Abstract
Thymic repopulation by transplanted hematopoietic progenitor cells (HPC) is likely to be important for long-term immune reconstitution and for successful gene therapy of diseases affecting the T-cell lineage. However, the T-cell progenitor potential of HPC, cultured in vitro for cell number expansion and gene transfer remains largely unknown. Here, we cultured highly purified human umbilical cord blood (CB) CD34+CD38− or CD34+CD38+ cells for up to 5 weeks in stroma-free cultures supplemented with various combinations of the cytokines thrombopoietin (TPO), stem cell factor (SCF), flt3/flk-2 ligand (FL), interleukin-3 (IL-3), and IL-6 and investigated thymus-repopulating ability of expanded cells in vitro and in vivo. After up to 5 weeks of culture in IL-3 + SCF + IL-6 or TPO + FL + SCF supplemented medium, the progeny of CD34+CD38− CB cells generated T cells and natural killer cells in the thymus. Limiting dilution experiments demonstrated increase in the number of T-cell progenitors during culture. After 3 weeks of culture, gene marked CD34+CD38− CB cells injected in the human thymus fragment transplanted in severe combined immunodeficient (SCID) mice (SCID-hu) generated thymocytes expressing the retroviral encoded marker gene GFP in vivo. Thus, our results show that the progeny of CD34+CD38− CB cells cultured for extensive periods, harbor thymus-repopulating cells that retain T-cell progenitor potential after expansion and gene transfer.
CLINICAL USE OF cord blood (CB) mononuclear cells in transplantation has indicated that successful engraftment depends on the number of nucleated cells injected per kg body mass.1,2 Given the limited number of nucleated cells that can be isolated from a CB donation, in vitro expansion of mononuclear cells and hematopoietic precursor cells (HPC) might overcome the current limitations for the use of CB for transplantation of larger children and adults.2 For retroviral gene transfer, cell cycle induction is beneficial, or even essential when using nonlentiviral vectors.3 Several investigators have reported extensive, growth factor-induced in vitro expansion of CB HPC. Early acting cytokines such as stem cell factor (SCF), thrombopoietin (TPO), flt3/flk-2 ligand (FL), interleukin-3 (IL-3), IL-6, erythropoietin (Epo), granulocyte colony-stimulating factor (G-CSF), and others were used to expand the number of CB cells over 1 or more weeks of culture.4-10 Besides total cell count, the number of colony-forming cells (CFC) and long-term culture-initiating cells (LTC-IC)5-8 also increased. Severe combined immunodeficient (SCID) mice repopulating cells (SRC), present at low frequency in the CD34+CD38−Lin− and the CD34−CD38−Lin−fraction of CB HPC,7,11,12 could be expanded modestly in short-term culture7,12 and in 1 report even extensively (up to 70-fold during 9 to 10 weeks of culture).9 Most of our knowledge of lymphoid precursor potential of expanded HPC is restricted to B-cell generation.7,13 Few data are available on T-cell precursor potential or the thymus-repopulating capacity of expanded HPC,4,10,14 as this cannot be assessed in suspension culture or in the nonobese diabetic (NOD)-SCID repopulation model.15 Thymic-dependent T-cell generation continues in adults16 and may contribute to immune reconstitution after HPC transplant.17,18 Gene therapy aimed at curing diseases affecting the T-cell lineage can only succeed if the genetically modified HPC can generate this lineage.17,19,20 We recently described an in vitro model that allows the study of thymic-dependent T-cell generation in fetal thymus organ culture (FTOC) from transduced CB HPC, cultured for 3 days in stroma-free suspension culture.21
Here, we show that highly purified CD34+Lin− umbilical CB cells harbor in both the CD38− and CD38+ fraction thymus-repopulating cells, which could generate T cells and natural killer (NK) cells in FTOC, even after several weeks of culture in cytokine-supplemented medium without stromal support. The number of T-cell progenitors present in the CD34+CD38−Lin− fraction increased during culture. After 3 weeks of culture, transduced CD34+CD38− cells could repopulate SCID-hu thymus, generating in vivo T cells, NK cells, and dendritic cells expressing the green fluorescent protein (GFP) marker gene.
MATERIALS AND METHODS
Monoclonal antibodies (MoAbs) and flow cytometry.
Mouse anti-human MoAbs used were CD56 (Immunotech, Marseille, France), anti–HLA-A3 (American Type Culture Collection [ATCC], Rockville, MD), and as described previously.21-23
MoAbs were labeled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC), or conjugated to biotin (BIO). Biotinylated antibodies were revealed by streptavidin (SA)-tricolor (TC) (Caltag Laboratories, San Francisco, CA), SA-PE (Becton Dickinson Immunocytometry Systems, Mountain View, CA [BDIS]), or SA-APC (BDIS). MoAbs from ATCC were FITC- or BIO-conjugated in our lab by standard methods. Flow cytometry and cell sorting was performed as described previously.21 23
Mice.
Fetal day 15 thymic lobes were isolated as described previously21 from C.B.-17 scid/scid (SCID) or NOD-LtSz-scid/scid (NOD-SCID) mice, bred in our specific pathogen-free breeding facility. For generation of SCID-hu mice, small pieces of fresh human fetal liver and thymus were transplanted under the SCID mouse kidney capsule as described previously.24Human tissue was obtained following the guidelines of the Medical Ethical Commission of the University Hospital of Ghent. Animals were treated according to the guidelines of the Laboratory Animal Ethical Commission of the University Hospital of Ghent.
Purification of CD34+ CB cells.
Umbilical CB was obtained from full-term, healthy newborns and used within 24 hours after collection for isolation of mononuclear cells as described previously.21 Briefly, the mononuclear cell fraction, containing on the average 11,250 CD34+ and 570 CD34+CD38− cells per 106CD45+ cells, was cryopreserved in liquid N2until use. After thawing, cells were stained with glycophorin-A, CD19, and CD7 FITC for immunomagnetic depletion. Unlabeled cells were recovered and stained with CD1 FITC, CD3 FITC, CD4 FITC, CD8 FITC, CD34 PE, and CD38 BIO. Cells that were CD34 PE++, lineage markers (Lin) FITC−, and either CD38 BIO/SA-TC+ or CD38 BIO/SA-TC− (called CD34+CD38+ or CD34+CD38−, respectively) were sorted on a fluorescence-activated cell sorting (FACS) Vantage (BDIS) cell sorter equipped with an argon-ion laser (488 nm). Sort gate for CD38− cells was set not to exceed fluorescence intensity of 99% of cells stained with isotypic control antibody. After sorting, on the average 1,100 CD34+CD38+Lin− cells and 425 CD34+CD38−Lin− were obtained per 106 mononuclear cells recovered after thawing. Sorted populations are collectively called CD34+Lin− cells. Sorted cells were checked for purity, which was always at least 99.0%.
