Key Points
PCPI identifies high-risk SMM.
Patients with an elevated proliferative index have a shorter time to progression, independent of conventional risk models.
Abstract
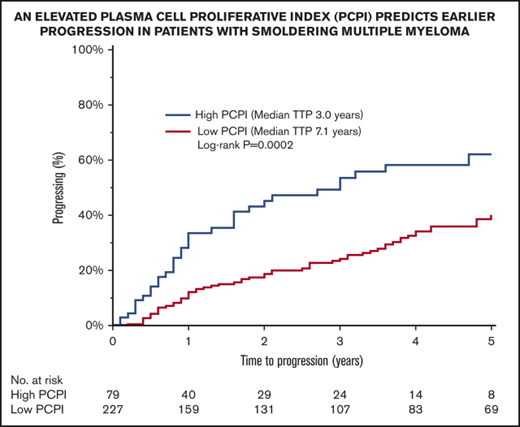
The plasma cell proliferative index (PCPI), determined by a slide technique or by flow cytometry, detects cells in the S phase of the cell cycle and is a useful prognostic tool in patients with plasma cell disorders such as multiple myeloma and amyloidosis. We conducted a retrospective review analyzing the prognostic effect of PCPI in 306 patients with smoldering multiple myeloma (SMM). Seventy-nine (26%) patients had an elevated PCPI (>0.5). An elevated PCPI predicted an inferior time to progression (median, 3.0 vs 7.1 years for those with a low PCPI; P = .0004). Within 24 months, the progression rate was significantly higher for patients with an elevated PCPI (49% vs. 20%; P < .0001). PCPI is a valuable tool in risk stratifying patients with SMM and identifies patients with earlier progression who may benefit from closer follow-up and consideration of early intervention trials.
Introduction
Smoldering multiple myeloma (SMM) is an asymptomatic clonal proliferative plasma cell disorder that encompasses a heterogeneous population of patients with varying risks of progression to multiple myeloma (MM) or immunoglobulin light chain (AL) amyloidosis.1,2 SMM is currently defined as an M-protein of at least 3 g/dL and/or 10% to 59% bone marrow clonal plasmacytosis without any myeloma-defining events or amyloidosis.3 The estimated incidence is 0.9 cases per 100 000 persons.4
The plasma cell proliferative index (PCPI) detects the cells in the S phase of the cell cycle and has been proven to be of prognostic value in patients with multiple myeloma and AL amyloidosis,5-7 It recognizes cells that are actively synthesizing DNA and gives an indication of the proliferative rate of the malignant plasma cells. Data are limited on the utility of this test in patients with SMM.8 Furthermore, recently, flow-cytometric methods developed to measure the PCPI are efficient, more reproducible, and applicable in many laboratories worldwide.9 Here, we report on the role the PCPI plays in predicting progression in patients with SMM.
Patients and methods
Study cohort
We conducted a retrospective study of patients with newly diagnosed SMM seen at Mayo Clinic between 1 July 1996 and 30 June 2016. The International Myeloma Working Group (IMWG) updated diagnostic criteria were used to determine eligibility.10 Patients were included in the analysis if they had a PCPI performed by a slide technique or flow cytometry within 6 months of diagnosis and before documentation of any evidence of progression. Patients were excluded if they had received any form of chemotherapy. Demographic and laboratory data were extracted from a prospectively maintained computerized database, as well as patients’ records. The data cutoff date was 30 June 2017.
The study was approved by the Mayo Clinic Institutional Review Board, according to federal regulations and in accordance with the Declaration of Helsinki. All patients consented to have their medical records reviewed according to institutional review board practices.
Outcome measures
Time to progression (TTP) was the primary end point and was calculated from the time of PCPI to the time of progression, which was defined as development of organ damage attributable to the plasma cell proliferative disorder, using the cutoffs proposed in the 2014 IMWG criteria for diagnosis of MM, initiation of therapy in the absence of CRAB (hypercalemia, renal failure, anemia, bony lesions) features, or development of immunoglobulin light chain amyloidosis.10 Patients were censored in the TTP analysis if they did not progress at the date of last follow-up, started therapy with an antimyeloma agent on a clinical trial for SMM, or initiated therapy with systemic corticosteroid or anticancer chemotherapy for any other indication.
