PROLIFERATION OF hematopoietic cells is controlled by a coordinated hierarchy of growth factors and their inhibitors. These growth factors form complex signaling networks either by stimulating or inhibiting cell proliferation and differentiation. They govern all growth stages of blood cells that emerge from a small common pool of ancestral stem cells. In addition, biologic effects are altered by the interaction of different growth factors, which adds another magnitude of complexity. Insulin-like growth factors (IGFs) are part of a number of growth factors and cytokines responsible of burst-like growth of early erythroid progenitor cells in vitro and may play a role in the ontogeny of marrow development. IGFs may not be considered as classical hematopoietic growth factors, but nonetheless their involvement in abberant hematopoietic growth regulation is intriguing. The complexity of the IGF system should be a challenge for conducting more research on this topic rather than a hurdle. This review aims to unravel the biological significance of IGFs in normal and malignant hematopoiesis as well as to stimulate further research in this rapidly expanding field.
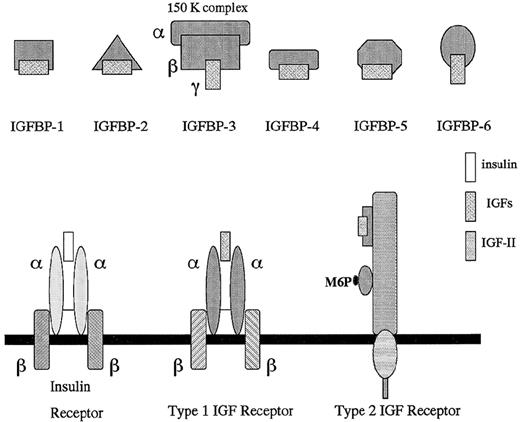
IGFs constitute a family of peptides capable of stimulating various cellular responses, including cell proliferation and differentiation.1 Both IGF-I and -II are mitogenic factors that are secreted by malignant cells as well.2 A steadily increasing number of IGF-binding proteins (IGFBP-1 to -6) and IGFBP-related proteins (IGFBP-rP1 to 4) are part of the IGF system. These IGFBPs either enhance or inhibit the IGF-mediated effects. The IGFBP-related proteins (IGFBP-rPs) are newly described factors that appear to be implicated in tumorigenesis.3 The type I (IGF-I-R) and type II (IGF-II-R) IGF receptor bind IGFs and mediate their effects (Figs 1, 2, and3). IGF-I-R is of particular interest because of its antiapoptotic role,4 and novel anticancer therapies may be designed aiming at this receptor.5
Schematic representation of the insulin-like growth factor axis: IGFs, IGFBPs, and IGF receptors.
Schematic representation of the insulin-like growth factor axis: IGFs, IGFBPs, and IGF receptors.
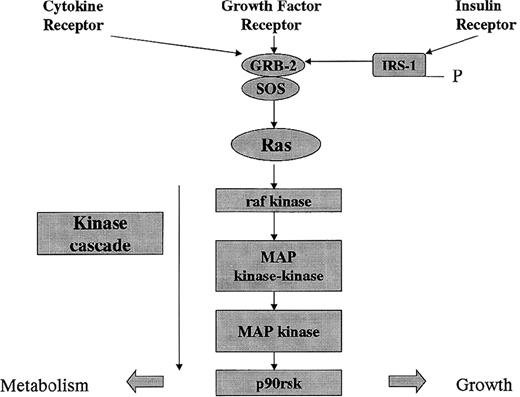
Signal pathway of receptor-activated tyrosine kinase receptors. Cytokines, growth factors, and insulin activate receptor-linked tyrosine kinases that induce receptor autophosphorylation. The GRB2 and son of sevenless (SOS) guanine nucleotide-releasing protein is recruited to the plasma membrane. SOS activates raf kinase, which initiates a phosphorylation cascade resulting in effects on metabolism and growth.
Signal pathway of receptor-activated tyrosine kinase receptors. Cytokines, growth factors, and insulin activate receptor-linked tyrosine kinases that induce receptor autophosphorylation. The GRB2 and son of sevenless (SOS) guanine nucleotide-releasing protein is recruited to the plasma membrane. SOS activates raf kinase, which initiates a phosphorylation cascade resulting in effects on metabolism and growth.
