Abstract
B cell acute lymphoblastic leukemia (B-ALL) accounts for 80% of total ALL cases and often occurs in children and adolescents. With contemporary therapies, the 5-year overall survival (OS) rate is over 80% in childhood ALL patients, but is merely 30%~40% for refractory/relapsed and/or adult B-ALL patients. In addition, pediatric patients with high-risk ALL often receive intensive chemotherapy, which may reduce quality of life due to acute and long-term toxicity and long-term morbidity. Thus, development of improved therapeutics with minimal side effects is urgently needed, especially for the patients with refractory/relapsed B-ALL.
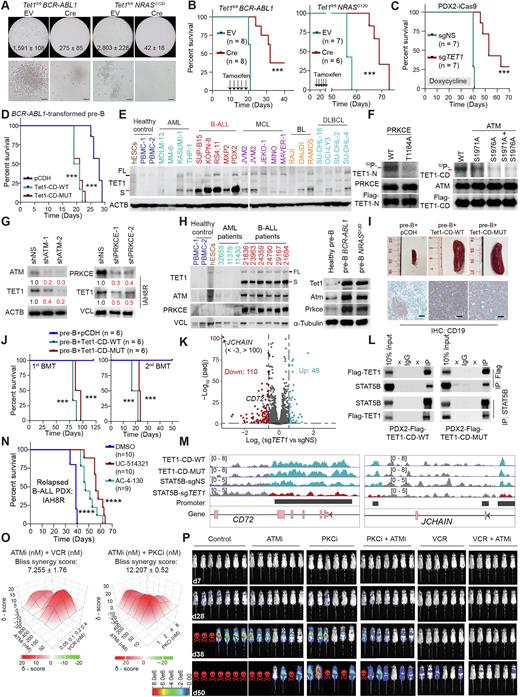
Ten-eleven translocation 1 (TET1) is a dioxygenase responsible for DNA demethylation and impacts broad transcriptional processes and genome stability in normal and pathological processes. It was reported that the deficiency of Tet family proteins (Tet1/2/3) promotes mature B-cell lymphoma development in a catalytic activity-dependent manner in mice (Cimmino et al., 2015; Shukla et al., 2022). Here, we found that induced ablation of Tet1 significantly inhibited the colony formation of BCR-ABL1- or NRASG12D-transformed murine precursor B (pre-B) cells in vitro (Fig. 1A) and delayed the onset of B-ALL in vivo, resulting in substantially prolonged overall survival (OS) in the immunocompromised NSG recipient mice (Fig. 1B). Induced knockout of TET1 recapitulated this phenotype in human B-ALL patient-derived xenotransplantation (PDX) model (Fig. 1C). Conversely, forced expression of either Tet1-catalytic domain (Tet1-CD-WT) or its catalytically dead mutant (Tet1-CD-MUT) significantly promoted BCR-ABL1-mediated transformation of normal pre-B cells and B-ALL development (Fig. 1D), indicating that TET1's oncogenic role is independent from its catalytic activity.
In contrast to its low mRNA level in B-ALL, TET1's protein, predominantly in a short isoform (TET1-S; Note: Refer to TET1 here for the simplicity) is aberrantly highly overexpressed in B-ALL patient samples, PDX cells and cell lines of diverse subtypes, relative to healthy controls, acute myeloid leukemia (AML) or lymphoma cells (Fig. 1E). Strikingly, we showed that TET1 protein is stabilized due to the phosphorylation at T1164, S1971 and S1976, which is mediated by protein kinase C epsilon (PRKCE) and the ATM serine/threonine kinase (ATM) (Figs. 1F and G); both PRKCE and ATM are also overexpressed in human and murine B-ALL cells, and their transcription is positively regulated by B-ALL oncoproteins (Fig. 1H). In line with the critical oncogenic role of TET1 in the initiation, progression, and maintenance of various major subtypes of B-ALL (e.g., those carrying BCR-ABL1 or NRAS mutations or MLL fusions), ectopic expression of either Tet1-CD-WT or Tet1-CD-MUT alone in normal pre-B cells is sufficient to transform the cells and cause full-blown B-ALL in mice within 3 months (Figs. 1I and J). Mechanistically, TET1 recruits STAT5B to the promotors of critical downstream target genes (e.g., CD72 and JCHAIN) and promotes their transcription independent of TET1's catalytic activity, which in turn promotes B-ALL development (Figs. 1K-M). Destabilization of TET1 protein by treatment with PRKCE or ATM inhibitors, or pharmacological targeting of its binding cofactor (STAT5B; UC-514321 and AC-4-130), greatly decreases B-ALL cell viability and inhibits B-ALL progression (Fig. 1N). The combination of ATM inhibitor (AZD0156) with PRKCE inhibitor (Staurosporine) or vincristine (a first-line chemotherapy agent for B-ALL) exhibits a synergistic effect on inhibition of relapsed B-ALL cell survival and leukemia progression (Figs. 1O and P).
Overall, our findings reveal that TET1 protein plays a critical oncogenic role independent of its demethylase activity in the development of precursor B-cell malignancies and demonstrate the therapeutic potential of targeting TET1 signaling by suppressing its upstream regulators (ATM and PRKCE) or binding cofactor (STAT5) in treating refractory/relapsed B-ALL. Thus, our studies not only provide profound novel insight into the molecular mechanism(s) underlying B-ALL pathogenesis by revealing the functional importance of the TET1 signaling in regulating gene expression and leukemogenesis independent of its DNA demethylase activity, but also highlight the therapeutic potential of targeting the TET1 signaling in treating refractory/relapsed B-ALL.
Disclosures
Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings.
Author notes
Asterisk with author names denotes non-ASH members.