Plasma cell proliferative index
Before May 2012, the PCPI was measured using the bromodeoxyuridine method, described previously.11 From May 2012 onward, the bromodeoxyuridine method was replaced by the DNA content measurement, using flow cytometry. Briefly, the bone marrow specimen was spun down and the pellet is lysed using 14 mL ACK (ammonium-chloride-potassium) lysing buffer (Thermo Fisher Scientific), followed by 2 washes with phosphate-buffered saline (PBS). The cell pellet was then resuspended in 0.2% bovine serum albumin/PBS with Azide (BD Pharmingen) and stained with the following antibodies: CD138 PerCPcy5.5, CD19 PE-cy7, CD38 FITC, and CD45 APC-H7 (all from BD Biosciences) for 15 minutes. After a wash with Caltag A reagent (Thermo Fisher Scientific), the pellet was resuspended in Caltag B reagent for permeabilization. Antibodies for cytoplasmic staining were added (κ APC and λ PE, both from Dako North America Inc.), and the specimen was incubated for 20 minutes. This was followed by the wash step and the incubation in the 1000 units/mL RNAse in PBS (Worthington Biochemical Corporation). A 21.4-μM working dilution of 4′,6-diamidino-2-phenylindole (Life Technologies) was added to the cell suspension and incubated at 4°C for 30 minutes. The cell pellet was then resuspended in 500 μL PBS. The flow cytometry fetal calf serum files were obtained on BD FACSCanto II instruments (500 000 events per specimen). The files are analyzed using Kaluza software (Beckman Coulter). Initial broad gates are set on CD138+CD38+ events. The clonal (abnormal) plasma cells are separated from the normal plasma cells, using differential expression of CD38, CD19, CD45, κ, and λ. 4′,6-Diamidino-2-phenylindole staining on polyclonal plasma cells is used to determine ploidy. The S-phase of clonal plasma cells was calculated by manually gating on G0G1 and G2M peaks and dividing the number of events in the S-window by the total number of abnormal plasma cells. A minimum of 300 abnormal plasma cells were needed to reliably calculate S-phase. The PCPI was reported as the percentage of clonal plasma cells actively proliferating in the S-phase of the cell cycle. After an initial analysis, we identified 2 cohorts of patients based on the PCPI level: those with low PCPI (PCPI≤0.5%; n = 227) and those with elevated PCPI (PCPI>0.5%; n = 79).
Statistical analysis
Statistical analysis was performed on JMP Pro software version 13.0 (SAS, Cary, NC). Patient- and disease-related factors were compared using the χ2 test for categorical variables and the Wilcoxon signed rank test for continuous variables. Progression analysis was performed using the Kaplan-Meier method. Groups were compared with the 2-tailed log-rank test. All statistical tests were 2-sided, and P < .05 was considered to be significant. Variables with P < .05 on the univariate analysis were used to construct a multivariable model.
Results
Patient characteristics
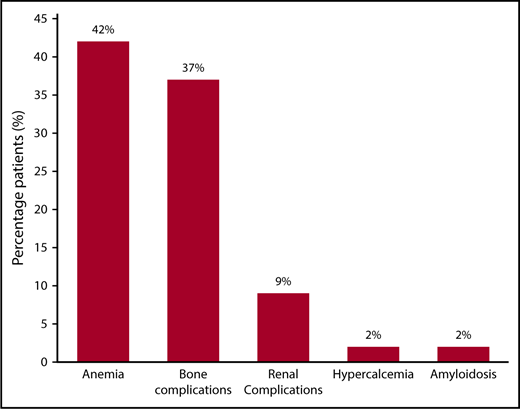
Three hundred six patients fulfilled the diagnostic criteria for SMM diagnosis and had a PCPI assessment within 6 months of diagnosis and before the documentation of any evidence of progression. Baseline demographic and laboratory data are outlined in Table 1. The median follow-up for the entire cohort was 10.2 years (95% confidence interval, 9.0-10.9 years). The median TTP was 5.9 years (95% confidence interval, 4.8-8.2 years). The median time between the diagnosis and PCPI date was 0 months (interquartile range, 0-1 months). The median age was 66 (range, 56-73) years, and 61% were men. Two hundred sixty (85%) patients had available free light chain levels at the time of diagnosis. Seventy-two (24%) patients had advanced imaging in the form of a positron emission tomography/computed tomography scan, computed tomography skeletal survey, or whole spine ± pelvic magnetic resonance imaging scan, and 10 (3%) had a combination of localized spine magnetic resonance imaging plus conventional X-ray skeletal survey, whereas 223 (73%) had a conventional X-ray skeletal survey only, documenting the absence of lytic lesions within 6 months of diagnosis. One-hundred sixty-nine (55%) patients were alive at the time of study analysis, whereas 118 (39%) had progressive disease. The distribution of symptomatic events at the time of progression is illustrated in Figure 1. Anemia (defined as a hemoglobin level <10 g/dL or a hemoglobin level >2 g/dL below the lower limit of normal) was present in 42%, and bone complications (37%) were the most common events at progression. Seventeen (14%) patients were categorized as having progression resulting from rapid progressive elevation in the serum-free light chains (sFLCs) and/or progressive increase in the size of their M-spike.
Baseline characteristics at the time of diagnosis grouped, according to PCPI
| Variable . | Entire cohort . | High PCPI . | Low PCPI . | P . |
|---|---|---|---|---|
| Patients, n (%) | 306 | 79 (26) | 227 (74) | |
| Median age , y | 66 | 69 | 64 | .008 |
| Age >65 y, n (%) | 159 (52) | 52 (66) | 107 (47) | .006 |
| Sex, male (%) | 186 (61) | 47 (59) | 139 (61) | .79 |
| Median hemoglobin, g/dL | 12.7 | 12.5 | 12.7 | .04 |
| Median β2 microglobulin, μg/mL | 2.5 | 2.9 | 2.4 | .03 |
| Median serum albumin, g/dL | 3.6 | 3.5 | 3.7 | .05 |
| Abnormal LDH, U/L, % | 16/227 (7) | 5/59 (8) | 11/168 (7) | .56 |
| Median creatinine, mg/dL | 1.1 | 1 | 1.1 | .19 |
| Bone marrow evaluation | ||||
| Median bone marrow plasma cells, % | 20 | 20 | 20 | .10 |
| BM plasma cells >20 (%) | 115 (38) | 38 (48) | 77 (34) | .03 |
| BM plasma cells ≤20 (%) | 191 (62) | 41 (52) | 150 (66) | |
| BM plasma cells ≥10 (%) | 302 (99) | 77 (97) | 225 (99) | .27 |
| BM plasma cells <10 (%) | 4 (1) | 2 (3) | 2 (1) | |
| FLC ratio (involved/uninvolved) ≥8 (%) | 123/260 (47) | 36/71 (51) | 87/189 (46) | .58 |
| Involved light chains (%) | .49 | |||
| κ | 196 (64) | 48 (61) | 148 (66) | |
| λ | 108 (36) | 31 (39) | 77 (34) | |
| Serum monoclonal protein | ||||
| Median M-spike, g/dL | 2.1 | 1.9 | 2.1 | .06 |
| ≥3 (%) | 62 (20) | 12/79 (17) | 50/226 (22) | .26 |
| <3 (%) | 243 (80) | 67/79 (83) | 176/226 (78) | |
| >2 (%) | 156 (51) | 33 (42) | 123 (54) | |
| ≤2 (%) | 149 (49) | 46 (58) | 103 (46) | |
| Median light chain concentration, mg/dL | ||||
| κ | 2.5 | 2.8 | 2.4 | .76 |
| λ | 1.2 | 1.3 | 1.2 | .14 |
| Ig heavy chain, mg/dL (%) | ||||
| IgG | 234 (76) | 58 (73) | 176 (78) | .45 |
| IgA | 57 (19) | 18 (23) | 39 (17) | .31 |
| IgM | 3 (1) | 0 (0) | 3 (1) | .57 |
| IgD | 2 (1) | 2 (3) | 0 (0) | .07 |
| Light chains only and others (%) | 10 (3) | 1 (1) | 9 (4) | .46 |
| Variable . | Entire cohort . | High PCPI . | Low PCPI . | P . |
|---|---|---|---|---|
| Patients, n (%) | 306 | 79 (26) | 227 (74) | |
| Median age , y | 66 | 69 | 64 | .008 |
| Age >65 y, n (%) | 159 (52) | 52 (66) | 107 (47) | .006 |
| Sex, male (%) | 186 (61) | 47 (59) | 139 (61) | .79 |
| Median hemoglobin, g/dL | 12.7 | 12.5 | 12.7 | .04 |
| Median β2 microglobulin, μg/mL | 2.5 | 2.9 | 2.4 | .03 |
| Median serum albumin, g/dL | 3.6 | 3.5 | 3.7 | .05 |
| Abnormal LDH, U/L, % | 16/227 (7) | 5/59 (8) | 11/168 (7) | .56 |
| Median creatinine, mg/dL | 1.1 | 1 | 1.1 | .19 |
| Bone marrow evaluation | ||||
| Median bone marrow plasma cells, % | 20 | 20 | 20 | .10 |
| BM plasma cells >20 (%) | 115 (38) | 38 (48) | 77 (34) | .03 |
| BM plasma cells ≤20 (%) | 191 (62) | 41 (52) | 150 (66) | |
| BM plasma cells ≥10 (%) | 302 (99) | 77 (97) | 225 (99) | .27 |
| BM plasma cells <10 (%) | 4 (1) | 2 (3) | 2 (1) | |
| FLC ratio (involved/uninvolved) ≥8 (%) | 123/260 (47) | 36/71 (51) | 87/189 (46) | .58 |
| Involved light chains (%) | .49 | |||
| κ | 196 (64) | 48 (61) | 148 (66) | |
| λ | 108 (36) | 31 (39) | 77 (34) | |
| Serum monoclonal protein | ||||
| Median M-spike, g/dL | 2.1 | 1.9 | 2.1 | .06 |
| ≥3 (%) | 62 (20) | 12/79 (17) | 50/226 (22) | .26 |
| <3 (%) | 243 (80) | 67/79 (83) | 176/226 (78) | |
| >2 (%) | 156 (51) | 33 (42) | 123 (54) | |
| ≤2 (%) | 149 (49) | 46 (58) | 103 (46) | |
| Median light chain concentration, mg/dL | ||||
| κ | 2.5 | 2.8 | 2.4 | .76 |
| λ | 1.2 | 1.3 | 1.2 | .14 |
| Ig heavy chain, mg/dL (%) | ||||
| IgG | 234 (76) | 58 (73) | 176 (78) | .45 |
| IgA | 57 (19) | 18 (23) | 39 (17) | .31 |
| IgM | 3 (1) | 0 (0) | 3 (1) | .57 |
| IgD | 2 (1) | 2 (3) | 0 (0) | .07 |
| Light chains only and others (%) | 10 (3) | 1 (1) | 9 (4) | .46 |
LDH, lactate dehydrogenase.
Risk for progression to myeloma or related disorder in 306 patients with SMM, using a PCPI cutoff of 0.5.
Risk for progression to myeloma or related disorder in 306 patients with SMM, using a PCPI cutoff of 0.5.
Patients with elevated PCPI
The median PCPI level in this cohort was 0.2 (interquartile range, 0-0.6). In this cohort, 79 (26%) patients had an elevated PCPI above 0.5%. Patients with an elevated PCPI were significantly older, with a median age of 69 years compared with 64 years for those with a low PCPI (P = .008). The percentage of patients with bone marrow plasma cells of more than 20% was higher in the high PCPI group, at 48 vs 34% (P = .03). Otherwise, baseline features were not significantly different between the 2 cohorts (Table 1).
Factors predicting risk for progression
An elevated PCPI predicted a shorter TTP (median, 3.0 vs 7.1 years for those with a low PCPI; P = .0004; Figure 2). At 24 months, the progression rate was significantly higher for patients with an elevated PCPI (49 vs 20%; P < .0001). As TTP increased, the proportion of patients with an elevated PCPI was reduced compared with those with a low PCPI (TTP <5 years, 70% for low PCPI vs 90% for elevated PCPI; TTP 5-10 years, 22% for low PCPI vs 6% for elevated PCPI; TTP >10 years, 8% for low PCPI vs 4% for elevated PCPI; P = .0015). We performed a subgroup analysis risk for progression in each PCPI cohort, according to the PCPI method used (bromodeoxyuridine method before May 2012, method 1; flow cytometry method from May 2012 onward, method 2). An elevated PCPI was predictive of a shorter TTP in method 1 (median TTP, 6.8 years for low PCPI vs 3 years for elevated PCPI; P = .0034). When calculated using method 2, an elevated PCPI showed a trend toward shorter TTP (median TTP, not reached for low PCPI vs 4.7 years for elevated PCPI; P = .08) without reaching statistical significance. However, this is likely to reflect the low numbers in this cohort (method 2; low PCPI, n = 28; elevated PCPI, n = 21).
We tested the conventional Mayo risk stratification assessment parameters for progression.12 Abnormal sFLC of more than 8 was associated with a shorter TTP (4.0 vs 10.4 years; P < .0001). The median TTP for patients with an elevated M-spike of at least 3 g/dL was 3.7 years compared with 7.4 years for patients with less than 3 g/dL (P = .001). The para-protein isotype (immunoglobulin A [IgA] vs others) was 6.5 vs 5.6 years (P = .49). The risk for progression for high-, intermediate-, and low-risk conventional Mayo grouping is 3.4, 5.3, and 11.7 years, respectively (P < .0001). One-hundred sixty-five patients had cytogenetic analysis performed; 34 (21%) were deemed high risk. High-risk cytogenetic features did not predict TTP (7.9 vs 6.8; P = .76).
We constructed 2 multivariable models using the conventional and recently proposed Mayo risk stratification tools for SMM.12,13 On both models, an elevated PCPI was an independent predictor of progression (Table 2).
Univariable and multivariable analysis
| Variable . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| Factors predicting TTP using the conventional Mayo risk stratification system | ||||||
| Bone marrow plasma cells (≥10%) | 0.3 | 0.1-5.1 | .31 | NA | ||
| Serum M-spike (≥3 g/dL) | 2.0 | 1.3-2.9 | .002 | 2.2 | 1.3-3.5 | .003 |
| sFLC ratio (≥8) | 2.2 | 1.5-3.4 | .0001 | 2.3 | 1.5-3.6 | .0002 |
| Elevated PCPI | 2.0 | 1.3-3.0 | .001 | 2.5 | 1.6-3.9 | <.0001 |
| Immunoparesis | 1.6 | 1.0-2.5 | .04 | 1.2 | 0.8-2.1 | .39 |
| Factors predicting TTP using the proposed new Mayo risk stratification system | ||||||
| Bone marrow plasma cells (>20%) | 2.7 | 1.8-4.1 | <.0001 | 2.2 | 1.4-3.5 | <0.0008 |
| Serum M-spike (>2 g/dL) | 2.2 | 1.5-3.2 | <.0001 | 1.9 | 1.2-3.0 | 0.007 |
| sFLC ratio (>20) | 2.7 | 1.8-4.1 | <.0001 | 2.7 | 1.7-4.2 | 0.22 |
| Elevated PCPI | 2.0 | 1.3-3.0 | .001 | 2.3 | 1.4-3.5 | 0.0007 |
| Immunoparesis | 1.6 | 1.0-2.5 | .04 | 1.0 | 0.6-1.7 | 0.97 |
| Variable . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| Factors predicting TTP using the conventional Mayo risk stratification system | ||||||
| Bone marrow plasma cells (≥10%) | 0.3 | 0.1-5.1 | .31 | NA | ||
| Serum M-spike (≥3 g/dL) | 2.0 | 1.3-2.9 | .002 | 2.2 | 1.3-3.5 | .003 |
| sFLC ratio (≥8) | 2.2 | 1.5-3.4 | .0001 | 2.3 | 1.5-3.6 | .0002 |
| Elevated PCPI | 2.0 | 1.3-3.0 | .001 | 2.5 | 1.6-3.9 | <.0001 |
| Immunoparesis | 1.6 | 1.0-2.5 | .04 | 1.2 | 0.8-2.1 | .39 |
| Factors predicting TTP using the proposed new Mayo risk stratification system | ||||||
| Bone marrow plasma cells (>20%) | 2.7 | 1.8-4.1 | <.0001 | 2.2 | 1.4-3.5 | <0.0008 |
| Serum M-spike (>2 g/dL) | 2.2 | 1.5-3.2 | <.0001 | 1.9 | 1.2-3.0 | 0.007 |
| sFLC ratio (>20) | 2.7 | 1.8-4.1 | <.0001 | 2.7 | 1.7-4.2 | 0.22 |
| Elevated PCPI | 2.0 | 1.3-3.0 | .001 | 2.3 | 1.4-3.5 | 0.0007 |
| Immunoparesis | 1.6 | 1.0-2.5 | .04 | 1.0 | 0.6-1.7 | 0.97 |
RR, relative risk; CI, confidence interval.
Discussion
SMM represents a heterogeneous population of patients with varying risks of progression to MM or AL amyloidosis.1 Several clinical and laboratory factors have been identified with increased risk for progression, including size of the monoclonal protein, extent of bone marrow involvement, high levels of circulating plasma cells, M protein isotype, abnormally high serum free light chain ratio, aberrant plasma cell immunophenotype, evolving changes in protein levels, high-risk cytogenetic abnormalities, and immunoparesis.1,12,14-22 Recently, our group attempted to further refine the previous thresholds used in our risk model.12,14 Although the current standard of care is watchful waiting, early therapeutic intervention has been attempted to delay progression.23 This is becoming more feasible with the introduction of more efficacious and less toxic therapy. Therefore, identification of patients with higher risk for progression is becoming more important.
Our study shows that an elevated PCPI predicts earlier progression with a significantly higher proportion of patients progressing within 2 years compared with those with a low PCPI. This was independent of other previously recognized factors influencing TTP. PCPI was previously measured using a slide-based method that was less efficient and not available in many centers. The current flow-cytometric technique can be performed relatively quickly and with a greater degree of reproducibility.9 Flow cytometry assessment of marrow samples at diagnosis is becoming the standard of care for patients with plasma cell disorders, and this renders measurement of the PCPI more applicable and widely available. Although 2 different methods were used to calculate the PCPI, we used a cutoff that was significant and applicable to both.
Our study has several strengths including the size of the cohort and long follow-up, but is limited by the inherent biases of a retrospective review. Because the study was conducted over a long period, 15% of patients did not have sFLC levels at the time of diagnosis, and only 27% of patients had advanced imaging performed. Thus, some patients who may have met criteria for a diagnosis of myeloma according to the updated IMWG diagnostic criteria may have been included in our study, and therefore affected the TTP. Moreover, cytogenetic data were available in only a small proportion of patients. Increasing data suggest that these are important predictors of progression risk in monoclonal gammopathy of undetermined significance and SMM.21,22,24 In addition, 2 different methods were used to calculate PCPI during the study period. Subgroup analysis by method of PCPI detection was limited because of the small numbers of patients with a PCPI calculated using the more recent flow cytometric method. The optimal cutoff and predictive value of the PCPI in SMM using the more sensitive flow cytometric method is yet to be determined and requires further study.
Despite these limitations, we have shown that PCPI is a useful tool in risk stratifying patients with SMM, and identifies patients with earlier progression who may benefit from closer follow-up and consideration for early intervention trials.
Authorship
Contribution: M.A.A. and M.H.S. designed the study, analyzed the data, wrote the first draft, and approved the final version of the manuscript; A.L., A.D., D.J., M.Q.L., F.K.B., D.D., E.M., A.L.F., S.R.H., M.A.H., W.I.G., R.W., T.V.K., Y.L.H., P.K., N.L., R.A.K., M.A.G., R.S.G., and S.V.R. performed patient management, revised the manuscript critically, and approved the final version of the manuscript; and S.K.K. designed the study, analyzed the data, wrote the first draft, approved the final version of the manuscript, and performed patient management.
Conflict-of-interest disclosure: M.A.G. received consultancy from Millennium and honoraria from Celgene, Millennium, Onyx, Novartis, SmithKline, Prothena, Ionis, and Amgen. S.K.K. received consultancy from Celgene, Millennium, Onyx, Janssen, and BMS and research funding from Celgene, Millennium, Novartis, Onyx, AbbVie, Janssen, and BMS. M.Q.L. received research funding from Celgene. D.D. received research funding from Karyopharm Therapeutics, Amgen, and Millennium Pharmaceuticals. P.K. received research funding from Takeda, Celgene, and Amgen. A.D. received research funding from Celgene, Millennium, Pfizer, and Janssen and a travel grant from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Shaji K. Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.
References
Author notes
M.A.A. and M.H.S. contributed equally to this manuscript.