Skip Nav Destination
Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease
Onsets of progression and second treatment determine survival of patients with symptomatic Waldenström macroglobulinemia
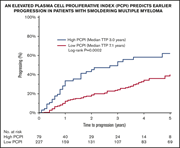
Plasma cell proliferative index is an independent predictor of progression in smoldering multiple myeloma
Comment on model-based meta-analysis to evaluate optimal doses of direct oral factor Xa inhibitors in atrial fibrillation patients
Issue Archive
Table of Contents
EDITORIAL
CLINICAL GUIDELINES
American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients
Holger J. Schünemann,Mary Cushman,Allison E. Burnett,Susan R. Kahn,Jan Beyer-Westendorf,Frederick A. Spencer,Suely M. Rezende,Neil A. Zakai,Kenneth A. Bauer,Francesco Dentali,Jill Lansing,Sara Balduzzi,Andrea Darzi,Gian Paolo Morgano,Ignacio Neumann,Robby Nieuwlaat,Juan J. Yepes-Nuñez,Yuan Zhang,Wojtek Wiercioch
American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism
Wendy Lim,Grégoire Le Gal,Shannon M. Bates,Marc Righini,Linda B. Haramati,Eddy Lang,Jeffrey A. Kline,Sonja Chasteen,Marcia Snyder,Payal Patel,Meha Bhatt,Parth Patel,Cody Braun,Housne Begum,Wojtek Wiercioch,Holger J. Schünemann,Reem A. Mustafa
American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy
Daniel M. Witt,Robby Nieuwlaat,Nathan P. Clark,Jack Ansell,Anne Holbrook,Jane Skov,Nadine Shehab,Juliet Mock,Tarra Myers,Francesco Dentali,Mark A. Crowther,Arnav Agarwal,Meha Bhatt,Rasha Khatib,John J. Riva,Yuan Zhang,Gordon Guyatt
American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism
Paul Monagle,Carlos A. Cuello,Caitlin Augustine,Mariana Bonduel,Leonardo R. Brandão,Tammy Capman,Anthony K. C. Chan,Sheila Hanson,Christoph Male,Joerg Meerpohl,Fiona Newall,Sarah H. O’Brien,Leslie Raffini,Heleen van Ommen,John Wiernikowski,Suzan Williams,Meha Bhatt,John J. Riva,Yetiani Roldan,Nicole Schwab,Reem A. Mustafa,Sara K. Vesely
American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy
Shannon M. Bates,Anita Rajasekhar,Saskia Middeldorp,Claire McLintock,Marc A. Rodger,Andra H. James,Sara R. Vazquez,Ian A. Greer,John J. Riva,Meha Bhatt,Nicole Schwab,Danielle Barrett,Andrea LaHaye,Bram Rochwerg
American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia
Adam Cuker,Gowthami M. Arepally,Beng H. Chong,Douglas B. Cines,Andreas Greinacher,Yves Gruel,Lori A. Linkins,Stephen B. Rodner,Sixten Selleng,Theodore E. Warkentin,Ashleigh Wex,Reem A. Mustafa,Rebecca L. Morgan,Nancy Santesso
EXCEPTIONAL CASE REPORT
Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease
Clinical Trials & Observations
Satoshi Kaito,Takashi Toya,Kota Yoshifuji,Shuhei Kurosawa,Kyoko Inamoto,Kozue Takeshita,Wataru Suda,Kazuhiko Kakihana,Kenya Honda,Masahira Hattori,Kazuteru Ohashi
REVIEW ARTICLES
CLINICAL TRIALS AND OBSERVATIONS
Onsets of progression and second treatment determine survival of patients with symptomatic Waldenström macroglobulinemia
Clinical Trials & Observations
Stephanie Guidez,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Julien Labreuche,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Elodie Drumez,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Loic Ysebaert,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Jana Bakala,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Caroline Delette,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Bénédicte Hivert,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Caroline Protin,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Hervé Declercq,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Mélanie Verlay,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Jean Pierre Marolleau,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Alain Duhamel,on behalf of the French Innovative Leukemia Organization (FILO) CLL group,Pierre Morel,on behalf of the French Innovative Leukemia Organization (FILO) CLL group
IMMUNOBIOLOGY AND IMMUNOTHERAPY
LYMPHOID NEOPLASIA
Plasma cell proliferative index is an independent predictor of progression in smoldering multiple myeloma
Clinical Trials & Observations
Mohammed A. Aljama,M. Hasib Sidiqi,Arjun Lakshman,Angela Dispenzieri,Dragan Jevremovic,Morie A. Gertz,Martha Q. Lacy,Francis K. Buadi,David Dingli,Eli Muchtar,Amie L. Fonder,Suzanne R. Hayman,Miriam A. Hobbs,Wilson I. Gonsalves,Rahma Warsame,Taxiarchis V. Kourelis,Yi Lisa Hwa,Prashant Kapoor,Nelson Leung,Ronald S. Go,Robert A. Kyle,S. Vincent Rajkumar,Shaji K. Kumar
MYELOID NEOPLASIA
THROMBOSIS AND HEMOSTASIS
GLOBAL ADVANCES
COMMENTARY
Comment on model-based meta-analysis to evaluate optimal doses of direct oral factor Xa inhibitors in atrial fibrillation patients
Clinical Trials & Observations
Stefan Willmann,Liping Zhang,Hannah Mayer,Hans-Ulrich Siegmund,Takahiko Tanigawa,Masato Kaneko,Gary Peters,Jeffrey I. Weitz,Scott D. Berkowitz,Rolf Burghaus
-
Cover Image
Cover Image
![issue cover]()
COVER FIGURE
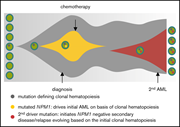
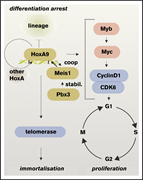
Model for clonal hematopoiesis driving relapse or secondary leukemia. The first leukemia is cured by chemotherapy based on the underlying clonal hematopoiesis. A second driver mutation then leads to overt relapse. See the article by Höllein et al. - PDF Icon Front MatterFront Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Advertisement intended for health care professionals