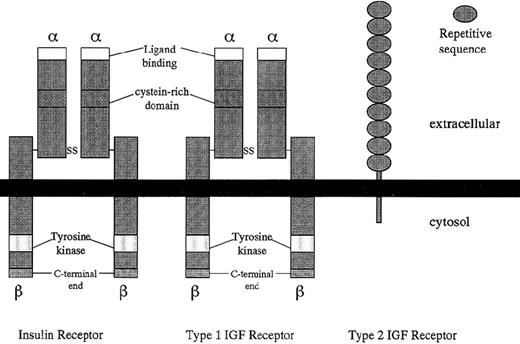
Structural comparison of insulin, type 1 (IGF-I), and type 2 (IGF-II) receptors.
IGFs are also of importance for blood formation.6 They stimulate both myeloid and lymphoid cells in culture.7,8Since epidemiologic studies have indicated that high birthweight is associated with an increased risk of infant leukemia,9 it has been postulated that IGF-I may both produce a larger baby and contribute toward leukemogenesis.10
INSULIN-LIKE GROWTH FACTORS
IGF-I and -II stimulate erythroid and myeloid progenitors.11-13 IGF-I induces granulopoiesis in human bone marrow culture granulocyte-monocyte colony-forming units (GM-CFU), and the effect is inhibited by monoclonal antibodies against the type I IGF receptor.14 IGF-II promotes GM colony formation of both normal and leukemic hematopoietic cells in vitro15 and was shown to function as a B-cell growth-promoting factor.16Bone marrow cells enriched for CD34+ cells and placed in liquid cultures supplemented with interleukin-3 (IL-3) and IGF-II showed an enhanced proliferation of granulocytes, macrophages, and erythroid cells.8 Infusion of human recombinant IGF-I stimulated erythropoiesis in hypophysectomized rats, evidenced by iron incorporation into red blood cells and an increase in reticulocyte numbers.11 Erythroid colony-forming cells stimulated with IGF-I, in addition to erythropoietin, showed an enhanced heme synthesis, cellular proliferation, and greatly enhanced nuclear condensation as well as enucleation in late erythroblasts.17 CD4+ T cells and splenic B cells increased in mice treated with IGF-I, indicating its likely role in augmenting the immune response.18,19 Both IGF-I and IGF-II were found to enhance T-cell proliferation 3-fold in the early activation process.7
Circulating erythroid progenitors in polycythemia vera (PV) are hypersensitive to IGF-I with respect to erythroid burst formation in serum-free medium and independent of erythropoietin.20 In peripheral blood mononuclear cells from PV patients, an increased basal tyrosine phosphorylation of the IGF-I receptor β-subunit and a hypersensitive receptor with respect to tyrosine phosphorylation was found.21 Elevated levels of IGFBP-1 were found in these patients and the effect of IGFBP-1 in the presence of IGF-I stimulated erythroid burst formation.22 Thus, these data indicate that IGFs are implicated in the pathogenesis of polycythemia vera.
Both insulin and IGF-I induce proliferation of the promyelocytic leukemia HL-60 cells in a dose-dependent manner.23-25 Both serum anti–IGF-I25 and antibodies directed against the type I IGF receptor23 abrogates the growth-promoting effect of IGF-I. IGF-I stimulates thymidine incorporation in K562 erythroleukemia cells26 and increases bone marrow blast colony numbers in patients with acute myeloid leukemia.27Murine ELM erythroleukemia cells underwent clonal extinction when serially cloned in IGF-I and became IGF-I–independent during long-term growth in IGF-I.28 IGF-I and IGF-II increase cell proliferation of AML-193 cells 4-fold and 2-fold, respectively. A synergistic effect of IGFs and GM colony-stimulating factor (GM-CSF) has been found in these cells.29 Casein kinase II, a key enzyme involved in the regulation of cell growth, is activated in human myeloblastic leukemia cell line ML-1 only when exposed to both IGF-I and transferrin.30 IGF-I and transferrin stimulate the expression of the c-myb and c-ets-1 proto-oncogenes which, as transcription factors, are involved in the regulation of cell proliferation and differentiation.31
Genomic imprinting is a non-Mendelian form of gene regulation resulting in differential allelic gene expression that plays a pivotal role in the transcriptional repression of developmental genes associated with normal growth. Relaxation of IGF-II genomic imprinting occurs in many different tumors and may represent a novel epigenetic mechanism. Loss of imprinting (LOI) of IGF-II was found in all 12 informative samples from acute myeloid leukemia (AML) patients, whereas both blood from normal individuals and 3 types of hematopoietic progenitor cells showed monoallelic expression. The fact that LOI of IGF-II was present in the earliest stage of disease (ie, myelodysplastic syndrome) and did not correlate with French-American-British (FAB) classification implies that LOI of IGF-II are both early and general epigenetic events in leukemogenesis.32 The significance of LOI of IGF-II lies in the upregulation of this autocrine growth factor during the development of leukemia.
Recently, loss of imprinting of the IGF-II gene has been found to be associated with disease progression in chronic myelogenous leukemia (CML). Whereas the importance of the Philadelphia chromosome translocation in CML has been recognized nearly 2 decades ago, factors that cause progression to blast crisis at a molecular level remain largely to be identified. LOI of IGF-II in accelerated phase and blast crisis but not in stable CML indicate a novel type of genetic alteration in CML related to disease progression and, thus, adverse prognosis.33 Abnormal DNA methylation at other loci such as calcitonin has been described in CML progression.34Furthermore, aberrant methylation has recently been shown in the major breakpoint cluster region (M-bcr) in CML where most Philadelphia chromosome breakpoints are located.35 Thus, the imbalance in DNA methylation may play a role in the progression of genetic instability characteristic for the advancing stages of CML.
IGF-I was found to increase the blast colony numbers in bone marrow of B-cell acute lymphoblastic leukemia (ALL) patients.27 However, neither a proliferative response nor clonal growth was induced by IGF-I in B-cell precursor ALL.36 Leukemic cells from patients with B-ALL do not require IGF-I for proliferation, which does not rule out the acquisition of an autocrine secretion.37 A possible role of IGF-I in etiology of bovine leukemia virus (BLV) infection and progression has also been suggested.38 Diet reduction appears to modulate mononuclear cell leukemia progression in Fischer rats via suppression of the GH:IGF-I axis and enhancement of host defences against tumor cells.39
Apoptosis or programmed cell death is a physiological process that eliminates damaged cells. Both anti-apoptotic proteins (Bcl-2, Bcl-xL and Bcl-w) and pro-apoptotic proteins (Bax, Bad, and Bcl-xS) are involved in the control of apoptosis. IGF-I was identified as an essential factor for R-Ras–induced suppression of cell death in the BaF3 pro-B cell line.40 IGF-I activated the extracellular signal-regulated kinase (ERK) and R-Ras and IGF-I cooperatively induced Bcl-xL expression in this cell line.41 The combination of IGF-I and IL-7 stimulates proliferation of pro-B cells42and cell survival may be a result of R-Ras activation by these factors.
IGF-I stimulates the proliferation of T-cell lymphoma lines that have phenotypic characteristics of thymic pre-T cells and may inhibit cell differentiation, which could be of importance in early lymphoma tumorigenesis.43 The in vitro proliferation of the murine lymphoid T-cell leukemia LB cells was enhanced by insulin but not IGF-I or IGF-II.44
Recombinant IGF-I stimulated the expression of immunoglobulin μ-heavy chain genes and potentiated the proliferative stimulus provided by IL-7, indicating a pivotal role for IGF-I in regulating primary B lymphopoiesis.45 Responsiveness to insulin and IGF-I was found to be less developed in 3 Epstein-Barr virus (EBV)-immortalized B-lymphoblastoid cell lines, in the Burkett lymphoma cell line Ramos, and in the non-EBV lymphoblastoid cell line HS Sultan as compared with human multiple myeloma cell lines.46 IGF-I causes a significant increase in proliferation of Burkitt’s lymphoma cells, which could be blocked by antiserum against IGF-I.25
IGF RECEPTORS
Specific receptors for IGFs are detectable on human erythrocytes47 as well as monocytes, B lymphocytes, and a marginal number of T lymphocytes.48 IGF-II exerts its effects on developmental regulation of human marrow erythroid progenitors via pathways involving the IGF-I-R.42 Type I and II IGF receptors are expressed on resting human T cells and are increased on activated T cells.49 It was found that expression of the type I IGF receptor after activation is skewed between CD4+ and CD8+ T-cell subpopulations.7 Relatively high numbers of the type I IGF receptor were found on monocytes, natural killer cells, and CD4+ T-helper cells.50 Jurkat T cells possess specific IGF-I-R and IGF-I induces phosphorylation of tyrosine kinase, which supports the notion that this receptor is involved in the induction of T-cell activation.51