Cell culture.
All cultures were performed at 37°C in a humidified atmosphere containing 7.0% (vol/vol) CO2 in air. The cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM), supplemented with penicillin (100 IU/mL), streptomycin (100 μg/mL), and 10% heat-inactivated fetal calf serum (complete IMDM; all products from Life Technologies, Paisley, UK). Cytokine-supplemented medium was refreshed at least twice a week. To avoid medium exhaustion,8 cell density was not allowed to exceed 2 × 106 cells/mL.
Single harvest cultures.
Sorted CD34+Lin− cells were resuspended in complete IMDM supplemented with cytokines as indicated and seeded in 96-well round-bottom tissue culture plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) at 2,500 to 7,500 cells in 150 μL medium per well. In the second week of culture, cells were transferred to 24-well plates, after resuspension and incubation of the well in Cell Dissociation Buffer (Life Technologies) to remove adherent cells from the well surface. For a third week of culture, the cells were transferred to 6-well tissue culture plates or to 25-cm2tissue culture flasks (Falcon). After the culture periods indicated, cells were harvested, counted, analyzed for CD34 and CD38 expression, and 90% of the cells were coincubated with 1 fetal thymic lobe to start FTOC. Expansion was calculated by dividing the number of cells obtained at the end of the culture period by the starting cell number.
For FTOC-limiting dilution experiments, 3 parallel wells containing 1,000 sorted CD34+CD38− cells each were seeded in medium supplemented with cytokines, as indicated, and cultured as described above. After 3, 10, and 17 days of culture, cells from 1 well were harvested. Resuspended cells were distributed over fetal thymic lobes: 1 lobe with 75% of the cells, 3 lobes with 5% of the cells each, and 5 to 8 lobes with 1% of the cells each. The latter lobes therefore received the progeny equivalent to that of 10 CD34+CD38− cells.
For SCID-hu thymus repopulation, 2,500 sorted CD34+CD38− cells were gene marked after 48 hours of culture. To this end, half of the medium volume was replaced with viral supernatant, supplemented with cytokines (to keep final cytokine concentration unchanged) and cells were seeded in 96-well flat-bottom non-tissue culture–treated plates (Falcon), coated with RetroNectin (Takara Biomedicals, Otsu Shiga, Japan) as instructed by the supplier. The retrovirus used encoded the marker gene, EGFP (Clontech, Palo Alto, CA), constructed and produced as described previously.21 The batch used in this report was shown to be free of replication competent retrovirus and contained after thawing 1 × 106 transforming units/mL (titrated on Jurkat T cells [ATCC], data not shown). Cells were further cultured for up to 3 days. After this period, wells were resuspended and incubated in cell dissociation buffer to remove adherent cells from the well surface. Transduction efficiencies of the cells were assayed by flow cytometry on a small sample, remaining cells were seeded in 24-well tissue culture plates and culture was continued as described above. After the culture periods indicated, cells were harvested, counted, and 90% of the cells were injected intrathymically as described below.
Sequential harvest cultures.
In a separate series of experiments, sorted CD34+Lin− cells were cultured for extended periods in wells that were harvested weekly, each time leaving one fourth of the cells in culture. To start the culture, freshly sorted cells were resuspended in complete IMDM supplemented with cytokines as indicated and seeded in 96-well round-bottom tissue culture plates at 6,500 cells in 150 μL medium per well. At day 3 of culture, cells were harvested by resuspension and incubation of the wells in cell dissociation buffer to remove adherent cells from the well surface. Each well was visually inspected to check for removal of all adherent cells. One fourth of the harvested cells was reseeded in the same well in cytokine-supplemented medium, while the remaining cells were analyzed for CD34 and CD38 expression and in some experiments, 90% of these cells were coincubated with 1 fetal thymic lobe to start FTOC. This procedure was repeated every week of culture, reducing the cell number to one fourth every week, for 6 weeks. In this way, at day 3 of culture, one fourth of the progeny of original cell input is reseeded. These cells are obtained at the second harvest (day 10 of culture) and at that moment, therefore, constitute of one fourth of the progeny of the original cell input. Again, one fourth of this progeny is reseeded and harvested 1 week later. Consequently, after 17 days of culture (third harvest), the harvested cells constitute 1/16th, and after 38 days of culture (sixth harvest), 1/1,024th of the progeny of original cell input. This means that without expansion, a well initiated with 6,500 cells would contain after 38 days of culture about 6 input cells. Expansion was calculated by multiplying the number of cells counted on harvest with 4n, where n is the number of harvests preceding, and dividing this product by the starting cell number.
FTOC.
Hanging drops were prepared in Terasaki plates by adding per well 25 μL complete IMDM containing the progeny or a fraction thereof of CD34+Lin− cells after washing in complete cytokine-free medium. To each well, 1 fetal thymic lobe was added, the plates were inverted to form hanging drops and incubated during 72 hours. After this incubation, at day 0 of FTOC, the lobes were removed, washed in complete medium, and cultured for 30 days as described previously.21 For limiting dilution experiments, harvested cells were coincubated with NOD-SCID lobes and irradiated CD34+ cells as described previously.25
Cytokines.
All cytokines were used at a fixed concentration during culture: 100 ng/mL recombinant human SCF (R&D Systems Europe, Abingdon, UK), 100 ng/mL recombinant human FL (R&D Systems Europe), 20 ng/mL TPO (R&D Systems Europe), 10 ng/mL recombinant human IL-3 (Innogenetics, Antwerp, Belgium), 100 U/mL recombinant human IL-6 (produced in yeast, kindly provided by Dr W. Fiers, University of Ghent, Belgium; specific activity was 280 U/ng, assayed as described previously26).
SCID-hu thymic repopulation.
SCID-hu mice, transplanted 5 to 20 months before, were given a sublethal dose of whole-body irradiation (200 cGy, 40 cGy/min) using a cobalt irradiation device. The next day, they were anesthetized and the kidney carrying the human tissue was dissected free. Donor cells were washed extensively, resuspended in 20 μL of complete cytokine-free medium, and injected intrathymically. Thymic implants were removed and analyzed by flow cytometry, 30 days postinjection. HLA mismatch between CB donors (HLA-A3+) and thymus acceptors (HLA-A3−) allowed differentiation between acceptor thymocytes and total donor or transduced donor-derived thymocytes by gating on donor HLA haplotype or on GFP expression, respectively.
RESULTS
Expansion of human CD34+Lin− CB cells in stroma-free liquid cultures.
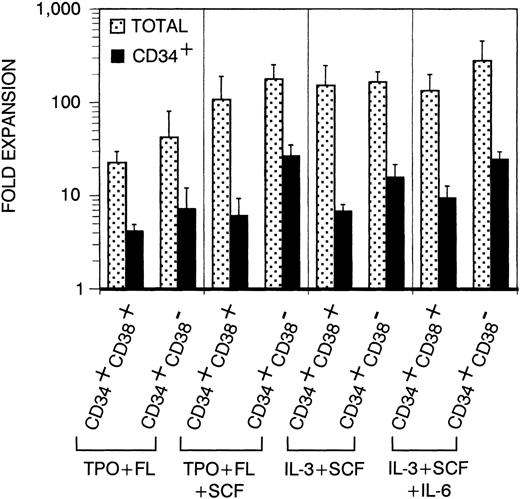
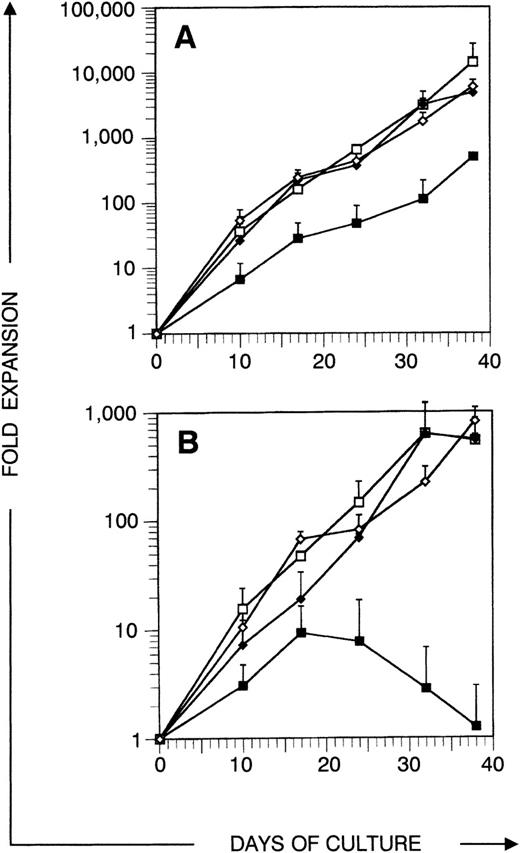
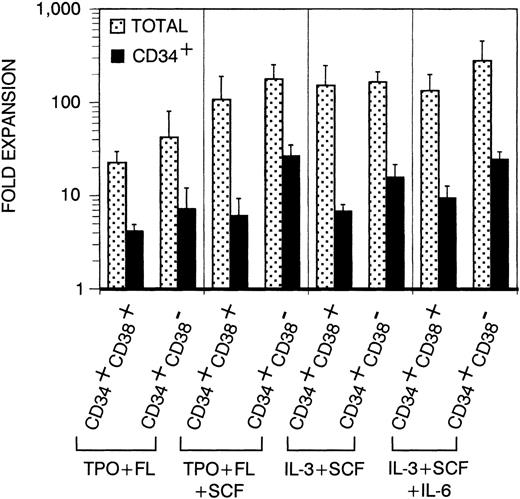
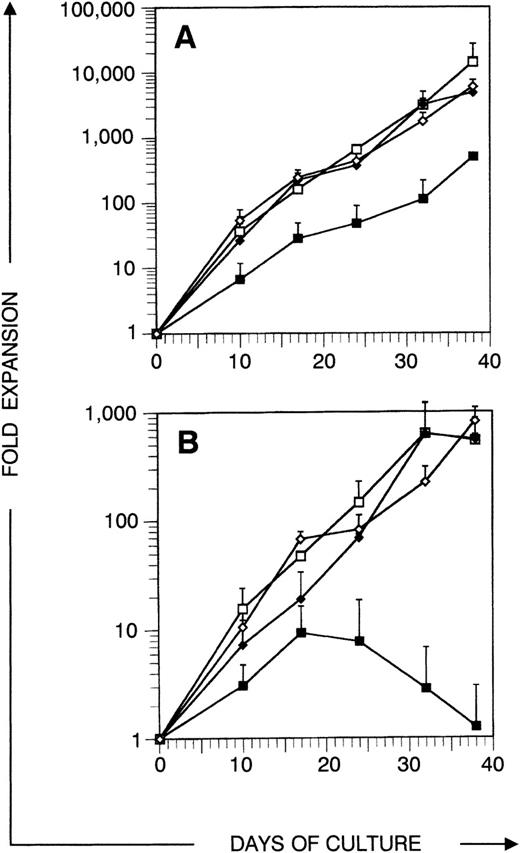
Before analyzing thymic repopulation by cultured CD34+ CB cells, we first compared efficacy of expansion in 4 different cytokine mixes: TPO + FL ± SCF and IL-3 + SCF ± IL-6. Highly purified CD34+CD38+ and CD34+CD38− CB cells were cultured in medium supplemented with these cytokine mixes. After 17 days of culture, the total progeny was harvested (single harvest culture). Total and especially CD34+ cell number expansion was higher in cultures started with CD34+CD38− cells compared with CD34+CD38+ cells (Fig 1). Expansion in TPO + FL-supplemented cultures was inferior to the other combinations tested. In cultures initiated with CD34+CD38+ or CD34+CD38− CB cells, supplemented with TPO + FL, CD34+ cell number expanded on the average 4.1- and 7.2-fold, respectively (resp). Addition of SCF to this mixture increased CD34+ cell number expansion to 6.1 resp 26.6-fold and total cell number expansion was 5-fold higher. Cultures supplemented with IL-3 + SCF and IL-3 + SCF + IL-6 showed comparable total and CD34+ cell number expansion as TPO + FL + SCF–supplemented cultures. Almost no viable cells were recovered from cells cultured in complete medium without cytokines (data not shown). To assay CD34+CD38− CB cell expansion for extensive culture periods in manageable culture dimensions, we performed a series of experiments in which total cell number was weekly reduced to a fourth (sequential harvest cultures). Cell number expansion was calculated as described in Materials and Methods. Total cell numbers expanded continuously over the culture period of 38 days. After 17 days of culture, total (Fig 2A) and CD34+ (Fig 2B) calculated cell number expansion was very similar to the values measured in single harvest culture (Fig 1). Again, TPO + FL was inferior in inducing total and CD34+cell number expansion compared with the other cytokine combinations. Moreover, the CD34+ cell content declined after more than 3 weeks of culture in TPO + FL–supplemented cultures. In the other combinations tested, CD34+ cell content increased during at least 30 days, exceeding a 100-fold expansion. As reported before,13 most of the progeny of CD34+CD38− cells express CD38 after more than 1 week of culture. However, a minority retained the CD38− phenotype over extended culture periods, most prominent in cultures supplemented with TPO + FL + SCF (Fig 3A and data not shown).
Expansion of total and CD34+ cell number in single harvest cultures. Sorted CD34+CD38+Lin− or CD34+CD38−Lin− cells were cultured for 17 days in medium supplemented with cytokines as indicated. Columns represent average expansion (fold increase of start cell number) from independent experiments with 6 different donors, error bars indicate standard deviation (SD). Dotted columns, all cells; filled columns, CD34+ cells.
Expansion of total and CD34+ cell number in single harvest cultures. Sorted CD34+CD38+Lin− or CD34+CD38−Lin− cells were cultured for 17 days in medium supplemented with cytokines as indicated. Columns represent average expansion (fold increase of start cell number) from independent experiments with 6 different donors, error bars indicate standard deviation (SD). Dotted columns, all cells; filled columns, CD34+ cells.
Expansion of total and CD34+ cell number in sequential harvest cultures. Sorted CD34+CD38−Lin− cells were cultured in medium supplemented with cytokines and harvested after different culture periods as indicated. Points represent average expansion (fold increase of start cell number) of all cells (A) or CD34+ cells (B) from independent experiments with 3 different donors, error bars indicate SD. (▪) TPO + FL, (□) TPO + FL + SCF, (⧫) IL-3 + SCF, (◊) IL-3 + SCF + IL-6.
Expansion of total and CD34+ cell number in sequential harvest cultures. Sorted CD34+CD38−Lin− cells were cultured in medium supplemented with cytokines and harvested after different culture periods as indicated. Points represent average expansion (fold increase of start cell number) of all cells (A) or CD34+ cells (B) from independent experiments with 3 different donors, error bars indicate SD. (▪) TPO + FL, (□) TPO + FL + SCF, (⧫) IL-3 + SCF, (◊) IL-3 + SCF + IL-6.
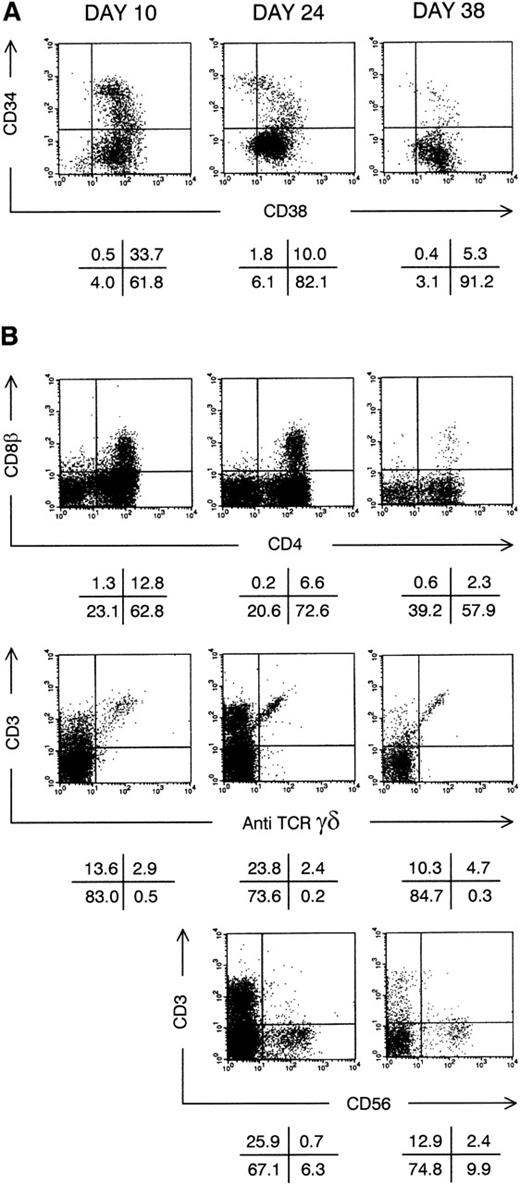
Thymic repopulation by cultured CD34+CD38−Lin− cells in vitro. (A) Flow cytometric analysis of the progeny of CD34+CD38−Lin− cells after pre-FTOC culture for 10, 24, and 38 days in TPO + FL + SCF. Dot plots show CD38 FITC versus CD34 PE staining. (B) Flow cytometric analysis of thymocytes recovered from FTOC initiated with cells shown above in (A). Dot plots show CD4 APC versus CD8β PE, anti-TCR–γδ FITC versus CD3 APC and CD56 PE versus CD3 APC stainings. The latter staining was not performed on thymocytes from FTOC initiated with CD34+CD38−Lin− cells cultured for 10 days. Quadrants were set to include 99% of cells stained with isotypic control antibody in lower left quadrants. Data shown are representative of 2 independent experiments with 2 different donors.
Thymic repopulation by cultured CD34+CD38−Lin− cells in vitro. (A) Flow cytometric analysis of the progeny of CD34+CD38−Lin− cells after pre-FTOC culture for 10, 24, and 38 days in TPO + FL + SCF. Dot plots show CD38 FITC versus CD34 PE staining. (B) Flow cytometric analysis of thymocytes recovered from FTOC initiated with cells shown above in (A). Dot plots show CD4 APC versus CD8β PE, anti-TCR–γδ FITC versus CD3 APC and CD56 PE versus CD3 APC stainings. The latter staining was not performed on thymocytes from FTOC initiated with CD34+CD38−Lin− cells cultured for 10 days. Quadrants were set to include 99% of cells stained with isotypic control antibody in lower left quadrants. Data shown are representative of 2 independent experiments with 2 different donors.
CD34+Lin− CB cells retain the potential to generate thymocytes in vitro after extensive culture.
To investigate whether the lymphoid precursor potential is retained in cultured CD34+Lin− CB cells, CD34+CD38+ and CD34+CD38− CB cells grown in single harvest cultures for 3, 10, and 17 days were assayed in FTOC. The results are summarized in Table 1. As described before,21 after 30 days of FTOC, thymocytes expressing CD4, CD8β, and CD3 were present if thymic repopulation succeeded (more than 80% of FTOCs performed with freshly sorted CD34+Lin− CB cells). FTOC phenotype after successful repopulation (scored by the presence of CD4+CD8β+ double-positive (DP) immature thymocytes and/or CD3+T-cell receptor [TCR]-γδ− thymocytes) was comparable to that obtained with CD34+Lin− CB cells grown in sequential harvest cultures (vide infra and Fig 3B). Both CD34+CD38+ and CD34+CD38− cells of almost all CB donors, grown for 3 days in any of the cytokine mixes tested, could generate CD3+ T cells and DP thymocytes in FTOC (Table 1). CD34+CD38+ cultured for 1 or 2 additional weeks showed a somewhat reduced T-cell generation success rate. Some FTOCs then contained only TCR-γδ+ cells and NK cells, but no DP or CD3+TCR-γδ− thymocytes. This FTOC phenotype was not considered as thymic repopulation, as TCR-γδ+ cells and NK cells can develop extrathymically.27 By contrast, with all CB donors, CD34+CD38− cells cultured for 17 days generated CD3+TCR-γδ− T cells and frequently also DP thymocytes in FTOC, irrespective of the cytokine mix used in the pre-FTOC culture (Table 1). CD3−CD56+ NK cells were present in most FTOCs (data not shown). These data indicate that the progeny of both the CD38+ and CD38− subsets of CD34+Lin− CB cells contained cells with T-cell and/or NK cell progenitor potential, even after 17 days of culture in the presence of cytokines.
By using the cells obtained during sequential harvest cultures as described above, we could address how long we could extend stroma-free culture while maintaining T-cell and NK cell progenitor potential. Due to previous harvests, a decreasing fraction of the progeny of original cell input is obtained as described in Materials and Methods. Quiescent cells are therefore diluted out. For 2 CB donors, we analyzed in vitro thymocyte generation from CD34+CD38+ and CD34+CD38− cells obtained weekly in sequential harvest cultures over more than 5 weeks. None of these 2 donors generated DP thymocytes in FTOC from CD34+CD38+ cells cultured for more than 17 days, irrespective of the cytokine mix used. The progeny of CD34+CD38− cells, cultured for a maximum of 24 days in medium supplemented with TPO + FL or IL-3 + SCF, could repopulate thymi (data not shown). By contrast, CD3+ T cells and DP thymocytes were generated in FTOC started with CD34+CD38− cells obtained after up to 38 days of sequential harvest culture in medium supplemented with TPO + FL + SCF or IL-3 + SCF + IL-6. As an example, Fig 3B shows the phenotype of FTOC initiated with CD34+CD38− cells from the same donor cultured for 10, 24, or 38 days in medium supplemented with TPO + FL + SCF. Both CD3+TCRγδ+ and CD3+TCRγδ− T cells and CD3−CD56+ NK cells were generated in these FTOCs (Fig 3B). T-cell development was still continuing at the end of FTOC, indicated by the presence of a population of DP thymocytes. With increasing pre-FTOC culture periods, the fraction of DP thymocytes generated by cultured CD34+Lin− cells decreased (Fig 3B and data not shown). As described in Materials and Methods, without expansion, wells harvested to start FTOC after 38 days of sequential harvest culture would contain about 6 sorted CD34+CD38− input cells. However, due to expansion, in TPO + FL + SCF resp IL-3 + SCF + IL-6–supplemented cultures, wells contained on the average of 90 × 103 resp 42 × 103cells, including 3.2 × 103 resp 5.9 × 103 CD34+ cells.
Expansion of thymus-repopulating cells in cultures of CD34+CD38−Lin−CB cells.
In single harvest cultures described above, almost the total progeny is put in FTOC. Quiescent cells present at the start of culture could in theory be ultimately harvested to initiate FTOC. However, T-cell generation from cultured CD34+CD38− cells obtained during sequential harvest culture seemed to indicate that the number of putative T-cell progenitors increased during culture. To demonstrate actual expansion of putative T-cell progenitors, we performed limiting dilution experiments as described in Materials and Methods with 3 different CB donors. Table 2shows representative results obtained with 1 of these donors. After 3 days of culture, the progeny equivalent to that of 750 CD34+CD38− cells could repopulate the thymic lobe, irrespective of the cytokine mix where these cells were cultured in (data not shown). However, the progeny equivalent to that of 50 or 10 cells could not in almost all FTOCs performed (Table 2). The cell number recovered after 3 days of culture of CD34+CD38− cells is about the same as the input number, as we previously described.21 After 10 days of pre-FTOC culture, the repopulated fraction in the lobes that received the progeny of 50 CD34+CD38−cells cultured in TPO + FL + SCF and IL-3 + SCF ± IL-6 increased (Table 2). FTOCs initiated with the progeny equivalent to that of 10 cells were only repopulated by cells cultured in medium supplemented with TPO + FL + SCF. An additional week of culture to a total of 17 days in this cytokine mix did not further increase repopulation frequency (Table 2). After 17 days of culture in IL-3 + SCF ± IL-6–supplemented medium, the progeny equivalent to that of 10 cells could now also repopulate thymic lobes. The progeny equivalent to that of 10 or 50 CD34+CD38− cells cultured in TPO + FL could not generate DP or CD3+TCR-αβ+ thymocytes in almost all FTOCs performed. Overall, these experiments showed an increase in the number of thymus-repopulating cells in the progeny of CD34+CD38− cells, when cultured in TPO + FL + SCF or in IL-3 + SCF ± IL-6–supplemented medium.
In vivo thymic repopulation by gene marked CD34+CD38−Lin− CB cells after extensive culture.
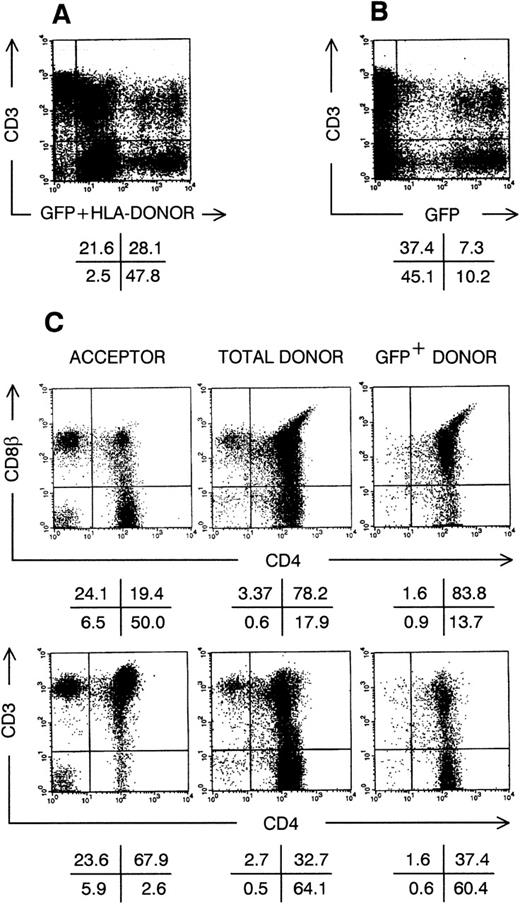
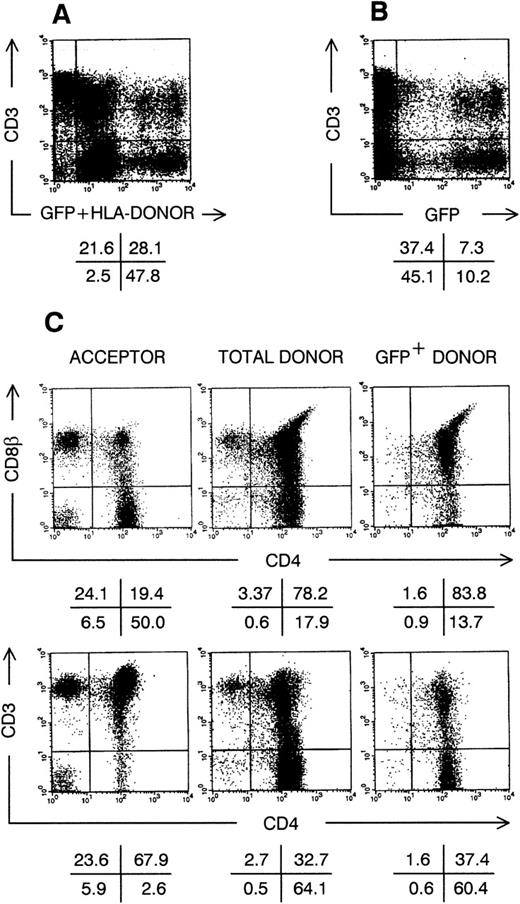
The experiments we performed indicated that after extended suspension culture a progeny equivalent to that derived from a few CD34+CD38− cells could generate thymocytes. Limiting dilution experiments indicated that the number of putative thymus-repopulating cells increased during culture. To prove that these cells divided in vitro and can generate thymocytes after gene transfer, we transduced CD34+CD38−cells cultured in TPO + FL + SCF or IL-3 + SCF + IL-6–supplemented medium with a Moloney murine leukemia virus-derived retrovirus encoding the marker gene GFP. One day after transduction, the percentage of cells expressing the retroviral encoded GFP was on the average (N = 3) 36%. Gene transfer requires division so that GFP+ cells are the progeny of cells that were replicating at the moment of transduction.21 We previously showed that after 3 days of culture in IL-3 + SCF-supplemented medium, transduced CD34+CD38− cells generate thymocytes expressing the marker gene in FTOC.21 In the present study, because culture of CB cells to increase graft size and gene transfer will probably last longer, we wanted to determine whether transduced CD34+CD38− cells, cultured for longer than 3 days, can repopulate thymus in vivo. Therefore, we performed a series of SCID-hu thymus repopulation23,28 experiments with almost the complete progeny of retrovirally transduced CD34+CD38− cells, cultured in medium supplemented with either TPO + FL + SCF or IL-3 + SCF + IL-6, and obtained with both cytokine mixes similar results. Figure 4 shows the phenotype of the human thymus, 1 month after intrathymic injection of the progeny of CD34+CD38− cells, cultured for 3 weeks in TPO + FL + SCF. In this example (Fig 4A), more than 75% of the SCID-hu thymocytes were from CB donor origin (HLA-A3+), of which about 1 in 4 expressed the marker GFP (Fig 4B). Acceptor thymocytes (HLA-A3−) were mainly mature, radioresistant CD3+, CD4+CD8β−single-positive (SP) or CD4−CD8β+ SP thymocytes (Fig 4C). Donor-derived thymocytes were predominantly of the immature DP phenotype, with variable CD3 cell surface expression levels. As observed previously in vitro,21 GFP-expressing thymocytes seem to develop somewhat slower compared with nontransduced donor thymocytes, as they generated less SP thymocytes 30 days after intrathymic injection. Lineage distribution in gene marked thymocytes was similar to that of other donor-derived thymocytes: a minority of the CD3hi cells were TCRγδ+, and small populations of GFP+CD3−CD56+NK cells, HLA-DRhiCD4+ dendritic cells and CD34+ precursor cells were present (data not shown).
Thymic repopulation by cultured CD34+CD38−Lin− cells in vivo. Flow cytometric analysis of SCID-hu thymocytes, 30 days after injection with transduced CD34+CD38−Lin− cells, precultured for 3 weeks in TPO + FL + SCF. (A) Dot plot shows anti-HLA–A3 FITC staining plus GFP fluorescence versus CD3 APC staining. (B) Dot plot shows GFP fluorescence versus CD3 APC staining; cells were not stained with anti–HLA-A3 FITC. (C) Dot plots show CD4 APC versus CD8β and CD4 APC versus CD3 PE staining for cells stained with anti–HLA-A3 FITC as in (A), gated on either FITC−GFP− cells (acceptor cells) or FITC+GFP+ cells (donor cells); and for cells not stained with anti-HLA–A3 FITC as in (B), gated on GFP+ cells (transduced GFP+ donor cells). Quadrants were set to include 99% of cells stained with isotypic control and GFP− cells in lower left quadrants. Data shown are representative of 2 independent experiments with 2 different donors.
Thymic repopulation by cultured CD34+CD38−Lin− cells in vivo. Flow cytometric analysis of SCID-hu thymocytes, 30 days after injection with transduced CD34+CD38−Lin− cells, precultured for 3 weeks in TPO + FL + SCF. (A) Dot plot shows anti-HLA–A3 FITC staining plus GFP fluorescence versus CD3 APC staining. (B) Dot plot shows GFP fluorescence versus CD3 APC staining; cells were not stained with anti–HLA-A3 FITC. (C) Dot plots show CD4 APC versus CD8β and CD4 APC versus CD3 PE staining for cells stained with anti–HLA-A3 FITC as in (A), gated on either FITC−GFP− cells (acceptor cells) or FITC+GFP+ cells (donor cells); and for cells not stained with anti-HLA–A3 FITC as in (B), gated on GFP+ cells (transduced GFP+ donor cells). Quadrants were set to include 99% of cells stained with isotypic control and GFP− cells in lower left quadrants. Data shown are representative of 2 independent experiments with 2 different donors.
DISCUSSION
In this report, we investigated thymus-repopulating potential of expanded CD34+Lin− CB cells. Our data indicate that expanded CD34+Lin− CB cells contain thymus-repopulating cells in both the CD38−and CD38+ fraction, even after several weeks in stroma-free culture. After 38 days of culture, the progeny of CD34+CD38− cells could generate thymocytes in vitro. During culture, the number of thymus-repopulating cells increased. After 3 weeks of culture, transduced CD34+CD38− cells could repopulate SCID-hu thymus, generating in vivo T cells, NK cells, and dendritic cells expressing the GFP marker gene. Collectively, our data indicate that thymus-repopulating cells can be expanded in vitro and can generate transduced thymocytes in vivo after extensive culture and retroviral gene transfer in vitro.
Clinical experience with the use of CB cells as a transplantable source of HPC indicates that myeloid and platelet engraftment is delayed if fewer than 20 to 30 million nucleated CB cells per kg body mass are injected.1,2 As the median number of nucleated cells in CB donations is about 1,000 million or less, expansion of CB cells might be necessary to use these widely accessible HPC in transplantation of adults.2 Therefore, considerable amount of data has been produced on growth factor–induced proliferation of primitive, hematopoietic CB cells in vitro.4-10 We compared 2 types of cytokine mixes known to act on primitive hematopoietic cells: TPO + FL ± SCF and IL-3 + SCF ± IL-6. TPO was recently described to act as a growth factor for CD34+ HPC of different origins, including CB.6,9,29,30 Combination of TPO with FL, the ligand for the flt3/flk-2 receptor tyrosine kinase, was reported by Piacibello et al6 to induce massive expansion of CD34+ and CD34+CD38− CB cells in sequentially harvested cultures. In their study, CD34+cells cultured in medium supplemented with TPO + FL expanded more than 1,000-fold in 3 to 5 weeks of culture. After 25 weeks of culture, CD34+CD38− cell numbers were calculated to have increased more than 10 million-fold.6 Recently, the same group reported extensive SRC expansion in TPO + FL–supplemented cultures of CD34+CB cells.9 In our experiments, TPO + FL was not able to sustain extensive expansion of sorted CD34+CD38− CB cells. In fact, addition of SCF increased total cell number expansion and was essential to maintain CD34+ cells in sequentially harvested cultures lasting longer than 3 weeks (Fig 2). Moreover, addition of SCF was necessary to expand thymus-repopulating cells (Table 2). This is in line with previous reports that addition of SCF to TPO + FL increases several-fold the expansion of CD34+ bone marrow cells after 6 to 14 days of culture.30,31 TPO or FL as single factors could support LTC-IC expansion from CD34+CD38− bone marrow cells cultured in serum-free medium for 10 days.29 However, this was not the case for TPO in CB LTC-IC expansion in serum-free culture for 10 days.8 In the latter study, addition of SCF was shown to be important for CB CFC expansion.8 We do not have an explanation why we could not reproduce the observations of Piacibello et al.6 These investigators have used cytokines from a different source and in slightly different concentrations than we did. In both their and our experiments, culture medium contains 10% (vol/vol) fetal calf serum. FCS is known to contain factors affecting human HPC proliferation and maintenance in vitro.32Serum-free culture media have been successfully used for expansion of CD34+ CB cells.7,8 Use of these media will eliminate the variability and potential biohazards introduced by FCS. As others and we observed,8 cultures of CD34+CB cells contain a portion of adherent cells. Transfer of cells between the adherent and the suspension phase could bias calculations in sequentially harvested cultures. We detached all adherent cells at each harvest. At the third harvest (17 days of culture), cell number expansion calculated from sequentially harvested cultures was comparable to that measured in single harvest cultures (compare Fig 2with Fig 1). Similarly, this bias was also excluded in the recent study of Piacibello et al.9
A classical cytokine cocktail frequently used to expand HPC is IL-3 + SCF + IL-6.4,11,33,34 We compared this mixture with the combination IL-3 + SCF, previously shown to maintain in vitro21 and in vivo28 thymus-repopulating ability of gene-marked CD34+ CB cells cultured for 3 to 4 days. Our experiments did not show a difference between cultures supplemented with either of these cocktails in expansion of total; CD34+, or thymus-repopulating cell number as estimated by the limiting dilution-FTOC experiments. Interestingly, CD34+CD38− cells cultured in IL-3 + SCF–supplemented medium for 7 days, but not freshly sorted CD34+CD38− cells, produced IL-6 bioactivity in medium conditioned for 4 days (data not shown). Therefore, endogenous IL-6 production in IL-3 + SCF–supplemented cultures may blur the difference induced by addition of exogenous IL-6. However, thymic repopulation by CD34+CD38− cells seemed to be maintained longer after culture when exogenous IL-6 was added to IL-3 + SCF. Presence of IL-6 at the start of the culture may be important. In single harvest cultures of CD34+CD38− CB cells lasting 10 days, multivariate analysis of cytokine combinations by Zandstra et al8 showed that IL-6 is important in CB LTC-IC and CFC expansion. In our experiments, the mix IL-3 + SCF + IL-6 seemed equivalent to TPO + FL + SCF in cell number expansion and thymic repopulation by expanded CD34+CD38− CB cells. However, the number of thymus-repopulating cells seemed to increase more prominently in TPO + FL + SCF compared with IL-3 + SCF + IL-6–supplemented cultures. In addition, similar results were obtained with single harvest cultures supplemented with all 5 cytokines (data not shown).
The progeny of CD34+CD38+ cells showed a reduced potential in generating T cells in vitro compared with that of CD34+CD38− cells with increasing culture duration, regardless of the cytokine mix used. This suggests that both the CD34+CD38+ and the CD34+CD38− population of CB cells contain thymus-repopulating cells, analogous to myeloid CFC. However, thymus-repopulating cells are lost after extended culture of CD34+CD38+ cells. By contrast, after more than 5 weeks in culture, thymus-repopulating cells were found in 1/1,024th of the progeny of 6,500 CD34+CD38−Lin− cells. Recently, the frequency of thymus-repopulating cells was estimated to be between 1/100 and 1/500 in freshly isolated CD34+ CB cells.25 The limiting dilution experiments and gene transfer experiments we performed show that the progeny of CD34+CD38− cells contains expandable thymus-repopulating cells. Such cells would represent long-term culture thymus-repopulating cells, similar to myeloid LTC-IC. After 38 days of culture, the progeny of CD34+CD38− cells cultured in medium supplemented with TPO + FL + SCF or IL-3 + SCF + IL-6 contained mainly myeloid lineage marker CD33+ cells, for the majority monocytes (CD14+HLA-DR+), but no T cells, NK cells, or B cells (data not shown). This indicates that the T cells and NK cells recovered from the FTOC were generated in the thymus from precursor cells. It is unclear whether these precursor cells might represent the human equivalent of a common lymphocyte progenitor (CLP), recently identified in mice.35 Our data indicate that thymus-repopulating CB T-cell and/or NK cell progenitors can be kept in culture in a stroma-free environment for more than 5 weeks. Previous work by Galy et al14 demonstrated SCID-hu thymus repopulation by CD34+ bone marrow cells, cultured for 3 weeks on autologous bone marrow stroma. Partially purified CB CD34+ cells were shown to repopulate SCID-hu thymus after 1 week of culture in IL-3 + SCF + IL-6–supplemented medium.4Also, lymphoid marker CD2-expressing cells could be found in TPO + FL–supplemented cultures of CD34+ CB cells, after 12 weeks of culture. However, this study did not clarify whether these cells were NK cells or possibly T cells.6
As we show here, transgenic thymocytes can be generated in SCID-hu thymus from transduced CD34+CD38− CB cells expanded during 3 weeks of stroma-free culture. This period is sufficient for reaching graft sizes that would contain enough nucleated cells and HPC to engraft adults. Retroviral marking in our experiments showed that thymocytes could be generated by cells that divided around day 2 of culture. We injected the cells directly into the human thymus, as thymic homing in SCID-hu mice is inefficient and not a validated model for human thymic seeding.36 Therefore, we cannot exclude that the thymus-repopulating cells we describe here might fail to home to the thymus after intravenous injection in humans.
The thymus remains functional and generates naive T cells in adults.16 In acquired immunodeficiency syndrome (AIDS) patients, thymic T-cell generation is responsible for the increase in naive T-cell counts during highly active antiretroviral therapy.16 Gene therapy for diseases affecting T cells, such as adenosine deaminase (ADA) deficiency, AIDS, and X-linked SCID, requires persistent lymphoid precursor potential of the genetically modified precursors.19,20 37 As our data show that the progeny of transduced CD34+CD38− cells can repopulate the thymus in vivo after 3 weeks of culture, thymic repopulation by transplanted CB cells after expansion and gene transfer may be possible, contributing to immune reconstitution and success of gene therapy.
ACKNOWLEDGMENT
We thank Christian De Boever for artwork, Achiel Moerman, Veronique Debacker, Isabelle Windey, Ilse Swennen, and Greet De Smet for animal care, the Departments of Obstetrics, Cardiac Surgery, and Pathology for the supply of human tissue, the Department of Radiotherapy and Nuclear Medicine for irradiation facilities, Dr Jo van Damme for measuring IL-6 bioactivity, and Dr G. Leclercq for critical reading of the manuscript.
Supported by grants from the Gezamelijk Overlegde Actie (GOA) University of Ghent; the Fund for Scientific Research, Flanders (Belgium); and the VIB. B.V. and T.K. are research assistants and D.V. is a postdoctoral research fellow of the Fund for Scientific Research, Flanders (Belgium). E.N. is a VIB employee.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Bruno Verhasselt, MD, Department of Clinical Chemistry, Microbiology and Immunology, University of Ghent, University Hospital of Ghent, 4 Blok A De Pintelaan 185, B-9000 Ghent, Belgium; e-mail: Bruno.Verhasselt@rug.ac.be.