Key Points
In MPL exon 10–mutated myeloproliferative neoplasms, the MPL-mutant allele burden varies considerably from about 1% to almost 100%.
High mutation burdens originate from acquired copy-neutral loss of heterozygosity of chromosome 1p and are associated with marrow fibrosis.
Abstract
We studied mutations of MPL exon 10 in patients with essential thrombocythemia (ET) or primary myelofibrosis (PMF), first investigating a cohort of 892 consecutive patients. MPL mutation scanning was performed on granulocyte genomic DNA by using a high-resolution melt assay, and the mutant allele burden was evaluated by using deep sequencing. Somatic mutations of MPL, all but one involving codon W515, were detected in 26/661 (4%) patients with ET, 10/187 (5%) with PMF, and 7/44 (16%) patients with post-ET myelofibrosis. Comparison of JAK2 (V617F)–mutated and MPL-mutated patients showed only minor phenotypic differences. In an extended group of 62 MPL-mutated patients, the granulocyte mutant allele burden ranged from 1% to 95% and was significantly higher in patients with PMF or post-ET myelofibrosis compared with those with ET. Patients with higher mutation burdens had evidence of acquired copy-neutral loss of heterozygosity (CN-LOH) of chromosome 1p in granulocytes, consistent with a transition from heterozygosity to homozygosity for the MPL mutation in clonal cells. A significant association was found between MPL-mutant allele burden greater than 50% and marrow fibrosis. These observations suggest that acquired CN-LOH of chromosome 1p involving the MPL location may represent a molecular mechanism of fibrotic transformation in MPL-mutated myeloproliferative neoplasms.
Introduction
Philadelphia-negative myeloproliferative neoplasms (MPN), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are characterized by clonal hematopoiesis and overproduction of mature myeloid cells.1 The unique JAK2 (V617F) mutation is found in about 95% of patients with PV and in 60% to 70% of those with ET and PMF.2-5 Several studies have been conducted to identify the somatic mutations underlying the remaining 30% to 40% of patients with JAK2 (V617F)–negative ET and PMF. The only consistent observation so far is that subsets of these patients carry activating somatic mutations of the MPL gene encoding the thrombopoietin receptor and that these mutations cluster in exon 10.6-14 Since MPL-mutant clones are often small, their detection is dependent on the sensitivity of the molecular method used and the source of DNA.15 Studies using sensitive methods in large patient populations indicate that MPL-mutated ET and PMF represent about 10% and 15%, respectively, of the respective JAK2 (V617F)–negative conditions.12-14
MPL exon 10 mutations affecting the juxtamembrane (W515L/K/A/R) or the transmembrane (S505N) domains result in a ligand-independent receptor activation with constitutive activation of downstream JAK-STAT signaling.6,16,17 Expression of MPL mutations in a murine bone marrow transplant model induced a myeloproliferative disorder characterized by leukocytosis, thrombocytosis, extramedullary hematopoiesis, bone marrow fibrosis, and splenomegaly.6 In the few studies that investigated the phenotypic associations of MPL mutations and their prognostic relevance, only minor relationships were found.9,10,12
We previously used high-resolution single neucleotide polymorphism (SNP) microarrays to detect chromosomal aberrations, such as loss of heterozygosity (LOH) and somatic copy number changes, in patients with MPN.18 Uniparental disomy (UPD) of chromosome 9p (9pUPD) involving JAK2 and UPD of chromosome 1p (1pUPD) involving MPL were found to be relatively common aberrations that are responsible for transition from heterozygosity to homozygosity for the JAK2 or MPL mutation, respectively.18 We also showed that the JAK2 (V617F)–mutant allele burden has a clinical effect in PV, representing in particular a risk factor for progression to myelofibrosis.19-21
In this study, we reasoned that the MPL-mutant allele burden, rather than the mere presence or absence of MPL mutations and their subtype, might have a clinical effect in MPL-mutated ET and PMF. Therefore, we did a mutation analysis of MPL exon 10 in patients with ET and PMF, assessed the mutant allele burden by using a deep sequencing approach, and then analyzed the clinical correlates of the MPL-mutant allele burden.
Patients and methods
Study population and definitions
This study was approved by the Institutional Ethics Committee (Comitato di Bioetica, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, Pavia, Italy). The procedures followed were in accordance with the Helsinki Declaration of 1975 (as revised in 2000), and samples were obtained after patients provided written informed consent.
The study design is schematically described in supplemental Figure 1. The main component was a prospective observational cohort study that included 892 patients with Ph− MPN other than PV diagnosed and followed at the Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Italy, between 2002 and 2012. Specifically, our cohort included 661 patients with ET, 187 patients with PMF, and 44 patients with post-ET myelofibrosis. The prospective observation started from the evaluation of MPL molecular status: 434 patients (49%) entered the study at diagnosis, and 458 (51%) were enrolled during follow-up.
Diagnosis of ET and PMF was in accordance with the World Health Organization (WHO) criteria.22,23 Post-ET myelofibrosis was diagnosed according to the criteria of the International Working Group of Myelofibrosis Research and Treatment (IWG-MRT)24 ; evolution into acute myeloid leukemia (AML) was defined according to the WHO criteria.23 For the assessment of bone marrow fibrosis, paraffin sections were stained with Gomori’s silver impregnation technique, and fibrosis was assessed semiquantitatively following the European consensus guidelines.25 Thrombotic events were defined as described in detail elsewhere.26 Patients were fundamentally treated according to the recommendations of the European LeukemiaNet.27
As described in supplemental Figure 1, in order to define the effect of MPL-mutant allele burden on clinical phenotype, we analyzed the Pavia cohort of 43 MPL-mutated patients together with a second cohort of 19 patients observed at the University of Florence, Italy, which was previously reported.10
JAK2 and MPL mutation analysis
Peripheral blood granulocytes and CD3+ T lymphocytes were isolated as previously described.28 DNA extraction was performed by using the Puregene Blood DNA isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s procedures. The granulocyte JAK2 (V617F) mutation burden was assessed by using a quantitative polymerase chain reaction (qPCR) –based allelic discrimination assay on a Rotor-Gene 6000 real-time analyzer (Qiagen) and applying the standard curve method.19 The sensitivity of this discrimination assay is approximately 0.2% mutant alleles.21
MPL mutation scanning was performed on granulocyte and T-lymphocyte genomic DNA by using a high-resolution melt (HRM) assay, and samples found to be MPL mutated at HRM analysis were further characterized by using direct sequencing, as previously described.13 MPL mutation analysis was performed at diagnosis or within 6 months from diagnosis in 30 of 62 patients. The remaining 32 patients were evaluated during follow-up (median time from diagnosis, 4.3 years; range, 0.5 to 19.5 years).
Assessment of granulocyte MPL-mutant allele burden
Primers used for the HRM screening were adapted for the multiplexed 454 GS-FLX ultramassive sequencing of granulocyte genomic DNA from MPL-positive patients, as described in detail in supplemental Methods and supplemental Table 1.
MPL exon 10–amplified regions were purified by MinElute columns (Qiagen, Valencia, CA) to remove the fragments shorter than 70 bp and were quantitated by PicoGreen DNA Quantitation Kit (Life Technologies, Monza, Italy). Thirteen amplicons were pooled and then amplified by emulsion PCR as required by the manufacturer’s instructions (Roche Diagnostics, Monza, Italy). Libraries were recovered by isopropanol emulsion breaking and enriched for positive reaction beads. Each enriched pooling sample was separately loaded onto one-eighth of the PicoTiterPlate and was sequenced according to the 454 GS-FLX Titanium protocol (Roche Diagnostics). Raw reads from the GS-FLX sequencing were demultiplexed by using Roche’s proprietary “sfffile” and “sffinfo” utilities and were mapped against a reference sequence of exon 10 of the MPL gene (isoform ENST00000372470 from Ensembl release 69 assembly) by using the Amplicon Variant Analyzer v. 2.6 (Roche Diagnostics). For each sequencing pool, a quality check of the percentage of sequences correctly mapped and carrying the appropriate multiplexing tag was performed. Afterward, by using a script developed in-house, we estimated the mutation burden in all the nucleotides of the reference for each sample. Variations in the sequence were then converted to the corresponding amino acid substitutions according to the selected reference isoform. Read alignments of samples with a very low mutation burden (<1%) were further manually inspected to exclude sequencing and alignment errors.
Detection of 1p LOH and analysis of MPL copy number
Granulocyte and T-lymphocyte DNA were genotyped for the rs760567, rs2073025, rs2297634, and rs1801574 loci by using HRM, as described in detail in supplemental Methods and supplemental Table 2. The number of copies of the MPL gene on chromosome 1p was determined by using TaqMan qPCR and comparing MPL and the haploid SRY gene; the housekeeping diploid ALB gene was used to normalize the data, as described in detail in supplemental Methods and supplemental Table 3.
Flow cytometry enumeration of circulating CD34+ cells
Circulating CD34+ cells were enumerated by flow cytometry using a single-platform assay as previously described.28
Statistical analysis
Numeric variables were summarized by their median and range. Categorical variables were described by count and relative frequency of each category. Hypothesis testing was carried out with a nonparametric approach. The Spearman coefficient was used to test for correlation between numerical variables. The Wilcoxon rank sum test was applied to the comparison of numerical variables between two groups, and the association between categorical variables (two-way tables) was investigated by means of Fisher’s exact test. All tests were two-tailed, and P values were considered significant when lower than .05. Microsoft Office Excel (Microsoft Corporation, Redmond, WA) and Stata 11.2 (StataCorp, Lakeway, TX) were used for data management and statistical analysis. The cumulative incidence of thrombotic complications and the cumulative incidence of leukemic evolution were estimated with a competing risk approach according to the Kalbfleisch-Prentice method.29 Death in the absence of the event of interest (thrombosis or leukemia) was considered as a competing event. Associations between JAK2 or MPL mutations status and marrow fibrosis were investigated by multivariate logistic regression analysis.
Results
JAK2 and MPL mutations in the Pavia cohort
The distribution of JAK2 and MPL mutations according to MPN subtype is reported in Table 1. Somatic MPL mutations, detected in granulocytes but not in T lymphocytes, were observed in 26 (4%) of 661 patients with ET, 10 (5%) of 187 with PMF, and 7 (16%) of 44 with post-ET myelofibrosis. The mutation status was significantly different among the three MPN subtypes (P = .021); in particular, Fisher’s exact test revealed significant differences in terms of MPL-mutated genotypes between ET and post-ET myelofibrosis (P = .004) and between PMF and post-ET myelofibrosis (P = .033), but no difference was observed between ET and PMF (P = .6).
Distribution of MPL and JAK2 mutations according to MPN diagnosis in the Pavia cohort
| Diagnosis . | JAK2 (V617F)–mutated (n = 533) . | MPL exon 10–mutated (n = 43) . | JAK2 (V617F)/MPL–unmutated (n = 316) . | All patients (n = 892) . |
|---|---|---|---|---|
| Essential thrombocythemia | 399 (60%) | 26* (4%) | 236 (36%) | 661 |
| Primary myelofibrosis | 114 (61%) | 10 (5%) | 63 (34%) | 187 |
| Post-essential thrombocythemia myelofibrosis | 20 (45%) | 7 (16%) | 17 (39%) | 44 |
| Diagnosis . | JAK2 (V617F)–mutated (n = 533) . | MPL exon 10–mutated (n = 43) . | JAK2 (V617F)/MPL–unmutated (n = 316) . | All patients (n = 892) . |
|---|---|---|---|---|
| Essential thrombocythemia | 399 (60%) | 26* (4%) | 236 (36%) | 661 |
| Primary myelofibrosis | 114 (61%) | 10 (5%) | 63 (34%) | 187 |
| Post-essential thrombocythemia myelofibrosis | 20 (45%) | 7 (16%) | 17 (39%) | 44 |
One patient with essential thrombocythemia carried both MPL and JAK2 (V617F) mutations; in subsequent analyses, this patient was included in the MPL group.
Demographic and clinical characteristics at diagnosis of patients with ET, PMF, and post-ET myelofibrosis according to their genotype are reported in supplemental Tables 4, 5, and 6, respectively. In ET, the three subgroups (MPL-mutated, JAK2-mutated, and JAK2/MPL-unmutated patients) showed significant differences in terms of clinical phenotype. In particular, MPL-mutated patients had lower hemoglobin levels (P = .0001) and lower platelet counts (P = .0001), and JAK2-positive patients had higher hemoglobin levels (P = .0001), higher WBC counts (P = .0001), and higher incidence of thromboembolic complications (P = .0001).
Relationship between JAK2 or MPL mutation status and risk of thromboembolic complications
The cumulative incidence of thrombosis was estimated with death as a competing risk. In ET, the 5-year cumulative incidence of thrombosis was 9.2% (95% CI, 1.5% to 25.6%) in MPL-mutated patients, 13.5% (95% CI, 9.6% to 18%) in JAK2-mutated patients, and 8.4% (95% CI, 4.7% to 13.4%) in JAK2/MPL-unmutated patients. JAK2-positive patients had a trend toward higher incidence of thrombotic events than JAK2/MPL-unmutated patients (P = .05).
In PMF, the 5-year cumulative incidence of thrombosis was 20% (95% CI, 0.9% to 57.3%) in MPL-mutated patients, 6.8% (95% CI, 2.7% to 13.4%) in JAK2-mutated patients, and 11.8% (95% CI, 4.8% to 22.2%) in wild-type patients. When comparing the three subgroups by pairs, we did not identify significant differences, possibly because of the low number of events.
In post-ET myelofibrosis, the 5-year cumulative incidence of thrombosis was 39.4% (95% CI, 12.3% to 66%) in JAK2-mutated patients, and no thrombotic event was observed in MPL-mutated and JAK2/MPL-unmutated patients.
Relationship between JAK2 or MPL mutation status and risk of leukemic evolution
During follow-up, 21 patients (2.3%) progressed to secondary AML, including 5 patients (0.7%) with ET, 12 (6.4%) with PMF, and 4 (9%) with post-ET myelofibrosis.
The cumulative incidence of leukemia was estimated with death as a competing risk. In ET, the 5-year cumulative incidence of leukemia was null in MPL-mutated and JAK2/MPL-unmutated patients and 1.2% (95% CI, 0.3% to 3.1%) in JAK2-mutated patients, without any significant difference among the 3 genotypic subgroups. In PMF, the 5-year cumulative incidence of leukemia was null in MPL-mutated and JAK2/MPL-unmutated patients and 9.5% (95% CI, 4.3% to 17%) in JAK2-mutated patients. JAK2-mutated patients showed a higher incidence of leukemic evolution in comparison with both MPL-mutated patients (P = .0019) and JAK2/MPL-unmutated patients (P = .013). In post-ET myelofibrosis, no leukemic evolution was observed in MPL-mutated patients; the 5-year cumulative incidence of AML was 12.7% (95% CI, 2% to 33.4%) in JAK2-mutated patients and 10% (95% CI, 0.5% to 35.8%) in JAK2/MPL-unmutated patients. JAK2-mutated patients showed a trend toward higher incidence of leukemic evolution in comparison with MPL-mutated patients (P = .051).
Relationship between JAK2 or MPL mutation status and survival
The whole cohort was observed for a median follow-up from diagnosis of 4.5 years (range, 0 to 21.1 years). Median overall survival (OS) was not reached in ET and post-ET myelofibrosis and was equal to 15.2 years in PMF. The molecular status (JAK2-mutated vs MPL-mutated vs JAK2/MPL-unmutated genotype) did not affect OS in patients with ET and post-ET myelofibrosis, but it did affect OS in patients with PMF (P = .011). In particular, in PMF, the median OS was 9.1 years in MPL-mutated patients, 12.9 years in JAK2-mutated patients, and not reached in JAK2/MPL-unmutated patients (supplemental Figure 2).
Effect of MPL-mutant allele burden on clinical phenotype
To assess the effect of MPL allele burden on clinical phenotype, we evaluated 62 MPL-mutated patients from Pavia (43 patients) and Florence (19 patients). Of these 62 patients, 40 patients were affected with ET, 14 with PMF, and 8 with post-ET myelofibrosis. Types of MPL mutations are reported in Table 2: MPL (W515L), MPL (W515K), and MPL (W515A) were the most common changes detected in 39, 11, and 7 patients, respectively. Four patients carried two MPL mutations, and one patient carried both MPL and JAK2 mutations.
Distribution of MPL mutations according to diagnosis in the Pavia and Florence merged cohorts
| Diagnosis . | W515L* . | W515K . | W515A . | W515R† . | W515S . | S505N . |
|---|---|---|---|---|---|---|
| Essential thrombocythemia | 22 (55%) | 7 (17.5%) | 6 (15%) | 2 (5%) | 2 (5%) | 1 (2.5%) |
| Primary myelofibrosis | 13 (92.9%) | 1 (7.1%) | 0 | 0 | 0 | 0 |
| Post-essential thrombocythemia myelofibrosis | 4 (50%) | 3 (37.5%) | 1 (12.5%) | 0 | 0 | 0 |
| Diagnosis . | W515L* . | W515K . | W515A . | W515R† . | W515S . | S505N . |
|---|---|---|---|---|---|---|
| Essential thrombocythemia | 22 (55%) | 7 (17.5%) | 6 (15%) | 2 (5%) | 2 (5%) | 1 (2.5%) |
| Primary myelofibrosis | 13 (92.9%) | 1 (7.1%) | 0 | 0 | 0 | 0 |
| Post-essential thrombocythemia myelofibrosis | 4 (50%) | 3 (37.5%) | 1 (12.5%) | 0 | 0 | 0 |
Two patients had another concomitant MPL mutation (S505C, V501A).
Two patients had another concomitant MPL mutation (V501A, Q516E).
The MPL mutant allele burden estimated by means of deep sequencing ranged from 0.8% to 94.7%, and the Kruskal-Wallis test showed a different distribution among different MPN subtypes (P = .0031), as shown in Figure 1. The median allele burden was significantly lower in ET compared with PMF (32.8% vs 59.4%; P = .0037) or post-ET myelofibrosis (32.8% vs 58.2%; P = .02), but there was no difference between PMF and post-ET myelofibrosis. In addition, the number of patients with a mutant allele burden greater than 50% was significantly different (P = .006) in the three MPN subtypes, being 8 (20%) of 40 patients in ET, 9 (64%) of 14 patients in PMF, and 4 (50%) of 8 patients in post-ET MF.
Granulocyte MPL-mutant allele burden in patients with different myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box), and outside values (dots). The Kruskal-Wallis test showed a different distribution among different MPN subtypes (P = .0031). The median allele burden was significantly lower in ET compared with PMF (32.8% vs 59.4%; P = .0037) or post-ET myelofibrosis (post-ET MF; 32.8% vs 58.2%; P = .02), but there was no difference between PMF and post-ET myelofibrosis.
Granulocyte MPL-mutant allele burden in patients with different myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box), and outside values (dots). The Kruskal-Wallis test showed a different distribution among different MPN subtypes (P = .0031). The median allele burden was significantly lower in ET compared with PMF (32.8% vs 59.4%; P = .0037) or post-ET myelofibrosis (post-ET MF; 32.8% vs 58.2%; P = .02), but there was no difference between PMF and post-ET myelofibrosis.
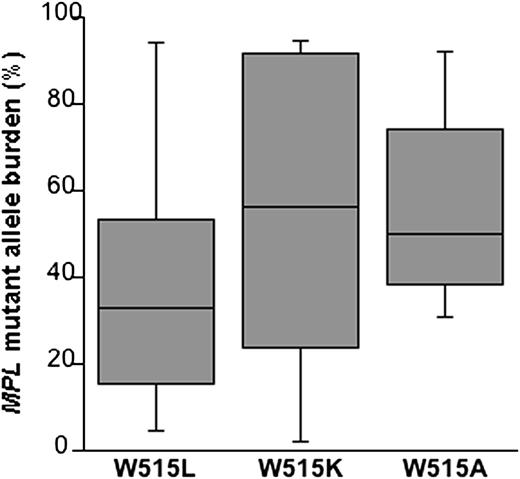
To establish whether the mutant allele burden was specifically related to subtypes of MPL mutation, we examined the most common MPL changes (Figure 2). The median allele burden was 32.9% (range, 4.7% to 94.4%) in the MPL (W515L) group, 56.2% (range, 2.0% to 94.7%) in the MPL (W515K) group, and 49.9% (range, 31.1% to 92.1%) in the MPL (W515A) group, with no significant difference among these median values.
Granulocyte MPL-mutant allele burden according to mutation type in patients with myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively) and lower and upper quartile with median value (box). The median allele burden was 32.9% in the MPL (W515L) group (39 patients), 56.2% in the MPL (W515K) group (11 patients), and 49.9% in the MPL (W515A) group (7 patients), with no significant difference between subgroups (W515L vs W515K P = .186; W515L vs W515A P = .079; and W515K vs W515A P = .821). When restricting the analysis to patients with ET, we found a significant difference among the 3 subgroups (P = .014). The median allele burden was 18.2% in the MPL (W515L) group (22 patients), 41.3% in the MPL (W515K) group (7 patients), and 51.1% in the MPL (W515A) group (6 patients) (W515L vs W515K P = .103; W515L vs W515A P = .0051; W515K vs W515A P = .568).
Granulocyte MPL-mutant allele burden according to mutation type in patients with myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively) and lower and upper quartile with median value (box). The median allele burden was 32.9% in the MPL (W515L) group (39 patients), 56.2% in the MPL (W515K) group (11 patients), and 49.9% in the MPL (W515A) group (7 patients), with no significant difference between subgroups (W515L vs W515K P = .186; W515L vs W515A P = .079; and W515K vs W515A P = .821). When restricting the analysis to patients with ET, we found a significant difference among the 3 subgroups (P = .014). The median allele burden was 18.2% in the MPL (W515L) group (22 patients), 41.3% in the MPL (W515K) group (7 patients), and 51.1% in the MPL (W515A) group (6 patients) (W515L vs W515K P = .103; W515L vs W515A P = .0051; W515K vs W515A P = .568).
We then analyzed the relationships between MPL-mutant allele burden and bone marrow fibrosis, circulating CD34+ cell count, or disease duration. The MPL-mutant allele burden was significantly higher (P < .0001) in patients with bone marrow fibrosis (median allele burden, 53.1%) than in those without fibrosis (median allele burden, 15.6%), as shown in Figure 3. The effect of mutation burden on fibrosis remained statistically significant (P = .0001), even when categorizing patients according to grade of fibrosis (Figure 3).25
Granulocyte MPL-mutant allele burden according to the degree of bone marrow fibrosis in patients with myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively) and lower and upper quartile with median value (box). (Upper panel, A) The proportion of MPL-mutant alleles was significantly higher in patients with bone marrow fibrosis than in those without bone marrow fibrosis (P < .0001). (Lower panel, B) The relationship also remained statistically significant (P = .0001) when categorizing patients according to grade of fibrosis (grade 0 vs grade 1 vs grade 2 to 3), defined according to the European consensus criteria.25
Granulocyte MPL-mutant allele burden according to the degree of bone marrow fibrosis in patients with myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively) and lower and upper quartile with median value (box). (Upper panel, A) The proportion of MPL-mutant alleles was significantly higher in patients with bone marrow fibrosis than in those without bone marrow fibrosis (P < .0001). (Lower panel, B) The relationship also remained statistically significant (P = .0001) when categorizing patients according to grade of fibrosis (grade 0 vs grade 1 vs grade 2 to 3), defined according to the European consensus criteria.25
The Spearman rank test showed a correlation between MPL-mutant allele burden and circulating CD34+ cells (ρ = .518; P = .0001). More specifically, patients with a mutant allele burden greater than 50% had a significantly higher number of circulating CD34+ cells than those with an allele burden below or equal to 50% (median number, 46.9 vs 3.6 × 106/L; P = .0028), as reported in Figure 4. A significant relationship was also found between MPL-mutant allele burden and disease duration (Spearman rank test ρ = .313; P < .013).
Relationship between circulating CD34+ cell count and granulocyte MPL-mutant allele burden in patients with myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box), and outliers (dots). Spearman rank test showed a significant direct relationship between circulating CD34+ cell count and MPL-mutant allele burden (P = .0001). Patients with mutant alleles >50% had a significantly higher number of circulating CD34+ cells than those with ≤50% (P = .0028).
Relationship between circulating CD34+ cell count and granulocyte MPL-mutant allele burden in patients with myeloproliferative neoplasms. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box), and outliers (dots). Spearman rank test showed a significant direct relationship between circulating CD34+ cell count and MPL-mutant allele burden (P = .0001). Patients with mutant alleles >50% had a significantly higher number of circulating CD34+ cells than those with ≤50% (P = .0028).
Multivariate logistic regression analysis of the association between marrow fibrosis and JAK2 or MPL-mutant allele burden in patients with ET or post-ET myelofibrosis
For the purpose of this analysis, patients with ET or post-ET myelofibrosis belonging to the Pavia cohort (Table 1) were considered as a phenotypic continuum in which the disease initially presents as ET and may progress to myelofibrosis over time.
A preliminary analysis comparing JAK2-mutated, MPL-mutated, and JAK2/MPL-unmutated patients (model 1 in Table 3) showed that MPL-mutated patients with ET had a higher risk of having marrow fibrosis than the JAK2 (V617F)–mutated ones (odds ratio, 3.22; P = .017). No significant difference was found between MPL-mutated and JAK2/MPL-unmutated ET patients.
Multivariate logistic regression analysis of the association between marrow fibrosis and JAK2- or MPL-mutant allele burden in patients with ET or post-ET myelofibrosis belonging to the Pavia cohort (see Table 1)
| Risk factors for the presence of fibrosis (covariates) . | Odds ratio . | P . | 95% CI . |
|---|---|---|---|
| Model 1 (including JAK2-mutated, MPL-mutated, and JAK2/MPL-unmutated patients) | |||
| Age at diagnosis (y) | 1.01 | .225 | 0.99-1.03 |
| MPL exon 10 mutation vs JAK2 (V617F) | 3.22 | .017 | 1.24-8.40 |
| MPL exon 10 mutation vs JAK2/MPL-unmutated patients | 1.64 | .323 | 0.62-4.37 |
| Model 2 (including JAK2-mutated and MPL-mutated patients exclusively) | |||
| Age at diagnosis (y) | 1.01 | .396 | 0.99-1.04 |
| MPL exon 10 mutation vs JAK2 (V617F) | 2.42 | .116 | 0.80-7.29 |
| Mutation burden, % | 1.05 | <.001 | 1.03-1.07 |
| Model 3 (including JAK2-mutated and MPL-mutated patients exclusively) | |||
| Age at diagnosis (y) | 1.01 | .227 | 0.99-1.04 |
| MPL exon 10 mutation vs JAK2 (V617F) | 2.67 | .066 | 0.94-7.57 |
| Mutation burden, >50% vs ≤50% | 15.45 | <.001 | 3.89-61.38 |
| Risk factors for the presence of fibrosis (covariates) . | Odds ratio . | P . | 95% CI . |
|---|---|---|---|
| Model 1 (including JAK2-mutated, MPL-mutated, and JAK2/MPL-unmutated patients) | |||
| Age at diagnosis (y) | 1.01 | .225 | 0.99-1.03 |
| MPL exon 10 mutation vs JAK2 (V617F) | 3.22 | .017 | 1.24-8.40 |
| MPL exon 10 mutation vs JAK2/MPL-unmutated patients | 1.64 | .323 | 0.62-4.37 |
| Model 2 (including JAK2-mutated and MPL-mutated patients exclusively) | |||
| Age at diagnosis (y) | 1.01 | .396 | 0.99-1.04 |
| MPL exon 10 mutation vs JAK2 (V617F) | 2.42 | .116 | 0.80-7.29 |
| Mutation burden, % | 1.05 | <.001 | 1.03-1.07 |
| Model 3 (including JAK2-mutated and MPL-mutated patients exclusively) | |||
| Age at diagnosis (y) | 1.01 | .227 | 0.99-1.04 |
| MPL exon 10 mutation vs JAK2 (V617F) | 2.67 | .066 | 0.94-7.57 |
| Mutation burden, >50% vs ≤50% | 15.45 | <.001 | 3.89-61.38 |
To determine the association between mutation burden and marrow fibrosis, we then performed a multivariate logistic regression analysis on MPL-mutated and JAK2-mutated patients, with presence of fibrosis as outcome and age at diagnosis, type of mutation (MPL vs JAK2 mutation), and mutation burden as covariates. As shown in Table 3 (models 2 and 3), there was no significant effect of mutation subtype (MPL or JAK2) when adjusting by mutant allele burden, although a trend toward a significantly higher odds ratio for the MPL mutation was observed in model 3. The most striking finding was the strong association between elevated mutant allele burden (greater than 50%) and the presence of marrow fibrosis, irrespective of the mutation involved.
LOH of chromosome 1p (1pLOH) with evidence of UPD in MPN patients with high MPL-mutant allele burden
The MPL gene is located on chromosome 1p34. Direct sequencing allowed us to identify the intronic T→C rs839995 SNP located 70 bases before the beginning of MPL exon 10. Informative patients with MPL mutation burden greater than 50% had a homozygous TT or CC genotype in granulocytes and a heterozygous TC genotype in T lymphocytes (data not shown), suggesting 1pLOH.
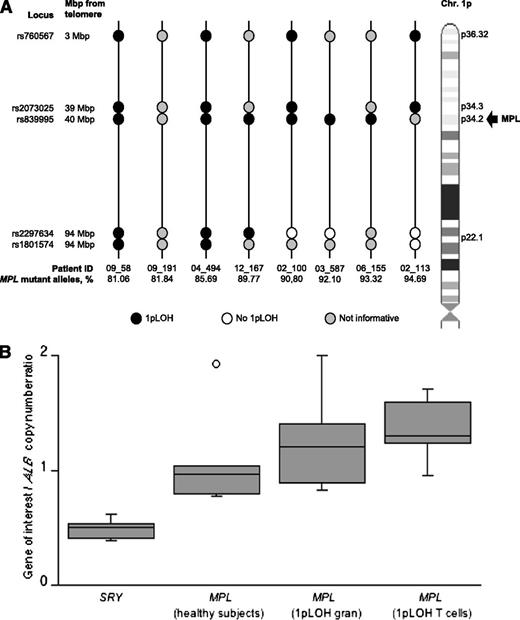
To unequivocally demonstrate 1pLOH, we studied 8 patients with an MPL-mutant allele burden greater than 75% and adequate DNA supply. We developed HRM assays for the analysis of the two telomeric SNPs rs760567 and rs2073025 mapping on chromosomes 1p36.32 and 1p34.3, respectively, and the two centromeric loci rs2297634 and rs1801574 located at 1p22.1. In myeloproliferative neoplasms, granulocytes typically belong to the malignant clone and T lymphocytes normally do not.4 Thus, 1pLOH is defined as a condition in which a homozygous genotype is detected in granulocytes, and a heterozygous one is found in T lymphocytes. Detection of a heterozygous genotype in both granulocytes and T lymphocytes indicates the absence of LOH, but detection of a homozygous genotype in both cell types means that the patient is not informative. The results of our analysis of these polymorphic markers are shown in Figure 5: although one patient was not informative, various patterns of LOH of chromosome 1p34 and its telomeric sequences were observed in granulocytes from the remaining patients.
Acquired CN-LOH of chromosome 1p in patients with MPN and high MPL-mutant allele burden. (Upper panel, A) Analysis of the two telomeric SNPs rs760567 and rs2073025 mapping on chromosome 1p36.32 and 1p34.3, respectively; the two centromeric SNPs rs2297634 and rs1801574 located at 1p22.1; and the intronic SNP rs839995 in 8 MPN patients with an MPL mutation burden >75%. Black circles indicate chromosome 1pLOH in granulocytes as detected by the corresponding polymorphic marker, white circles indicate the absence of 1pLOH, and gray circles indicate noninformative loci. Vertical lines represent individual patients. This analysis showed 1pLOH, always involving the location of MPL in 1p34, in 7 of the 8 patients studied; the remaining patient (09_191, second patient from left) was not informative. With respect to chromosome 1p34, telomeric sequences were involved in 1pLOH in all informative patients, although centromeric sequences were involved in only a subset of patients. (Lower panel, B) Evaluation of number of copies of MPL in patients with 1pLOH. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box) and outliers (dots). No significant difference in MPL copy number was found between granulocytes (gran) and T lymphocytes from patients with 1pLOH (P = .54), as well as between patients and healthy participants (P = .12), although significant differences were found with respect to the SRY gene (P < .001).
Acquired CN-LOH of chromosome 1p in patients with MPN and high MPL-mutant allele burden. (Upper panel, A) Analysis of the two telomeric SNPs rs760567 and rs2073025 mapping on chromosome 1p36.32 and 1p34.3, respectively; the two centromeric SNPs rs2297634 and rs1801574 located at 1p22.1; and the intronic SNP rs839995 in 8 MPN patients with an MPL mutation burden >75%. Black circles indicate chromosome 1pLOH in granulocytes as detected by the corresponding polymorphic marker, white circles indicate the absence of 1pLOH, and gray circles indicate noninformative loci. Vertical lines represent individual patients. This analysis showed 1pLOH, always involving the location of MPL in 1p34, in 7 of the 8 patients studied; the remaining patient (09_191, second patient from left) was not informative. With respect to chromosome 1p34, telomeric sequences were involved in 1pLOH in all informative patients, although centromeric sequences were involved in only a subset of patients. (Lower panel, B) Evaluation of number of copies of MPL in patients with 1pLOH. Data are shown in a box plot depicting the lower and upper adjacent values (lowest and highest horizontal line, respectively), lower and upper quartile with median value (box) and outliers (dots). No significant difference in MPL copy number was found between granulocytes (gran) and T lymphocytes from patients with 1pLOH (P = .54), as well as between patients and healthy participants (P = .12), although significant differences were found with respect to the SRY gene (P < .001).
To evaluate whether 1pLOH resulted from deletion of the telomeric portion of one chromosome 1 or from mitotic recombination events, we used qPCR to determine the number of copies of MPL. Only one copy of DNA for the MPL locus would be expected in case of deletion, whereas two copies should be detected in case of mitotic recombination. To discriminate between these two possibilities, we determined the number of copies of MPL in granulocytes of patients with 1pLOH. Numbers of copies of MPL in healthy participants and in T cells from patients with 1pLOH were considered as two-copy DNA controls, whereas the number of copies of the haploid gene SRY on chromosome Y was used as a one-copy DNA control. As shown in Figure 5, no significant MPL copy number variation was found between granulocytes and T lymphocytes from patients with 1pLOH (P = .54), as well as between patients and healthy participants (P = .12), but significant differences were found with respect to the SRY gene (P < .001). Taken together, these results clearly indicate copy-neutral LOH (CN-LOH) of chromosome 1p in the patients studied.30
Discussion
Most of MPL mutations identified in this study affected the amino acid W515 (W515L/K/A/R), which belongs to the RWQFP juxtamembrane domain of the MPL receptor (ie, to a domain that prevents spontaneous activation of the receptor).31 We also found a somatic mutation affecting the transmembrane domain (S505N); this mutation, originally identified in hereditary thrombocytosis and later observed in sporadic MPN, may promote or facilitate receptor dimerization.9,16,17 Four patients had a second rare substitution (S505C, V501A, and Q516E), likely devoid of significant transforming activity.17
In agreement with previous observations,12 we found that compared with JAK2 (V617F), MPL mutation had little distinctive phenotypic and clinical effect. When comparing JAK2-mutated with MPL-mutated ET patients, the latter group was found to have lower hemoglobin levels, as expected.32 With respect to clinical outcome, MPL-mutated patients had lower incidence of thrombotic complications and lower risk of leukemic evolution compared with JAK2-mutated patients.
The most remarkable findings of this study are that the MPL-mutant allele burden is highly variable in MPL-mutated myeloproliferative neoplasms and that higher mutation burdens originate from acquired CN-LOH of chromosome 1p and are associated with marrow fibrosis.
Acquired CN-LOH represents a common molecular mechanism of disease in myeloid malignancies.30 A paradigmatic example is acquired CN-LOH of chromosome 9p, responsible for the transition from heterozygosity to homozygosity for the JAK2 (V617F) mutation and in turn for high JAK2 (V617F)–mutant allele burden.4,33 A recent study has shown recurrent acquisition of JAK2 (V617F) homozygosity in both PV and ET patients, with different homozygous subclones being found in individual patients.34 This is consistent with the notion that abnormal mitotic events occur frequently in hematopoietic cells from JAK2 (V617F)–mutated patients and give rise to multiple subtypes of acquired CN-LOH of chromosome 9p. The selective advantage of a dominant homozygous subclone, which might reflect additional genetic or epigenetic changes, would drive erythrocytosis and transition from ET to PV. Over time, a high mutation burden almost inevitably leads to secondary myelofibrosis, as we previously demonstrated.19,21
Acquired CN-LOH of chromosome 1p involving MPL was previously described in two patients with refractory anemia with ring sideroblasts associated with marked thrombocytosis carrying the MPL (W515L) mutation35 and in a patient with PMF with the same mutation.36 In addition, a few MPN patients with high MPL-mutant allele burden and/or evidence of homozygous MPL mutation have been described.9,11 However, the clinical effects of acquired CN-LOH of chromosome 1p and high MPL-mutant allele burden have not been investigated systematically in patients with MPN.
In the 62 MPL-mutated patients in this study, the granulocyte mutant allele burden ranged from 1% to 95% and was significantly higher in patients with PMF or post-ET myelofibrosis than in those with ET, confirming previous findings by Jones et al.37 Unlike Jones et al, however, we did not find any significant difference in mutation load between patients carrying MPL (W515K) and those with MPL (W515L). Taking into account statistical variability, a mutant allele burden greater than 50% would by definition indicate the existence of at least a subclone of cells that are homozygous for the mutation. In myeloid malignancies, this subclone is typically a result of mitotic events that generate acquired CN-LOH.30 To demonstrate this, we studied 8 patients with a mutant allele burden greater than 75%, unambiguously indicating the existence of a dominant homozygous clone. In all the informative patients, we indeed detected acquired CN-LOH of chromosome 1p, consistently involving the location of MPL on chromosome 1p34 (Figure 5).
The association between high MPL-mutant allele burden and marrow fibrosis was supported by different univariate and multivariate analyses. In particular, when we did a multivariate logistic regression analysis of patients with ET or post-ET myelofibrosis considered as a phenotypic continuum, the MPL-mutant allele burden was found to be the most significant risk factor for marrow fibrosis (Table 3). Our observation that MPL-mutated ET patients are more likely to directly progress to post-ET myelofibrosis than JAK2 (V617F)–mutated ET patients has a coherent pathophysiological explanation. In fact, acquired CN-LOH of chromosome 9p in a JAK2 (V617F)–mutated ET patient is expected to first determine a transition from ET to PV34 and later on, progression to post-PV myelofibrosis.21
Although the relationship between high MPL-mutant allele burden and marrow fibrosis is strong, the underlying causal mechanisms remain to be defined. The thrombopoietin receptor is expressed not only in megakaryocytes but also in hematopoietic stem cells and plays a crucial role in maintaining the quiescence of the latter.38 In PMF, MPL (W515L/K) mutations occur in multipotent hematopoietic stem cells and induce spontaneous megakaryocyte differentiation.39 In a mouse model, the expression of the MPL (W515A) mutation induced a myeloproliferative phenotype with severe marrow fibrosis.40 A number of studies have suggested that the abnormal megakaryocytes of patients with MPN release profibrotic growth factors in the bone marrow,41 and more recently, abnormal proplatelet formation has been described in MPN patients with marrow fibrosis.42 Thus, if somatic mutations of MPL involving the W515 residue are relevant to the abnormal proliferation and differentiation of megakaryocytes, these abnormalities are expected to be more severe in clonal cells that are homozygous compared with those that are heterozygous for the mutation.
In conclusion, acquired CN-LOH of chromosome 1p, involving location of MPL, likely represents a mechanism of disease progression in MPL-mutated MPN, since it generates a subclone that is homozygous for the MPL mutation and expands, leading to a high mutant allele burden. This mechanism may specifically play a role in the progression from MPL-mutated ET to post-ET myelofibrosis. With increasing adoption of deep sequencing in diagnostic laboratories, assessing the MPL-mutant allele burden may become part of the diagnostic work-up of MPN, allowing clinicians to identify ET patients at high risk of fibrotic transformation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (Milan, Italy), Special Program Molecular Clinical Oncology 5x1000, to Associazione Italiana per la Ricerca sul Cancro-Gruppo Italiano Malattie Mieloproliferative, project number 1005; by grant number RBAP11CZLK from “Fondo per gli investimenti della ricerca di base” (A.V. and M.C.); by grant number 2010NYKNS7 from “Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale” (A.V. and M.C.); by grant number GR‐2010‐2312855 from the Italian Ministry of Health and a grant award from the Italian Society of Experimental Hematology (E.R.); and by a grant from Regione Toscana, “Programma per la ricerca regionale in materia di salute 2009” (P.G.). A detailed description of the “Associazione Italiana per la Ricerca sul Cancro-Gruppo Italiano Malattie Mieloproliferative” project is available at http://www.progettoagimm.it.
Authorship
Contribution: E.R., D.P., and M.C. conceived this study, collected and analyzed data, and wrote the manuscript; P.G., I.C., E.S., C.A., F.P., and A.V. collected clinical data; R.B., M.S., A.Pi., and G.D.B. performed deep sequencing investigations; D.P. and C.M. performed HRM, LOH, and MPL copy number analyses; A.Pa. and G.R. did PCR analyses; V.F., E.F., and C.P. did statistical analyses; E.B. studied bone marrow fibrosis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisa Rumi, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: elisarumi@hotmail.com; and Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.
References
Author notes
E.R. and D.P. contributed equally to this study.