Type I IGF receptors were found on the human promyelocytic leukemia cell line HL-60 and their number decreases while differentiating to macrophage-like cells.52 The treatment of the erythropoietin (EPO)-dependent cell line, F-36P, with a combination of EPO and IGF-I enhanced EPO-induced tyrosine phosphorylation of STAT5 and mRNA expression of c-CIS, which is a target molecule of STAT5. It was therefore concluded that IGF-I may augment EPO-induced proliferation by enhancing tyrosine phosphorylation of STAT5 and that Ras may be involved in this process.53 It was suggested that IGF-I-R is either not expressed or expressed at low levels on normal hematopoietic progenitor cells but was easily detectable in more differentiated cells derived from day 6 burst-forming unit-erythroid (BFU-E) and CFU-GM colonies.54 IGF-I-R are present on K562 erythroleukemia cells and IGF-I binding sites decrease in the course of differentiation.26 A novel human ryk tyrosine kinase cDNA originally identified as a polymerase chain reaction (PCR)-amplified cDNA fragment in K 562 cells55 was shown to possess homology to the IGF-I-R tyrosine kinase.56
Receptors for insulin, IGF-I, and IGF-II are present on T- and B-lymphoblasts.57 Several T-cell lines infected with human T-lymphotropic virus (HTLV)-I and -II showed significantly higher type I IGF receptor mRNA transcripts as compared with uninfected cells, suggesting that deregulation of this receptor contributes toward proliferation and transformation in HTLV-I and -II infected cell lines.58 High numbers of type I IGF receptors (IGF-I-R) were found in immature (stage I) as well as pre-B ALL cell lines, and cross-linking revealed IGF-I-R α-subunits of 135 and 116 kD in the HSB2 T-ALL cell line.59 The IGF-I-R appears to mediate the IGF-I effect in early differentiated T-cell lines HSB2 and HUT78 as well as the B-cell line REH, whereas both the IGF-I-R and IGF-II-R seem to be involved in the proliferation of the differentiated T-ALL Jurkat and JMP cell lines.60 The presence of EBV in Burkitt’s lymphoma cells causes a reduction of the IGF-I-R number but an increase of insulin receptors in these cells.61
IGF-BINDING PROTEINS
Malignant cells synthesize IGF-binding proteins and these peptides have been found to be useful tumor markers.1 Human leukemic B lymphoblasts synthesize IGFBP-2 and -4 but not IGFBP-1 and -3.62 High amounts of IGFBP-2 as determined by radioimmunoassay were detectable in culture supernatant of T-cell lines and promyelocytic HL-60 cells, whereas normal B cells secreted only small amounts of IGFBP-2.63 The addition of IGF-II, and to a lesser extent IGF-I, to leukemic T cells increased IGFBP-2 secretion significantly.64 Thus, IGFBPs may modulate the biology of leukemic cells conferring either a growth-enhancing or growth-inhibitory effect upon them.
In children with leukemia and non-Hodgkin’s lymphoma (NHL), serum concentrations of IGF-I, IGF-II, and IGFBP-3 were significantly decreased whereas IGFBP-2 levels were elevated.65 After hematological remission, all 4 parameters had normalized, which is a further indication that IGFBP-2 levels may directly relate to the proliferation of lymphoblasts.66 Thus, IGFBP analysis could be of relevance for the surveillance of remission and/or early diagnosis of disease relapse.
In conclusion, future research should therefore provide us with a more profound insight into the role of IGFs for the proliferation and differentiation of hematological malignancies. Above all, novel therapeutic approaches that target the IGF system may be designed, which should improve the outcome of malignant hematological disease.
Supported by Grants Bu 10-0361 from the Deutsche Krebshilfe, BioRegio 0311661 TP5 and TP10 from the Bundesministerium für Bildung und Forschung, and the Elterninitiative Kinderkrebsklinik e.V.
REFERENCES
Author notes
Address reprint requests to Walter Zumkeller, MD, Department of Hematology/Oncology, Martin-Luther-University Halle-Wittenberg, Children’s Hospital Medical Center, 06097 Halle, Germany; e-mail: walter.zumkeller@medizin.uni-halle.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal