis of TAM patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the TAM patients and their families for their participation. The authors thank Drs Akira Niwa, Masashi Sanada, Hironao Numabe, Tomoki Kawai, Takahiro Yasumi, and Ryuta Nishikomori for technical advice.
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and from the Japanese Ministry of Health, Labor and Welfare.
Authorship
Contribution: S.S., I.K., T.M., H.F., and K.U. performed sample collection and processing; S.S., K.T., K.Y., and R.W. performed experiments; A.S.-O. performed microarray analysis (accession number GSE44739); Y.S. and S.M. provided expert statistical analysis; S.S., Y.O., and T.T. analyzed results and made the figures; M.I. and T.N. generated NOG mice; and S.S., K.W., H.H., S.A., E.I., S.O., and T.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshio Heike, Department of Pediatrics, Graduate School of Medicine, Kyoto University, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto, 606-8507, Japan; e-mail: heike@kuhp.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal