Key Points
Leukocyte recruitment is ontogentically regulated during fetal life.
A new intravital imaging model of leukocyte recruitment has been established in the mouse.
Abstract
In adult mammals, leukocyte recruitment follows a well-defined cascade of adhesion events enabling leukocytes to leave the circulatory system and transmigrate into tissue. Currently, it is unclear whether leukocyte recruitment proceeds in a similar fashion during fetal development. Considering the fact that the incidence of neonatal sepsis increases dramatically with decreasing gestational age in humans, we hypothesized that leukocyte recruitment may be acquired only late during fetal ontogeny. To test this, we developed a fetal intravital microscopy model in pregnant mice and, using LysEGFP (neutrophil reporter) mice, investigated leukocyte recruitment during fetal development. We show that fetal blood neutrophils acquire the ability to roll and adhere on inflamed yolk sac vessels during late fetal development, whereas at earlier embryonic stages (before day E15), rolling and adhesion were essentially absent. Accordingly, flow chamber experiments showed that fetal EGFP+ blood cells underwent efficient adhesion only when they were harvested on or after E15. Fluorescence-activated cell sorter analysis on EGFP+ fetal blood cells revealed that surface expression of CXCR2 and less pronounced P-selectin glycoprotein ligand-1 (PSGL-1) begin to increase only late in fetal life. Taken together, our findings demonstrate that inflammation-induced leukocyte recruitment is ontogenetically regulated and enables efficient neutrophil trafficking only during late fetal life.
Introduction
In recent years, genetic manipulation of mice and advances in bio-imaging tools have markedly expanded our understanding of how different subsets of leukocytes navigate throughout the body to exert their biological functions.1 One of the crucial steps during navigation involves the recruitment of circulating leukocytes from the intravascular compartment into tissues. This process follows a multistep adhesion cascade that consists of tightly regulated adhesion and signaling events, leading to preferential recruitment of specific leukocyte subsets that are needed in the extravascular compartment. In general, recruitment into tissue begins with leukocyte tethering to and rolling along the endothelium. Both tethering and rolling are in most cases mediated by selectins, which bind to fucosylated and sialylated glycans that are presented on glycoproteins and glycolipids.2 During rolling, leukocytes engage in intimate contact with the endothelial surface and thus are afforded sufficient time to screen the luminal surface for activation signals, such as chemokines and other chemoattractants that interact with their cognate G-protein–coupled receptor(s) on the leukocyte surface. Together with signaling events transduced by selectin-selectin ligand interactions,3,4 chemokine receptor triggering leads to the activation of leukocyte-expressed integrins, enabling firm leukocyte arrest on and transmigration through the endothelium.1,5,6 Genetic defects in either the selectin or integrin-dependent adhesion steps result in leukocyte adhesion deficiency (LAD) syndromes, which predispose affected individuals to severe recurrent bacterial and fungal infections.1,2
Although the molecular mechanisms of leukocyte recruitment into inflamed tissues are well defined in adults, the requisite steps for leukocyte trafficking in the fetus are still unknown.7 This is of relevance because epidemiologic data show that the risk of severe sepsis in neonates increases dramatically with decreasing gestational age. In fact, close to 60% of extremely premature infants suffer from bacterial sepsis, compared with <2% in late preterm and term neonates.8 Thus, we set out to test the hypothesis that the reduced immune response in premature infants is, at least in part, a consequence of the inability of leukocytes to extravasate into inflamed tissue. Considering the increasing number of prematurely delivered infants in recent years8 and the high mortality rate of neonatal sepsis in this population, studies investigating leukocyte recruitment during fetal development are warranted and could provide valuable pathophysiological insights into the regulation and maturation of the innate immune system during fetal life.
Previous studies of fetal leukocyte recruitment were mostly conducted either in nonmammalian organisms (zebrafish and chicken9,10 ) or performed under in vitro conditions using human leukocytes isolated from cord blood.7,11-14 Several of these earlier studies have suggested that leukocyte recruitment may be impaired during fetal life. For example, investigations of adhesion molecule expression on umbilical cord blood neutrophils from neonates and preterm infants revealed a reduced expression of the β2 integrin Mac-1 and of l-selectin.15,16 In addition, Lorant et al17 found a reduced expression of P-selectin in endothelial cells of neonatal rats and human premature infants. These findings are complemented by reports that posttranslational glycosylation of selectin ligands, a prerequisite for binding of selectin ligands to selectins, changes during leukocyte maturation.18
It is currently unclear whether and how the functional characteristics of fetal neutrophils or endothelial cells translate into a biologically relevant impairment of leukocyte recruitment during infection. Indeed, to date, the intravascular behavior of fetal mammalian leukocytes has not been directly observed in situ. To address this question, we developed a new intravital microscopy (IVM) model to define the dynamics and molecular mechanisms of leukocyte recruitment during murine fetal life. Using this model, we found that circulating leukocytes acquire the ability to roll and adhere only relatively late in fetal ontogeny. Similar results have been obtained in the human system as very recently reported by Nussbaum et al.19 Additional experiments exploring the molecular mechanisms of this observation revealed reduced expression of P-selectin glycoprotein ligand-1 (PSGL-1) and CXCR2 on fetal neutrophils and changes in the composition of systemic white blood cells as the main contributing factors.
Methods
Surgical preparation of the yolk sac and fetus
For mice and antibodies used in this study, please refer to the supplemental Methods on the Blood website. All animal experiments were approved by the local Animal Care and Use Committee. Pregnant mice were anesthetized intraperitoneally with ketamine (5 mg/mL) and xylazine (1 mg/mL) in 10 mL/kg of normal saline. A PE-10 polyethylene catheter was inserted into the carotid artery for injection of reagents. To expose one fetus, the uterine horn was carefully exteriorized through an abdominal wall incision, and the uterus was opened with a short, semicircular incision in the uterine wall, opposite the site of placenta attachment (Figure 1). The intact yolk sac (YS), with the fetus inside, was further exteriorized through a uterine incision. The fetus was allowed to rest on the stage of the platform within a Petri dish (35 × 10 mm) containing prewarmed (37°C) sterile saline or Ringer solution. A 24 × 50-mm glass coverslip was placed over the preparation, and additional solution was added as needed to ensure complete submersion of the fetal tissues under the coverslip. Up to 3 neighboring fetuses could be exposed and filmed consecutively. In some experiments, exteriorized fetuses were superfused with a 1 or 10 µM N-formyl-methionyl-leucyl-phenylalanine (fMLP) superfusion buffer.
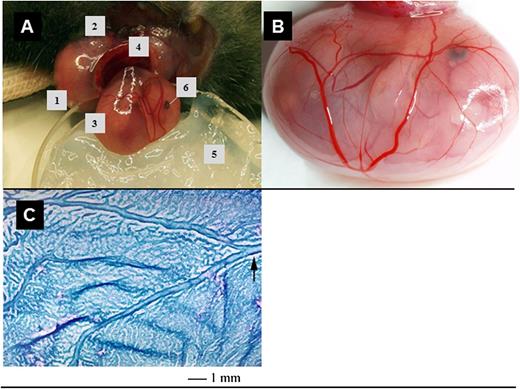
Surgical preparation and exposure of fetal tissues for IVM. (A) An anesthetized pregnant mouse is placed on a plexiglass stage, the right uterine horn (1) exteriorized after lateral incision (2) and opened to expose one fetus (3) contained within its intact yolk sac and connected to the placenta (4). The fetus is then placed in a vacuum grease–containing Petri dish (5) and superfused with superfusion buffer. Fetal eye (6). (B) To visualize yolk sac microvessels, a coverslip (not shown) is placed over the exposed yolk sac. (C) A whole mount preparation of a wildtype YS (E14), fixed and stained with modified Giemsa reagent, shows the extensive network of developing blood vessels that are accessible for IVM and the villous nature of the tissue. The arrow points out one of the larger vitelline vessels.
Surgical preparation and exposure of fetal tissues for IVM. (A) An anesthetized pregnant mouse is placed on a plexiglass stage, the right uterine horn (1) exteriorized after lateral incision (2) and opened to expose one fetus (3) contained within its intact yolk sac and connected to the placenta (4). The fetus is then placed in a vacuum grease–containing Petri dish (5) and superfused with superfusion buffer. Fetal eye (6). (B) To visualize yolk sac microvessels, a coverslip (not shown) is placed over the exposed yolk sac. (C) A whole mount preparation of a wildtype YS (E14), fixed and stained with modified Giemsa reagent, shows the extensive network of developing blood vessels that are accessible for IVM and the villous nature of the tissue. The arrow points out one of the larger vitelline vessels.
The study duration ranged from 1 to 2 hours, depending on the type of experiment performed. Fetal stage was confirmed immediately after filming by analysis of anatomical features and size.20
Intravital microscopy
Animals were transferred to an intravital microscope (La Vision Biotech, Braunschweig, Germany, and Olympus BX51, Hamburg, Germany) equipped with a ×20/NA0.95 water-immersion objective (Olympus), as described in the supplemental Methods and by Frommhold et al.6 Rolling of acridine orange (AO)- or EGFP-labeled cells observed during IVM was assessed as rolling flux (rolling cells per minute) and rolling flux fraction (flux per total number of cells passing the vessel within 1 minute).21 Adhesion of AO- or EGFP-labeled cells was quantified as the number of (>30 seconds) adherent cells per square millimeter of vascular surface area.21
Adhesion of fetal LysEGFP+ cells in the flow chamber
To investigate the ability of fetal EGFP+ cells to interact with defined adhesion molecules, we performed flow chamber assays using rectangular microflow chambers (20 × 200-µm cross section; Vitro Tubes) coated with either recombinant murine (rm)P-selectin (2 µg/mL), rm intercellular adhesion molecule (ICAM)-1 (1 µg/mL; both R&D), and rmCXCL1 (5 µg/mL; Peprotech, Hamburg, Germany) or rmE-selectin (2 µg/mL; R&D), rmICAM-1, and rmCXCL1. This combination of adhesion molecules has been demonstrated to mediate firm adhesion of neutrophils perfused through the flow chamber.6 Fetal blood was collected in Petri dishes after decapitation of LysEGFP+ fetuses. After centrifugation at 1000 rpm for 7 minutes, the leukocyte pellet was resuspended in phosphate-buffered saline, and the number of EGFP+ cells was counted. After isolation of blood cells from E14 to E18 embryos, LysEGFP newborn (<24 hours old) mice, and LysEGFP adult mice, EGFP+ cells were adjusted to 100 000 cells/mL in phosphate-buffered saline containing 1 mmol/L Ca2+ and 1 mmol/L Mg2+ and perfused through the flow chamber at 1 dyne/cm2 using a high precision perfusion pump (Harvard Apparatus).
Fetal cell counts and differentiation, immunoperoxidase staining (IPS), and whole mount yolk sac preparation
Fetal blood cells were obtained by incising the umbilical cord at the abdominal wall, with subsequent exsanguination into ice-cold medium. Cyto-centrifuge preparations of cells were fixed in acetone for 10 minutes (4°C) and air-dried. Slides were treated with 1% toluidine blue in methanol for 5 minutes or with modified Giemsa (1:20 dilution) for 60 minutes. For IPS, fetal tissue was snap frozen in optimal cutting temperature embedding medium, sectioned (5 µm), fixed in 4% paraformaldehyde, and immunostained with antibodies, following standard procedures.22 To investigate fMLP-induced neutrophil recruitment into yolk sac tissue, the yolk sac was fixed in 4% paraformaldehyde and stained with Giemsa, as described.5,6 For flow cytometric analysis and systemic counts of fetal blood cells, please refer to supplemental Methods.
Quantitative real-time reverse transcriptase-PCR
EGFP+ neutrophils were isolated from whole blood of E13 and E18 LysEGFP embryos, as well as from newborn and adult LysEGFP mice, as described above, and used for extraction of mRNA. Quantitative real-time polymerase chain reaction (PCR) was conducted as described.23 Further methodological details are provided in supplemental Methods.
Results
Microvascular anatomy of the yolk sac
Murine wildtype or LysEGFP fetuses at E13 to E18 were prepared for in situ imaging. A uterine horn was exteriorized from an anesthetized pregnant female, and the uterine wall was microsurgically dissected to reveal the highly vascularized, transparent YS (Figure 1A-B), while taking care not to puncture the YS or to induce bleeding from the placenta. For fetuses younger than E13, it was not possible to exteriorize the intact fetus from the uterine cavity to enable sufficient access for IVM. Fetuses older than E18 showed incipient YS involution and were not used for evaluation of leukocyte adhesion in YS vessels. The incised maternal uterine wall was retracted from the intact YS whereby the fetus inside the YS remained attached to the placenta via the umbilical cord and vitelline vessels. The fetus, placenta, and retracted uterine wall were submerged in an observation reservoir filled with warmed (37°C) buffer and covered with a glass coverslip. The YS vasculature was visualized and investigated using epifluorescence microscopy. A stained (modified Giemsa) whole mount preparation of the vitelline vein and its draining network is depicted in Figure 1C.
The anatomic description of vessels is based not only on function, but also on structural characteristics in adult mice, such as the presence of smooth muscle surrounding vessels. Because we were not able to detect these surrounding structures readily in our system, we categorized YS and also intrafetal vessels as afferent (arterial), bridging (capillary or sinusoid), or efferent (venous), based on size and the direction of blood flow within them. For example, afferent vessels channel blood flow into a larger number of smaller vessels, bridging vessels receive blood from afferent vessels and connect to other vessels of similar size, and efferent vessels discharge blood from smaller into larger vessels (venular or veinlike). Numerous loops were present, some of which developed anastomoses across the loop at its base, essentially reducing or eliminating flow through them. Therefore, it should be emphasized that in E13 to E18 fetuses, a clear separation into afferent or efferent vessel segments was not always possible.
Nucleated blood cells in the fetal circulation during development
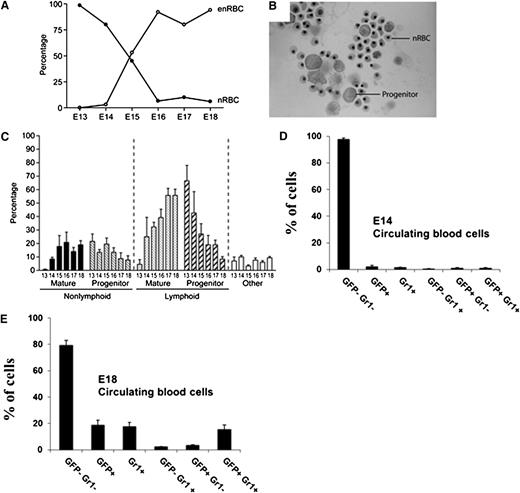
To determine the identity of fetal circulating leukocytes at different gestational times, we prepared cytospin preparations of fetal blood collected by exsanguination through the transected umbilical cord, as well as from newborn mice. By E18, <1% to 10% of all red blood cells in the periphery were nucleated (nRBCs), whereas virtually all RBCs contained nuclei on E13 (Figure 2A), consistent with earlier reports.24 At E18, terminally differentiated neutrophils constituted ∼20% of the circulating nucleated cell pool (white blood cells and nRBCs), and mononuclear cells comprised ∼66% (data not shown). The concentration of circulating white blood cells increased daily between E17 to birth, averaging 0.5 × 103/µL at E17, 1.0 × 103/µL at E18, and 1.5 × 103/µL at parturition (data not shown).
Phenotypic analysis of cell subsets in the circulation at different days of gestation. (A) The graph shows the frequency of nRBCs vs enucleated RBCs (enRBCs) between E13 and E18 (determined as percentage of total RBCs). (B) At E15, nRBCs and large mononuclear cells with features typical of progenitors are readily found on cytospin preparations stained with modified Giemsa. (C) The differential white blood cell count in fetal blood between E13 and E18 is shown. Cells included within the category “other” include primordial stem cells, monocytes, basophils, and eosinophils. (D-E) In addition, fetal LysEGFP+ and LysEGFP− blood leukocytes were investigated for their expression of the neutrophil marker Gr1 at (D) E14 and (E) E18.
Phenotypic analysis of cell subsets in the circulation at different days of gestation. (A) The graph shows the frequency of nRBCs vs enucleated RBCs (enRBCs) between E13 and E18 (determined as percentage of total RBCs). (B) At E15, nRBCs and large mononuclear cells with features typical of progenitors are readily found on cytospin preparations stained with modified Giemsa. (C) The differential white blood cell count in fetal blood between E13 and E18 is shown. Cells included within the category “other” include primordial stem cells, monocytes, basophils, and eosinophils. (D-E) In addition, fetal LysEGFP+ and LysEGFP− blood leukocytes were investigated for their expression of the neutrophil marker Gr1 at (D) E14 and (E) E18.
Interestingly, there was a steady decrease in the frequency of circulating progenitor cells after E13 (Figure 2B-C); this corresponded with a steady rise from E13 onward in the frequency of mature white blood cells, reaching a peak at parturition.
Next, we investigated circulating fetal blood cells from LysEGFP fetuses by fluorescence-activated cell sorter (FACS) and correlated the expression of EGFP with the neutrophil differentiation marker Gr-1. LysEGFP mice are known to express EGFP under the lysosome M promoter, leading to EGFP expression in monomyelocytic cells (primarily neutrophils, but also some monocytes).25 At E14, we observed only 1.9% EGFP+ cells and 1.4% Gr-1+ cells, whereas 1.0% of cells were double positive (Figure 2D). In contrast, the frequency of circulating EGFP+ cells, Gr-1+ cells, and EGFP+ Gr-1+ double positive cells rose steadily thereafter, reaching 19%, 17%, and 15%, respectively, of circulating fetal nucleated blood cells in E18 fetuses (Figure 2E).
Leukocyte rolling is ontogenetically regulated in the mouse fetus in vivo
To investigate the ability of fetal leukocytes to roll and adhere on inflamed fetal microvessels, we performed IVM on the exteriorized yolk sac of LysEGFP fetuses. Measurement of vascular dimensions and tracking of individual free-flowing blood cells allowed us to determine microvascular and hemodynamic parameters, which are given in Table 1. After exteriorization of the YS, we observed interactions of EGFP+ cells in YS vessels of E13 to E18 fetuses (Figure 3A). Rolling of EGFP+ cells was almost completely absent in E13 and E14 fetuses; however, from E15 to E18 fetuses, rolling flux continually rose (supplemental Movies 1 and 2). To investigate whether the increase in rolling flux between E15 and E18 is caused by changes in the systemic concentration of EGFP+ cells in the circulation, we also assessed the rolling flux fraction of EGFP+ cells by dividing the number of rolling EGFP+ cells by the number of all EGFP+ cells passing through a vessel during a given time interval. Similar to the increase in rolling flux in Figure 3A, the rolling flux fraction of EGFP+ cells also increased during fetal development (Figure 3B), indicating that the increase in rolling during fetal maturation is not merely a reflection of changes in systemic leukocyte counts, but likely caused, at least in part, by enhanced leukocyte and/or microvascular adhesiveness.
Microvascular parameters such as vessel diameter, centerline blood flow velocity, and wall shear rate in the yolk sac at different days of gestation
| Microvascular parameters . | Embryonic day (fluorophore) . | |||||
|---|---|---|---|---|---|---|
| 13 (GFP) . | 14 (GFP) . | 15 (GFP) . | 16 (AO + GFP) . | 17 (AO + GFP) . | 18 (GFP) . | |
| Number of vessels | 24 | 35 | 37 | 24 | 31 | 16 |
| Diameter (μm) | 56 ± 2 | 59 ± 2 | 53 ± 2 | 55 ± 3 | 48 ± 2 | 44 ± 3 |
| Vmean (μm/s) | 1100 ± 60 | 1000 ± 70 | 1000 ± 60 | 1200 ± 60 | 1100 ± 10 | 1000 ± 100 |
| γ (s−1) | 480 ± 30 | 420 ± 30 | 500 ± 30 | 570 ± 45 | 580 ± 55 | 590 ± 60 |
| Microvascular parameters . | Embryonic day (fluorophore) . | |||||
|---|---|---|---|---|---|---|
| 13 (GFP) . | 14 (GFP) . | 15 (GFP) . | 16 (AO + GFP) . | 17 (AO + GFP) . | 18 (GFP) . | |
| Number of vessels | 24 | 35 | 37 | 24 | 31 | 16 |
| Diameter (μm) | 56 ± 2 | 59 ± 2 | 53 ± 2 | 55 ± 3 | 48 ± 2 | 44 ± 3 |
| Vmean (μm/s) | 1100 ± 60 | 1000 ± 70 | 1000 ± 60 | 1200 ± 60 | 1100 ± 10 | 1000 ± 100 |
| γ (s−1) | 480 ± 30 | 420 ± 30 | 500 ± 30 | 570 ± 45 | 580 ± 55 | 590 ± 60 |
Leukocyte rolling and adhesion in inflamed yolk sac vessels during fetal development. (A) Rolling flux (number of rolling EGFP+ or AO+ cells per minute, mean ± standard error of the mean), (B) rolling flux fraction (rolling EGFP+ or AO+ cells to all EGFP+ or AO+ cells, respectively, passing the vessel per minute), and (C) adhesion (cells per square millimeter; mean ± standard error of the mean) of EGFP+ or AO+ blood cells in murine yolk sac vessels (n > 3 mice per group and n > 3 vessels per mouse). In addition, induction of EGFP+ (D) cell adhesion in yolk sac vessels and (E) extravasation into perivascular tissue are shown following local stimulation (superfusion) with the formyl peptide fMLP (n > 3 mice per group). Significant differences (P < .05) vs E18 (A-C) are indicated by the asterisk. anti-P, P-selectin blocking antibody.
Leukocyte rolling and adhesion in inflamed yolk sac vessels during fetal development. (A) Rolling flux (number of rolling EGFP+ or AO+ cells per minute, mean ± standard error of the mean), (B) rolling flux fraction (rolling EGFP+ or AO+ cells to all EGFP+ or AO+ cells, respectively, passing the vessel per minute), and (C) adhesion (cells per square millimeter; mean ± standard error of the mean) of EGFP+ or AO+ blood cells in murine yolk sac vessels (n > 3 mice per group and n > 3 vessels per mouse). In addition, induction of EGFP+ (D) cell adhesion in yolk sac vessels and (E) extravasation into perivascular tissue are shown following local stimulation (superfusion) with the formyl peptide fMLP (n > 3 mice per group). Significant differences (P < .05) vs E18 (A-C) are indicated by the asterisk. anti-P, P-selectin blocking antibody.
Several murine IVM models of trauma-induced inflammation in postnatal tissues, including the exteriorized cremaster muscle and the mesentery, have demonstrated that rolling induced by surgical tissue manipulation is primarily mediated by P-selectin.26 To test if trauma-induced rolling of EGFP+ cells in exteriorized YS is also mediated by P-selectin, we microinjected anti–P-selectin blocking mAb RB40.34 (1 µg) into the fetal circulation of E17 fetuses and assessed leukocyte rolling in exteriorized YS vessels. EGFP+ cell rolling was almost completely abrogated after injection of anti–P-selectin (Figure 3A), demonstrating that rolling in this setting is primarily dependent on P-selectin.
To assess whether EGFP− fetal blood cells contributed to rolling, we also performed IVM in C57BL/6 fetuses (E16 and E17) where fetal circulating blood cells were labeled with AO. This dye was injected intravenously into the maternal circulation and readily crossed the placenta into the fetal circulation where it accumulated in nucleated cells. As demonstrated in Figure 3A-B, rolling of AO-labeled fetal circulating cells during fetal development was comparable to rolling of EGFP+ cells, suggesting that rolling cells are mostly EGFP+ cells. Subsequently, we systemically injected AO into females carrying E18 LysEGFP fetuses and assessed rolling in exteriorized yolk sac vessels before and after injection of AO. There was no significant change in the number of rolling cells after AO injection (10 ± 3 cells/min before vs 9 ± 3 cells/min after AO injection, data not shown), demonstrating that EGFP− cells do not contribute to rolling in this setting.
Chronological acquisition of firm arrest by fetal leukocytes
Analysis of the number of adherent EGFP+ cells in LysEGFP fetuses was conducted in the same vessels that were used for quantification of rolling events. No sticking cells were detected in fetuses at E13 and E14 (Figure 3C), consistent with the absence of rolling behavior during this time of gestation (Figure 3A-C; supplemental Movies 1 and 2).
To test if EGFP− cells adhere on inflamed endothelium, we injected AO into the maternal circulation of wild-type mice. The number of adherent AO-labeled fetal circulating cells in surgically prepared vessels was comparable to the number of adherent EGFP+ cells, suggesting that EGFP+ cells are also the predominant cell type capable of arresting within inflamed microvessels in this setting (Figure 3C).
fMLP-induced adhesion of EGFP+ fetal blood cells
To investigate whether adhesion of EGFP+ circulating blood cells can be boosted by a proinflammatory stimulus mimicking bacterial infection, we superfused the yolk sac with a formyl peptide, fMLP (1 µM), over 15 minutes and recorded the number of adherent EGFP+ cells. Studies in adult mice have shown that local stimulation with fMLP induces strong ICAM-1– and LFA-1–dependent firm leukocyte arrest in venules.27,28 Thus, we assessed the number of adherent EGFP+ cells in YS vessels of E15, E17, and E18 fetuses before and after 15-minute fMLP superfusion. Microvascular parameters are given in supplemental Table 3 and showed no significant differences in vessel diameter, blood flow velocity, and wall shear rate before and after fMLP superfusion. Although we observed a trend toward an increase in the number of adherent EGFP+ cells during fMLP superfusion, we did not find any statistically significant changes in EGFP+ cell adhesion over time of perfusion at E15, E17, and E18 compared with the number of adherent EGFP+ cells at 0 minutes (Figure 3D). This suggests that fMLP-induced adhesion is compromised during early fetal development, which is in line with other studies reporting reduced responsiveness of neutrophils from premature infants following stimulation with fMLP, with respect to upregulation of CD11b or reactive oxygen species production.29,30
We next investigated perivascular extravasation of neutrophils in E15, E17, and E18 fetuses after 15-minute superfusion of the YS with 10 µM fMLP. For analysis of neutrophil extravasation, fMLP-treated YSs were fixed and stained with Giemsa stain. Whole mount YS preparations were then analyzed for perivascular neutrophils. Although we did not find extravasated neutrophils in untreated fetuses, exposure to 10 µM fMLP led to a significant increase in the number of extravasated cells in E17 and E18 fetuses (P < .05 vs control) but not in E15 fetuses (Figure 3E), indicating that the hyporesponsiveness of intravascular leukocytes to fMLP in E15 fetuses leads to attenuated extravasation of neutrophils in those fetuses.
Adhesion of fetal LysEGFP+ cells in the flow chamber
Next, we investigated the ability of isolated fetal EGFP+ cells to adhere to a set of immobilized adhesion-relevant molecules using a microflow chamber assay.6 Microflow chambers (20 × 200 µm, cross section) were coated with either rmP-selectin, rmICAM-1, and rmCXCL1 or rmE-selectin, rmICAM-1, and rmCXCL1, a neutrophil-specific chemokine demonstrated to be up-regulated during fetal inflammation.31 EGFP+ blood cells isolated from E14 to E18 LysEGFP embryos, LysEGFP newborn mice, and LysEGFP adult mice were adjusted to 1 × 105 cells/mL and perfused through the flow chamber at 1 dyne/cm2. Similar to data obtained with in vivo experiments, we found only a few adherent EGFP+ cells from E13/E14 fetuses in chambers coated with different combinations of trafficking-related molecules (Figure 4A-B). For later time points, the number of adherent EGFP+ cells steadily increased in both groups but did not reach levels observed with blood cells from newborns or adults (Figure 4A-B). Taking into account the significant increase of EGFP+ cells in the fetal circulation during ontogeny (Figure 2D-E), the results from the flow chamber experiments not only confirm our in vivo observations, but also demonstrate that even at the same concentration of EGFP+ cells, adhesion of those cells depends on the fetal developmental state. Adhesion of adult neutrophils in flow chambers coated with rmP-selectin, rmE-selectin, or buffer alone did not lead to any significant adhesion (Figure 4C).
Microflow chamber experiments. Isolated EGFP+ cells from E13 to E18 fetuses and microflow chambers (20 × 200-µm rectangular capillaries) coated with either (A) rmP-selectin, rmICAM-1, and rmCXCL1 or with (B) rmE-selectin, rmICAM-1, and rmCXCL1 were used to observe adhesion of EGFP+ cells (adherent cells per field of view [FOV], mean ± standard error of the mean from n > 3 mice per group) in the flow chamber at a wall shear stress of 1 dyne/cm2. (C) Adhesion of adult neutrophils in flow chambers coated with P-selectin or E-selectin or buffer alone. Significant differences (P < .05) in rolling to E18 are indicated by the asterisk.
Microflow chamber experiments. Isolated EGFP+ cells from E13 to E18 fetuses and microflow chambers (20 × 200-µm rectangular capillaries) coated with either (A) rmP-selectin, rmICAM-1, and rmCXCL1 or with (B) rmE-selectin, rmICAM-1, and rmCXCL1 were used to observe adhesion of EGFP+ cells (adherent cells per field of view [FOV], mean ± standard error of the mean from n > 3 mice per group) in the flow chamber at a wall shear stress of 1 dyne/cm2. (C) Adhesion of adult neutrophils in flow chambers coated with P-selectin or E-selectin or buffer alone. Significant differences (P < .05) in rolling to E18 are indicated by the asterisk.
Chronological acquisition of adhesion molecule expression by YS endothelium
To determine whether the poor adhesiveness of leukocytes at early stages of gestation was because of endothelial hypoadhesiveness, we performed IPS to examine YS vessels at E11, −14, −15, −17, and −18 (Table 2). Within the limit of detection achievable by this method, no selectin/selectin ligands, other than CD34, were detectable on YS vessels at any stage of development. CD34 is a sulfated sialomucin that can present glycan ligands for CD62L/l-selectin in high endothelial venules of lymph nodes, but it is unclear whether it can support hematopoietic progenitor cell adhesion in adult nonlymphoid organs or fetal microvascular beds.32 ICAM-1 and ICAM-2 expression became apparent with advanced gestational age, beginning at approximately E14 on a subset of vessels, whereas CD34 and CD31/platelet endothelial cell adhesion molecule were present on YS endothelium at all gestational periods examined. The integrin CD29/β1 was also found on YS vessels at all time points tested (E11-E18), whereas mucosal addressin cell adhesion molecule (MAdCAM-1), a member of the immunoglobulin superfamily that binds α4β7+ leukocytes in intestinal microvessels,33 and vascular cell adhesion molecule-1 (interacting with α4β1 integrin) were not detectable in the YS (Table 2). We also examined by IPS and postintravital filming, tissues whose vessels supported rolling, but we could not detect expression of vascular cell adhesion molecule-1 nor P-selectin (using either polyclonal or monoclonal antibodies for P-selectin, data not shown). This might reflect insufficient surface density of P-selectin for detection by IPS, similar to reports by others who were unable to detect surface expressed P-selectin on human umbilical vein endothelial cells, although human umbilical vein endothelial cell monolayers supported P-selectin–dependent rolling in flow chambers.34
Immunohistological analysis of adhesion-related molecules in yolk sac vessels from C57Bl/6 mice at different days of gestation
| Adhesion molecules . | E11 . | E14/15 . | E17/18 . |
|---|---|---|---|
| Selectins/ selectin ligands | |||
| MAdCAM | ND | ND | ND |
| PNAd | ND | ND | ND |
| E-selectin | ND | ND | ND |
| P-selectin | ND | ND | ND |
| L-Selectin | ND | ND | ND |
| PSGL-1 | ND | ND | ND |
| CD34 | All vessels | All vessels | All vessels |
| Integrins and integrin ligands | |||
| β7 integrin | ND | ND | ND |
| β1 integrin (CD29) | All vessels | All vessels | All vessels |
| VCAM-1 (CD106) | ND | ND | ND |
| ICAM-1 (CD54) | ND | ± | All vessels |
| ICAM-2 (CD102) | ND | Some vessels | Some vessels |
| PECAM-1 (CD31) | All vessels | All vessels | All vessels |
| Adhesion molecules . | E11 . | E14/15 . | E17/18 . |
|---|---|---|---|
| Selectins/ selectin ligands | |||
| MAdCAM | ND | ND | ND |
| PNAd | ND | ND | ND |
| E-selectin | ND | ND | ND |
| P-selectin | ND | ND | ND |
| L-Selectin | ND | ND | ND |
| PSGL-1 | ND | ND | ND |
| CD34 | All vessels | All vessels | All vessels |
| Integrins and integrin ligands | |||
| β7 integrin | ND | ND | ND |
| β1 integrin (CD29) | All vessels | All vessels | All vessels |
| VCAM-1 (CD106) | ND | ND | ND |
| ICAM-1 (CD54) | ND | ± | All vessels |
| ICAM-2 (CD102) | ND | Some vessels | Some vessels |
| PECAM-1 (CD31) | All vessels | All vessels | All vessels |
Tissue sections from frozen yolk sacs were immunostained via the immunoperoxidase method, as previously described.21 Vessels were sized as small, moderate, or large in size, depending on diameter width: small, <25 μm; moderate, 25 to 75 μm; large, >75 μm. ND, not detectable on endothelium; PECAM-1, platelet endothelial cell adhesion molecule-1; VCAM, vascular cell adhesion molecule; ±, weak expression detected on a subset of vessel; PNAd, Peripheral node addressin.
Expression of adhesion molecules on fetal circulating Gr-1+ cells
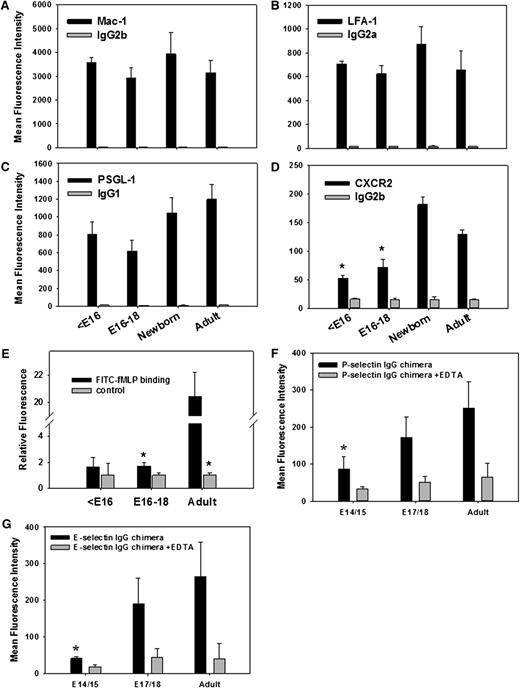
We also investigated the expression of adhesion molecules on Gr-1+ blood cells isolated from E13 to E18 fetuses, newborns, and adult mice. In contrast to reports of an up-regulation of the β2 integrin Mac-1 on myeloid cells during human fetal development and postnatally, Mac-1 expression on murine fetal Gr-1+ cells was similar at all time points tested (Figure 5A; supplemental Figure 1A). Similarly, LFA-1 expression on fetal Gr-1+ cells did not change during fetal development (Figure 5B; supplemental Figure 1B). For PSGL-1, the most prominent selectin ligand during inflammation, we found a trend toward lower expression on Gr-1+ cells early during fetal development with a moderate increased at later time points. However, this did not reach statistical significance (Figure 5C; supplemental Figure 1C). In contrast, expression of CXCR2, a chemokine receptor that contributes to neutrophil recruitment during inflammation in adults, was very low on Gr-1+ cells in <E16 fetuses, but increased considerably late in gestation (Figure 5D; supplemental Figure 1D). These findings imply that leukocyte recruitment during fetal life may be compromised, at least in part, because of low surface expression of CXCR2 and possibly PSGL-1.19
FACS analysis of adhesion relevant molecules on Gr-1+ blood cells isolated from E13 to E18 fetuses, newborn, and adult mice. Expression of the surface molecules (A) Mac-1, (B) LFA-1, (C) PSGL-1, and (D) CXCR2 including appropriate isotype controls are shown as mean fluorescence intensity values (mean ± standard error of the mean) from ≥3 experiments per group. (E) In addition, FITC-labeled fMLP binding to Gr-1+ fetal and adult cells was investigated in the absence or presence of excess (500-fold) unlabeled fMLP. Finally, (F) P-selectin and (G) E-selectin IgG chimeric protein binding (±EDTA) to Gr-1+ blood cells is shown (mean fluorescence intensity values, mean ± standard error of the mean from n ≥ 3 mice per group). Significant differences (P < .05) vs adult values are indicated by the asterisk.
FACS analysis of adhesion relevant molecules on Gr-1+ blood cells isolated from E13 to E18 fetuses, newborn, and adult mice. Expression of the surface molecules (A) Mac-1, (B) LFA-1, (C) PSGL-1, and (D) CXCR2 including appropriate isotype controls are shown as mean fluorescence intensity values (mean ± standard error of the mean) from ≥3 experiments per group. (E) In addition, FITC-labeled fMLP binding to Gr-1+ fetal and adult cells was investigated in the absence or presence of excess (500-fold) unlabeled fMLP. Finally, (F) P-selectin and (G) E-selectin IgG chimeric protein binding (±EDTA) to Gr-1+ blood cells is shown (mean fluorescence intensity values, mean ± standard error of the mean from n ≥ 3 mice per group). Significant differences (P < .05) vs adult values are indicated by the asterisk.
Subsequently, we assessed binding of fluorescein isothiocyanate (FITC)-labeled fMLP to fetal and adult Gr-1+ blood cells in the absence or presence of excess (500-fold) unlabeled fMLP. Although we found a reduction in FITC-fMLP binding to adult and to E16 to E18 fetal Gr-1+ cells in the presence of unlabeled fMLP compared with FITC-fMLP alone, excess unlabeled fMLP did not change binding of FITC-fMLP to Gr-1 high cells of <E16 fetuses (Figure 5E), suggesting that fMLP binding to its formyl peptide receptors is strongly reduced on Gr-1 high blood cells on and before E16.
To further characterize the ability of fetal Gr-1+ blood cells to bind to selectins, we analyzed binding of P- and E-selectin Fc chimeric proteins to Gr-1+ cells of E14/E15 fetuses, E17/E18 fetuses, and adult mice by FACS analysis. Similar to the in vivo results, we found markedly reduced binding of both selectins to fetal Gr-1+ blood cells compared with adult neutrophils, further confirming that selectin ligand activity is significantly reduced during fetal life (Figure 5F-G).
Because Gr-1+ cells also include some monocytes, we conducted a separate series of FACS experiments using the neutrophil-specific epitope Ly6G. As shown in supplemental Figure 2, the pattern of adhesion molecule expression on Ly6G+ cells of <E16 fetuses, E16 to E18 fetuses, and adults showed a similar pattern to that found for CXCR-2 and Mac-1 on Gr-1–positive cells, respectively. In contrast, no changes could be observed for the expression of PSGL-1 between Ly6G+ cells from <E16, E16 to E18, and adults. Furthermore, LFA-1 expression was greatest in <E16 fetuses (supplemental Figure 2).
mRNA levels of neutrophil traffic molecules
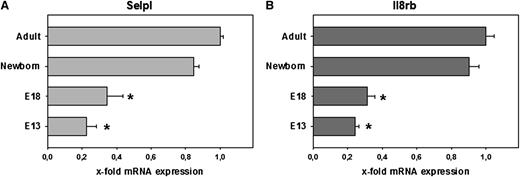
EGFP+ blood cells were isolated from E13 and E18 LysEGFP embryos and from newborn and adult LysEGFP mice. Quantitative real-time PCR was performed to measure mRNA levels of adhesion relevant gene products. Consistent with our flow cytometry results, Selpl (PSGL-1) expression doubled from E13 to E18 (Figure 6A). Interestingly, mRNA levels for PSGL-1 in EGFP+ cells continued to increase postnatally, reaching peak levels only in adulthood. Also, in line with our FACS data, the mRNA expression of Il8rb (CXCR2) was low early during development (E13) and increased significantly at later time points (E18; Figure 6B). At E13 and E18, the expression of Il8rb was about 14% and 75% of that in adult mice, respectively (Figure 6B).
Quantitative real-time reverse transcription-PCR of adhesion relevant genes. Differential expression of (A) Selpl encoding for PSGL-1 and (B) Il8rb encoding for CXCR2 in neutrophils obtained from E13 fetuses, E18 fetuses, newborn mice, and adult mice. Real-time PCR was performed using neutrophil cDNA reversely transcribed from mRNA with the help of primers specific for adhesion relevant genes as indicated. Gyc and B2M (housekeeping genes) were used to create ΔCT values of adhesion relevant genes tested. Gene expression in adult mice is set to 1 (2−ΔΔCT method), and x-fold expression is shown for all other time points. Values represent mean ± standard error of the mean from ≥3 independent experiments per group. Significant differences to adult values are indicated by the asterisk.
Quantitative real-time reverse transcription-PCR of adhesion relevant genes. Differential expression of (A) Selpl encoding for PSGL-1 and (B) Il8rb encoding for CXCR2 in neutrophils obtained from E13 fetuses, E18 fetuses, newborn mice, and adult mice. Real-time PCR was performed using neutrophil cDNA reversely transcribed from mRNA with the help of primers specific for adhesion relevant genes as indicated. Gyc and B2M (housekeeping genes) were used to create ΔCT values of adhesion relevant genes tested. Gene expression in adult mice is set to 1 (2−ΔΔCT method), and x-fold expression is shown for all other time points. Values represent mean ± standard error of the mean from ≥3 independent experiments per group. Significant differences to adult values are indicated by the asterisk.
Discussion
We described a new fetal IVM model in the mouse that allows us to investigate the trafficking of fetal blood cells during mouse ontogeny. We found a dramatic disability of fetal circulating leukocytes to interact with inflamed fetal endothelium, and we showed that leukocyte rolling and adhesion occur only at embryonic day 15 or later. Similar results are reported for the human system by Nussbaum et al.19 Together, these findings support the hypothesis that the dramatic increase in the incidence of severe sepsis observed in very premature infants8 may be related to the inability of circulating leukocytes to extravasate into infected tissue and fight invading microorganisms. Several studies in LAD mice or patients with LAD types I-III, which mechanistically mirror the situation of severely compromised leukocyte adhesion during early fetal life, also report a high incidence of severe infections.35
Many different IVM models have been used to study the microcirculation in adult tissues, but to our knowledge, this is the first real-time IVM model to study prenatal leukocyte adhesion dynamics at the single-cell level in mice. Our aim was to investigate the developmental regulation of fetal circulating cell interactions with the endothelium and to determine whether molecules used in adult mice for leukocyte-endothelial cell interactions are regulated in a similar manner in utero.
The fetal model differs from adult models in several ways, including the type of microvascular beds that can be observed and the day-to-day variability in circulating cell phenotypes. This model permits a rigorous analysis of the fetal YS microvasculature, including the onset and progressive increase in frequency of rolling and adhesion events starting as early as E15. Rolling fluxes in the circulation increased chronologically as did the frequency of nonerythroid, terminally differentiated cells such as neutrophils, which are critical for functional innate immune defenses. Corroborative evidence for the acquisition of rolling events during fetal development comes also from an experimental model in the developing chick.10 In this model, leukocyte-endothelial interactions in intestinal venules were reduced in the fetus compared with postnatal life.10 Our flow chamber experiments using isolated fetal EGFP+ blood cells from E14 to E18 LysEGFP embryos also demonstrated a developmentally regulated acquisition of rolling and adhesion, consistent with the idea that differentiated neutrophils released into the fetal circulation start to roll and adhere late in fetal ontogeny.
As neutrophil rolling in mice is heavily dependent on posttranslationally modified PSGL-1,26,36 the rise in the expression of PSGL-1 on Gr-1+ cells and concomitant selectin binding activity on EGFP+ cells with advancing gestation may explain, at least in part, why EGFP+ cells only roll at later time points during fetal life. Similar results for PSGL-1 have been found for human fetal neutrophils.19 On the other hand, our FACS analysis of PSGL-1 expression on Ly6G+ cells could not demonstrate significant changes in PSGL-1 expression during fetal ontogeny, which could indicate that selectin ligand activity might be regulated at the posttranslational level rather than at the expression level. In this context, it should also be pointed out that hematopoietic stem and progenitor cells (which are released into the blood circulation early during fetal development at select time points) in adults express abundant selectin ligands and readily home to adult bone marrow.37,38 However, we did not observe any rolling of EGFP− cells (including hematopoietic stem and progenitor cells) in the fetal circulation, suggesting that the inability of early fetal blood cells to roll and adhere rather reflects fetal immaturity than the state of hematopoietic cell differentiation.39
We also noted a severe decrease in fMLP-induced adhesion and extravasation of neutrophils in the inflamed YS prior to E18. This was accompanied by a marked impairment in fMLP binding to Gr-1high blood cells from <E16 fetuses compared with E16 to E18 fetuses and adults. Using Scatchard plot analysis, Nunoi et al40 also found reduced binding of fMLP to human fetal neutrophils, as well as reduced intracellular response after stimulating fetal neutrophils with fMLP, compared with adult neutrophils. This implies both a reduced expression of fMLP receptors and attenuated receptor-mediated signaling. Surprisingly, we also found a significant reduction in CXCR2 expression, a key chemokine receptor on neutrophils that triggers integrin-mediated firm arrest on activation in vivo and in vitro.41 Therefore, reduced expression of CXCR2 may be another factor contributing to reduced neutrophil adhesion in fetal ontogeny.
Taken together, our study provides strong evidence that maturation of neutrophils in the fetus underlies an intrinsic program regulating the gradual acquisition of adhesive functions of neutrophils during regular gestation and therefore providing a functional innate immune system after birth. The intrinsic nature of fetal neutrophil maturation is also strongly supported by analogous findings in the human system, which demonstrate that neutrophil function in human fetuses is strictly dependent on postconceptional age but is independent of postnatal age.19 Recent reports have described several mechanisms by which transcription of specific sets of genes can be regulated. Such mechanisms may be involved in controlling neutrophil maturation in the fetus. For example, changes in chromatin accessibility and nucleosome remodeling were shown to contribute to inducible gene transcription for a specific set of genes during an inflammatory response.42 Elucidating how maturation of neutrophil adhesive functions during fetal development is controlled on the transcriptional level might carry great potential for the development of new therapeutic strategies aiming to accelerate fetal neutrophil maturation in preterm infants. Applying this strategy may eventually lower the incidence and severity of neonatal sepsis. On the other hand, it must be considered that the relative inability of fetal neutrophils could also confer benefits during term pregnancies. For example, the absence of neutrophil-mediated inflammation and extravasation might reduce the likelihood that the maternal immune system is exposed to fetal alloantigens in an immunostimulatory context. Further work will be needed to address these questions in vivo.
This article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Dietmar Vestweber, Barry Wolitzky, and Timothy Springer for providing antibodies. Drs Jens Stein and Irina Mazo provided excellent technical and scientific advice. The authors also thank Brigitte Häussle, Susanne Bierschenk, and Simone Hofmann for invaluable help in the assessment of fetal differential counts, flow chamber assays, and quantitative real-time PCR.
This work was supported by the Pfizer Scholars Grant for New Faculty, awarded to E.J.Q., by the DFG Collaborative Research Center grant SFB 914, project B1, and the FöFoLe program of Ludwig Maximilians University, Munich, and by National Institutes of Health, National Institute of Allergy and Infectious Diseases grants AI069259, AI078897, and AI095261 to U.H.v.A. E.J.Q. was also supported by the Amy Potter Fellowship, kindly granted to the Center for Blood Research by Mrs Nancy Potter (Westin, MA).
Authorship
Contribution: M.S. and E.J.Q. designed the project, performed and analyzed experiments, and wrote the manuscript; J.A., N. Sushkova, C.N., S.S., M.P., A.K., A.M., A.S., N. Schweiger, I.B., N.K., D.F., and U.J. planned and performed experiments and analyzed data; L.B. provided invaluable reagents for the project; O.G.-B. provided valuable suggestions on the project and revised the manuscript; and U.H.v.A. designed and supervised the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.J.Q. is Agennix Inc., Princeton, NJ.
Correspondence: Markus Sperandio, Walter-Brendel-Center of Experimental Medicine, Ludwig-Maximilians-Universität, Marchioninistrasse 27, 81377 Munich, Germany; e-mail: markus.sperandio@med.uni-muenchen.de; and Ulrich H. von Andrian, Department of Microbiology and Immunobiology, Harvard Medical School, 77 Avenue Louis Pasteur, Boston, MA 02115; e-mail: uva@hms.harvard.edu.
References
Author notes
M.S. and E.J.Q. contributed equally to this work.


![Figure 4. Microflow chamber experiments. Isolated EGFP+ cells from E13 to E18 fetuses and microflow chambers (20 × 200-µm rectangular capillaries) coated with either (A) rmP-selectin, rmICAM-1, and rmCXCL1 or with (B) rmE-selectin, rmICAM-1, and rmCXCL1 were used to observe adhesion of EGFP+ cells (adherent cells per field of view [FOV], mean ± standard error of the mean from n > 3 mice per group) in the flow chamber at a wall shear stress of 1 dyne/cm2. (C) Adhesion of adult neutrophils in flow chambers coated with P-selectin or E-selectin or buffer alone. Significant differences (P < .05) in rolling to E18 are indicated by the asterisk.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/21/10.1182_blood-2012-07-447144/4/m_e118f4.jpeg?Expires=1767738328&Signature=kfGwtnkZBU5KyBqoZo2WDMvzh-DDtDZi2k4-izoHM3NVm7X6NiG8~YG27z~V4ToPaKcxkibdGsS5ez49ul9f1C7M-ydU9G~JHpvfDGWD3oHhSbKMPl1yImUeqcfyDd3cBKN8WY8T~eXBrfn5qvMG13CVzHFAToIlC5swx9vPOg1G4zbD2d99fILp-pn7HRAyw-MRveVN8jPNzDrKyTL~06B2zuQQd5ZOtIwmGQLPq5W~VxGmi8lNVAbIyvBIzp7iNfYQcHpLICz9Vv6SOtLfXbqb1-7zwe9Pr6Vu75Uv0-TBgKn3zEE6KvuSpVB0GPXJ3l834-tsp0JjXe4ISuKB3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal